Insulin Inclusion into a Tragacanth Hydrogel: An Oral Delivery System for Insulin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterisation
2.2.1. Microparticle Preparation
2.2.2. Acid-Induced Gelation
2.2.3. Particle Size and Zeta Potential Analysis
2.2.4. Measurement of Loading Efficiency
2.2.5. DSC (Differential Scanning Calorimetry) Analysis
2.2.6. FTIR Analysis
2.2.7. Scanning Electron Microscope (SEM)
2.3. Statistics
3. Result and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sonia, T.A.; Sharma, C.P. An overview of natural polymers for oral insulin delivery. Drug Discov. Today 2012, 17, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Nur, M.; Vasiljevic, T. Can natural polymers assist in delivering insulin orally? Int. J. Biol. Macromol. 2017, 103, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Kavimandan, N.J. Nanoscale analysis of protein and peptide absorption: Insulin absorption using complexation and pH-sensitive hydrogels as delivery vehicles. Eur. J. Pharm. Sci. 2006, 29, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ferreira, D.C.; Jorgensen, L.; van de Weert, M. Probing insulin’s secondary structure after entrapment into alginate/chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2007, 65, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Martins, S.; Ribeiro, A.; Veiga, F.; Neufeld, R.; Ferreira, D. Development and comparison of different nanoparticulate polyelectrolyte complexes as insulin carriers. Int. J. Pept. Res. Ther. 2006, 12, 131–138. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- De, S.; Robinson, D. Polymer relationships during preparation of chitosan–alginate and poly-l-lysine–alginate nanospheres. J. Control. Release 2003, 89, 101–112. [Google Scholar] [CrossRef]
- Lee, K.Y.; Park, W.H.; Ha, W.S. Polyelectrolyte complexes of sodium alginate with chitosan or its derivatives for microcapsules. J. Appl. Polym. Sci. 1997, 63, 425–432. [Google Scholar] [CrossRef]
- Lin, Y.H.; Sonaje, K.; Lin, K.M.; Juang, J.H.; Mi, F.L.; Yang, H.W.; Sung, H.W. Multi-ion-crosslinked nanoparticles with pH-responsive characteristics for oral delivery of protein drugs. J. Control. Release 2008, 132, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Avadi, M.R.; Sadeghi, A.M.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and arabic gum with ionic gelation method. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Sarmento, B.; Souto, E.B.; Ferreira, D.C. Insulin-loaded alginate microspheres for oral delivery-effect of polysaccharide reinforcement on physicochemical properties and release profile. Carbohydr. Polym. 2007, 69, 725–731. [Google Scholar] [CrossRef]
- Moses, L.R.; Dileep, K.J.; Sharma, C.P. Beta cyclodextrin–insulin-encapsulated chitosan/alginate matrix: Oral delivery system. J. Appl. Polym. Sci. 2000, 75, 1089–1096. [Google Scholar] [CrossRef]
- Wang, K.; He, Z. Alginate-konjac glucomannan-chitosan beads as controlled release matrix. Int. J. Pharm. 2002, 244, 117–126. [Google Scholar] [CrossRef]
- Onal, S.; Zihnioglu, F. Encapsulation of insulin in chitosan-coated alginate beads: Oral therapeutic peptide delivery. Artif. Cell Blood Substit. Immobil. Biotechnol. 2002, 30, 229–237. [Google Scholar] [CrossRef]
- Silva, C.M.; Ribeiro, A.J.; Figueiredo, I.V.; Gonçalves, A.R.; Veiga, F. Alginate microspheres prepared by internal gelation: Development and effect on insulin stability. Int. J. Pharm. 2006, 311, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Klibanov, A.M. Controling the rate of protein release from polyelectrolyte complexes. Biotechnol. Bioeng. 2003, 82, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W.; Woiszwillo, J.; Sims, R.C.; Middaugh, C.R. Insulin containing polyethylenimine–dextran sulfate nanoparticles. Int. J. Pharm. 2003, 255, 139–151. [Google Scholar] [CrossRef]
- Nur, M.; Ramchandran, L.; Vasiljevic, T. Tragacanth as an oral peptide and protein delivery carrier: Characterization and mucoadhesion. Carbohydr. Polym. 2016, 143, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Mathiowitz, E.; Jacob, J.S.; Jong, Y.S.; Carino, G.P.; Chickering, D.E.; Chaturvedi, P.; Santos, C.A.; Vijayaraghavan, K.; Montgomery, S.; Bassett, M.; et al. Biologically erodable microspheres as potential oral drug delivery systems. Nature 1997, 386, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, K.; Ghaemy, M. Synthesis of new thermo/pH sensitive drug delivery systems based on tragacanth gum polysaccharide. Int. J. Biol. Macromol. 2016, 87, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Qomarudin, Q.; Orbell, J.D.; Ramchandran, L.; Gray, S.R.; Stewart, M.B.; Vasiljevic, T. Properties of beta-lactoglobulin/alginate mixtures as a function of component ratio, pH and applied shear. Food Res. Int. 2015, 71, 23–31. [Google Scholar] [CrossRef]
- Sonia, T.A.; Sharma, C.P. Oral Delivery of Insulin; Elsevier Science: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Kasapis, S.; Bannikova, A. Chapter 2-Rheology and Food Microstructure. In Advances in Food Rheology and Its Applications; Ahmed, J., Ptaszek, P., Basu, S., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 7–46. [Google Scholar]
- Jadhav, K.R.; Pacharane, S.S.; Koshy, V.P.; Kadam, J.V. Smart polymers and their role in drug delivery: A review. Curr. Drug Ther. 2010, 5, 250–261. [Google Scholar] [CrossRef]
- De Lima, G.G.; Kanwar, D.; Macken, D.; Geever, L.; Devine, D.M.; Nugent, M.J.D. Smart hydrogels: Therapeutic advancements in hydrogel technology for smart drug delivery applications. In Handbook of Polymers for Pharmaceutical Technologies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–16. [Google Scholar]
- Zhao, C.; Nie, S.; Tang, M.; Sun, S. Polymeric pH-sensitive membranes—A review. Prog. Polym. Sci. 2011, 36, 1499–1520. [Google Scholar] [CrossRef]
- Reyes-Ortega, F. 3-pH-responsive polymers: Properties, synthesis and applications. In Smart Polymers and Their Applications; Woodhead Publishing: Cambridge, UK, 2014; pp. 45–92. [Google Scholar]
- Lim, H.-P.; Tey, B.-T.; Chan, E.-S. Particle designs for the stabilization and controlled-delivery of protein drugs by biopolymers: A case study on insulin. J. Control. Release 2014, 186, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications, 2nd ed.; CRC Press: Hoboken, NJ, USA, 2014. [Google Scholar]
- Farzi, M.; Yarmand, M.S.; Safari, M.; Emam-Djomeh, Z.; Mohammadifar, M.A. Gum tragacanth dispersions: Particle size and rheological properties affected by high-shear homogenization. Int. J. Biol. Macromol. 2015, 79, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, M.; Bowyer, A.; Eisenthal, R.; Hubble, J. A novel pH- and ionic-strength-sensitive carboxy methyl dextran hydrogel. Biomaterials 2005, 26, 4677–4683. [Google Scholar] [CrossRef] [PubMed]
- Black, K.A.; Priftis, D.; Perry, S.L.; Yip, J.; Byun, W.Y.; Tirrell, M. Protein encapsulation via polypeptide complex coacervation. ACS Macro Lett. 2014, 3, 1088–1091. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Raj, N.K.K.; Sharma, C.P. Oral insulin—A perspective. J. Biomater. Appl. 2003, 17, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Al-Kurdi, Z.; Chowdhry, B.; Leharne, S.; Al Omari, M.; Badwan, A. Low molecular weight chitosan–insulin polyelectrolyte complex: Characterization and stability studies. Mar. Drugs 2015, 13, 1765–1784. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.; Larijani, B.; Ahmadian, S.; Junginger, H.E.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and its quaternized derivatives. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zohuriaan, M.J.; Shokrolahi, F. Thermal studies on natural and modified gums. Polym. Test. 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Mimmo, T.; Marzadori, C.; Montecchio, D.; Gessa, C. Characterisation of Ca- and Al-pectate gels by thermal analysis and FT-IR spectroscopy. Carbohydr. Res. 2005, 340, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Ecletica Quimica 2004, 29, 57–63. [Google Scholar] [CrossRef]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoparticle- and Microparticle-Based Delivery Systems: Encapsulation, Protection and Release of Active Compounds; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Gombotz, W.R.; Wee, S. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Brange, J.; Langkjœr, L. Insulin structure and stability. In Stability and Characterization of Protein and Peptide Drugs; Wang, Y.J., Pearlman, R., Eds.; Springer: New York, NY, USA, 1993; Volume 5, pp. 315–350. [Google Scholar]
- Tahtat, D.; Mahlous, M.; Benamer, S.; Khodja, A.N.; Oussedik-Oumehdi, H.; Laraba-Djebari, F. Oral delivery of insulin from alginate/chitosan crosslinked by glutaraldehyde. Int. J. Biol. Macromol. 2013, 58, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, A.; Petrini, P.; Munarin, F.; Shokoohinia, Y.; Golozar, M.A.; Varshosaz, J.; Tanzi, M.C. Polysaccharides derived from tragacanth as biocompatible polymers and gels. J. Appl. Polym. Sci. 2013, 129, 2092–2102. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Silva, C.; Ferreira, D.; Veiga, F. Chitosan-reinforced alginate microspheres obtained through the emulsification/internal gelation technique. Eur. J. Pharm. Sci. 2005, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Piccirilli, F.; Mangialardo, S.; Postorino, P.; Lupi, S.; Perucchi, A. Ftir analysis of the high pressure response of native insulin assemblies. J. Mol. Struct. 2013, 1050, 159–165. [Google Scholar] [CrossRef]
- Mitrevej, A.; Sinchaipanid, N.; Rungvejhavuttivittaya, Y.; Kositchaiyong, V. Multiunit controlled-release diclofenac sodium capsules using complex of chitosan with sodium alginate or pectin. Pharm. Dev. Technol. 2001, 6, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Guo, X.D.; Zhang, L.J.; Jiang, W.; Ling, L.; Qian, Y.; Chen, Y. Solvent mediated microstructures and release behavior of insulin from pH-sensitive nanoparticles. Colloids Surf. B Biointerfaces 2012, 94, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Lin, Y.-H.; Juang, J.-H.; Wey, S.-P.; Chen, C.-T.; Sung, H.-W. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials 2009, 30, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Djabourov, M.; Nishinari, K.; Ross-Murphy, S.B. Physical Gels from Biological and Synthetic Polymers; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Nejatian, M.; Hatami, M.; Mohammadifar, M.A. Effect of gum tragacanth exuded by three iranian astragalus on mixed milk protein system during acid gelation. Int. J. Biol. Macromol. 2013, 53, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Matia-Merino, L.; Lau, K.; Dickinson, E. Effects of low-methoxyl amidated pectin and ionic calcium on rheology and microstructure of acid-induced sodium caseinate gels. Food Hydrocoll. 2004, 18, 271–281. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Huang, Q.; Tong, Z.; Yao, J.; Kong, X. A composite hydrogel system containing glucose-responsive nanocarriers for oral delivery of insulin. Mater. Sci. Eng. C 2016, 69, 37–45. [Google Scholar] [CrossRef] [PubMed]
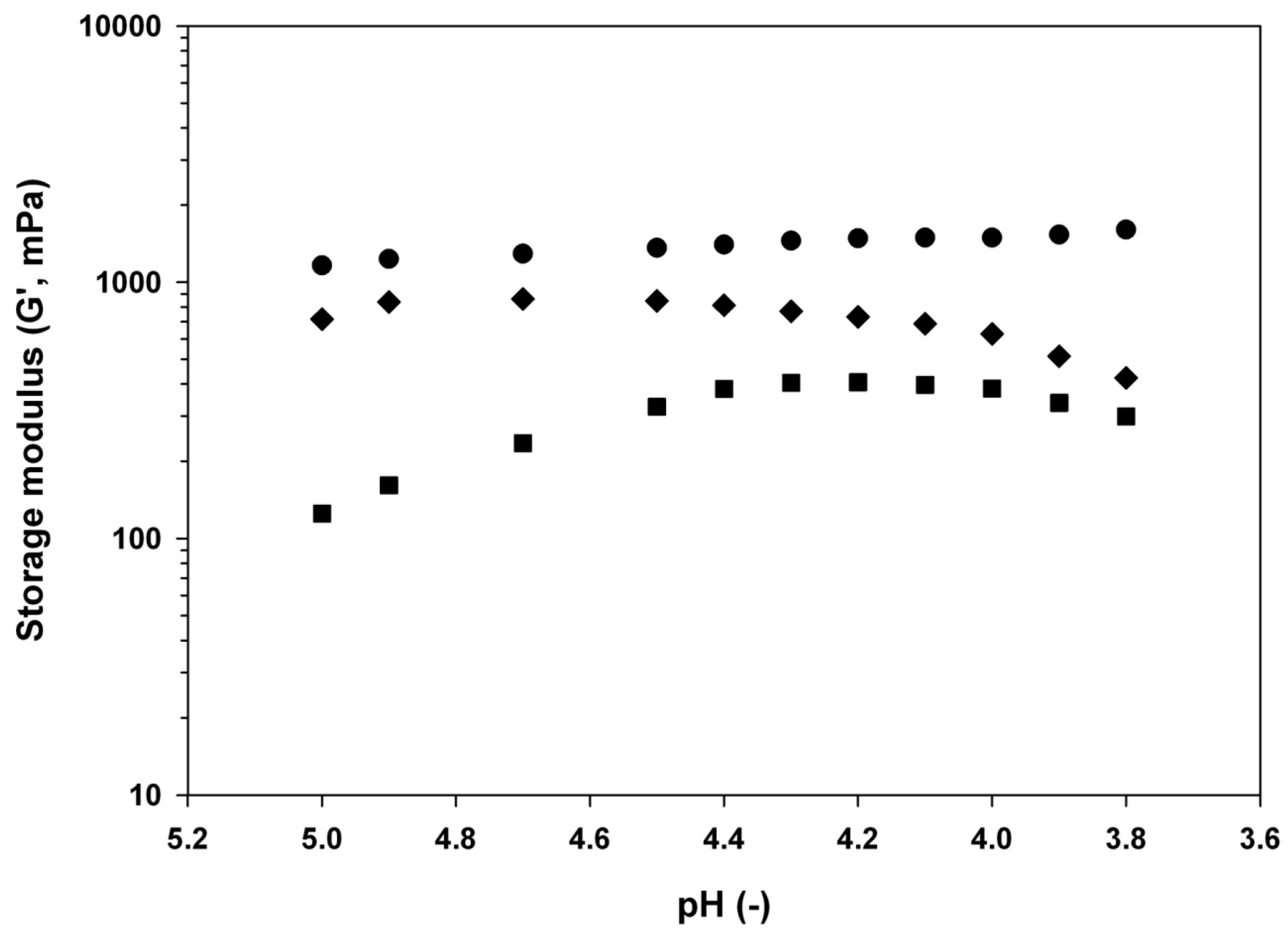
 −0.1% w/w,
−0.1% w/w,  −0.5% w/w, and
−0.5% w/w, and  −1% w/w) at different pH (3.7, 4.3, 4.6, or 6) adjusted by addition of GDL at room temperature and equilibration under very low magnetic stirring overnight.
−1% w/w) at different pH (3.7, 4.3, 4.6, or 6) adjusted by addition of GDL at room temperature and equilibration under very low magnetic stirring overnight.
 −0.1% w/w,
−0.1% w/w,  −0.5% w/w, and
−0.5% w/w, and  −1% w/w) at different pH (3.7, 4.3, 4.6, or 6) adjusted by addition of GDL at room temperature and equilibration under very low magnetic stirring overnight.
−1% w/w) at different pH (3.7, 4.3, 4.6, or 6) adjusted by addition of GDL at room temperature and equilibration under very low magnetic stirring overnight.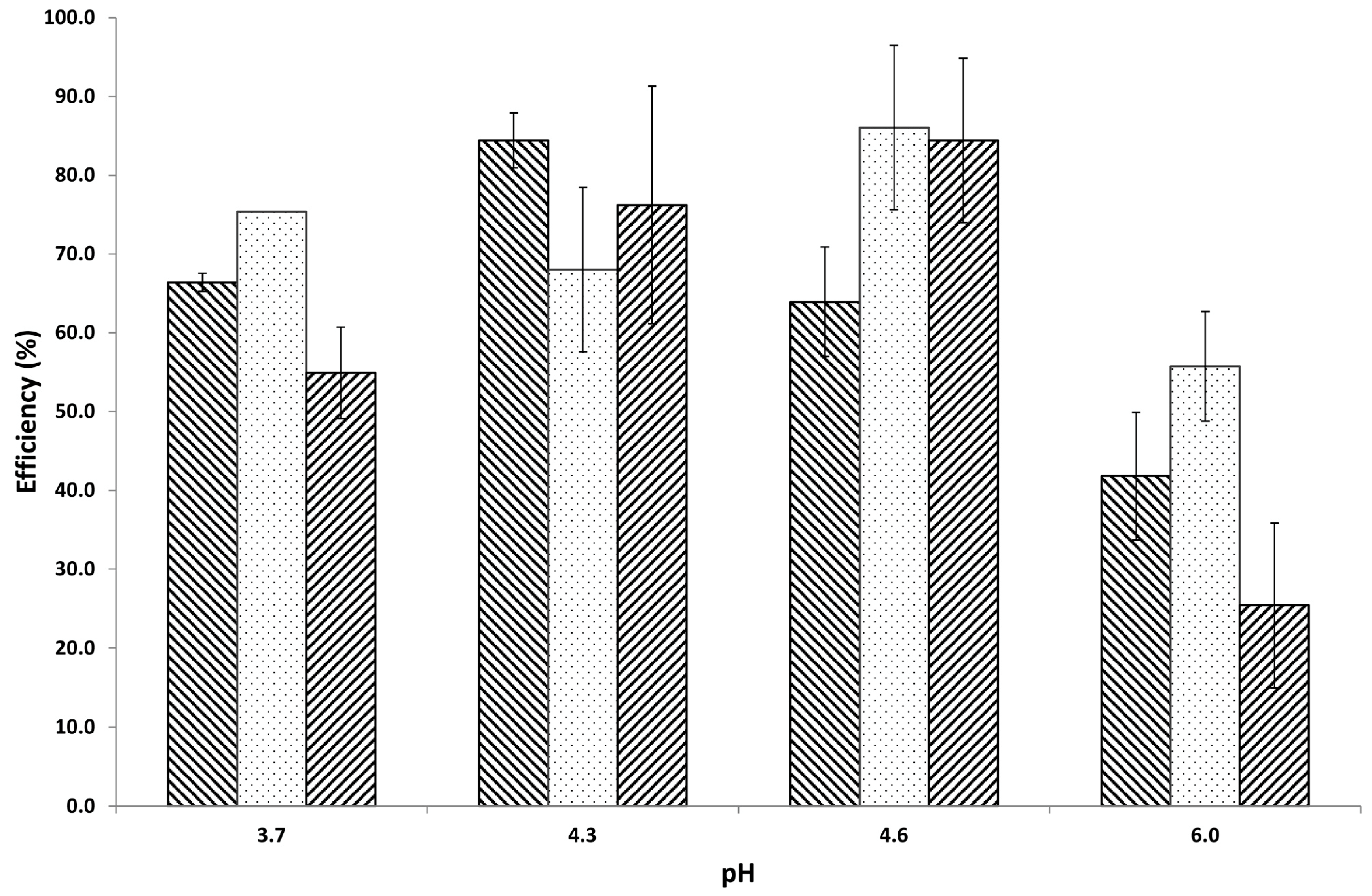
 tragacanth (control);
tragacanth (control);  insulin (control);
insulin (control);  mixture at pH 3.7;
mixture at pH 3.7;  mixture at pH 4.3;
mixture at pH 4.3;  mixture at pH 4.6; and,
mixture at pH 4.6; and,  mixture at pH 6.0. Arrows and numbers indicate the temperature of phase transition.
mixture at pH 6.0. Arrows and numbers indicate the temperature of phase transition.
 tragacanth (control);
tragacanth (control);  insulin (control);
insulin (control);  mixture at pH 3.7;
mixture at pH 3.7;  mixture at pH 4.3;
mixture at pH 4.3;  mixture at pH 4.6; and,
mixture at pH 4.6; and,  mixture at pH 6.0. Arrows and numbers indicate the temperature of phase transition.
mixture at pH 6.0. Arrows and numbers indicate the temperature of phase transition.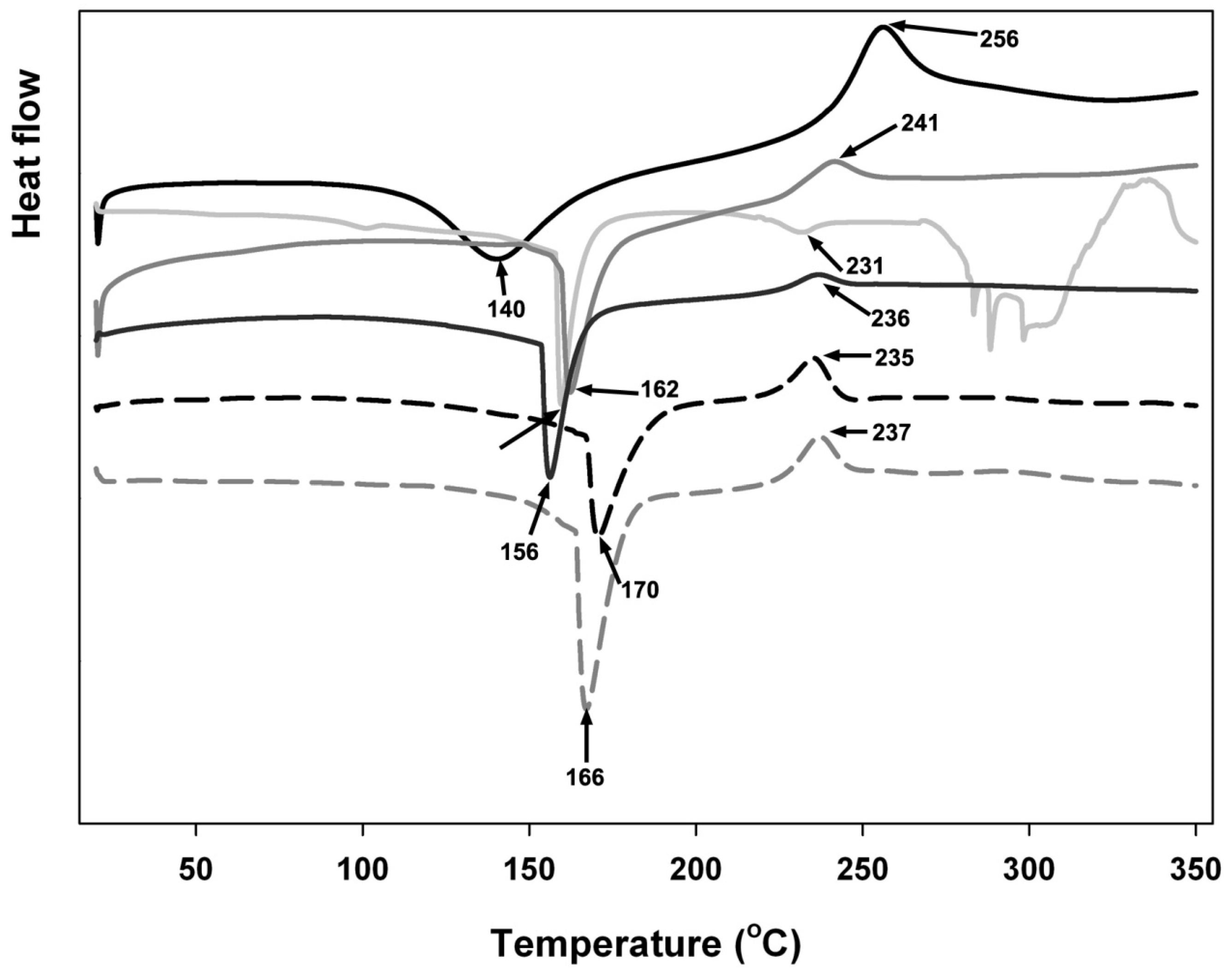
 tragacanth (control);
tragacanth (control);  insulin (control);
insulin (control);  mixture at pH 3.7;
mixture at pH 3.7;  mixture at pH 4.3;
mixture at pH 4.3;  mixture at pH 4.6; and,
mixture at pH 4.6; and,  mixture at pH 6.0. Arrows and numbers indicate a wavenumber of a particular structural change.
mixture at pH 6.0. Arrows and numbers indicate a wavenumber of a particular structural change.
 tragacanth (control);
tragacanth (control);  insulin (control);
insulin (control);  mixture at pH 3.7;
mixture at pH 3.7;  mixture at pH 4.3;
mixture at pH 4.3;  mixture at pH 4.6; and,
mixture at pH 4.6; and,  mixture at pH 6.0. Arrows and numbers indicate a wavenumber of a particular structural change.
mixture at pH 6.0. Arrows and numbers indicate a wavenumber of a particular structural change.


| pH | Particle Size, nm | ||
|---|---|---|---|
| Tragacanth concentration, % w/w | |||
| 0.1 | 0.5 | 1 | |
| 3.7 | 1105 ± 48 | 1382 ± 141 | 839 ± 22 |
| 4.3 | 667 ± 37 | 811 ± 20 | 957 ± 56 |
| 4.6 | 651 ± 09 | 601 ± 19 | 649 ± 25 |
| 6 | 566 ± 23 | 373 ± 08 | 719 ± 05 |
| pH | Zeta Potential, mV | ||
|---|---|---|---|
| Tragacanth concentration, % w/w | |||
| 0.1 | 0.5 | 1 | |
| 3.7 | −22.3 ± 0.6 | −26.0 ± 0.6 | −29.1 ± 0.5 |
| 4.3 | −38.9 ± 1.2 | −19.2 ± 2.5 | −30.9 ± 1.9 |
| 4.6 | −36.3 ± 1.7 | −7.5 ± 0.4 | −18.1 ± 0.6 |
| 6 | −38.1 ± 1.3 | −39.5 ± 4.9 | −40.8 ± 8.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nur, M.; Vasiljevic, T. Insulin Inclusion into a Tragacanth Hydrogel: An Oral Delivery System for Insulin. Materials 2018, 11, 79. https://doi.org/10.3390/ma11010079
Nur M, Vasiljevic T. Insulin Inclusion into a Tragacanth Hydrogel: An Oral Delivery System for Insulin. Materials. 2018; 11(1):79. https://doi.org/10.3390/ma11010079
Chicago/Turabian StyleNur, Mokhamad, and Todor Vasiljevic. 2018. "Insulin Inclusion into a Tragacanth Hydrogel: An Oral Delivery System for Insulin" Materials 11, no. 1: 79. https://doi.org/10.3390/ma11010079





