Study of CeO2 Modified AlNi Mixed Pillared Clays Supported Palladium Catalysts for Benzene Adsorption/Desorption-Catalytic Combustion
Abstract
:1. Introduction
2. Experimental
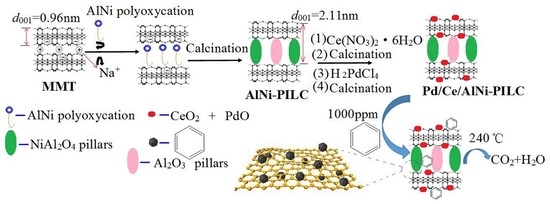
2.1. Synthesis
2.2. Catalytic Activity Tests
2.3. Characterization
3. Results and Discussion
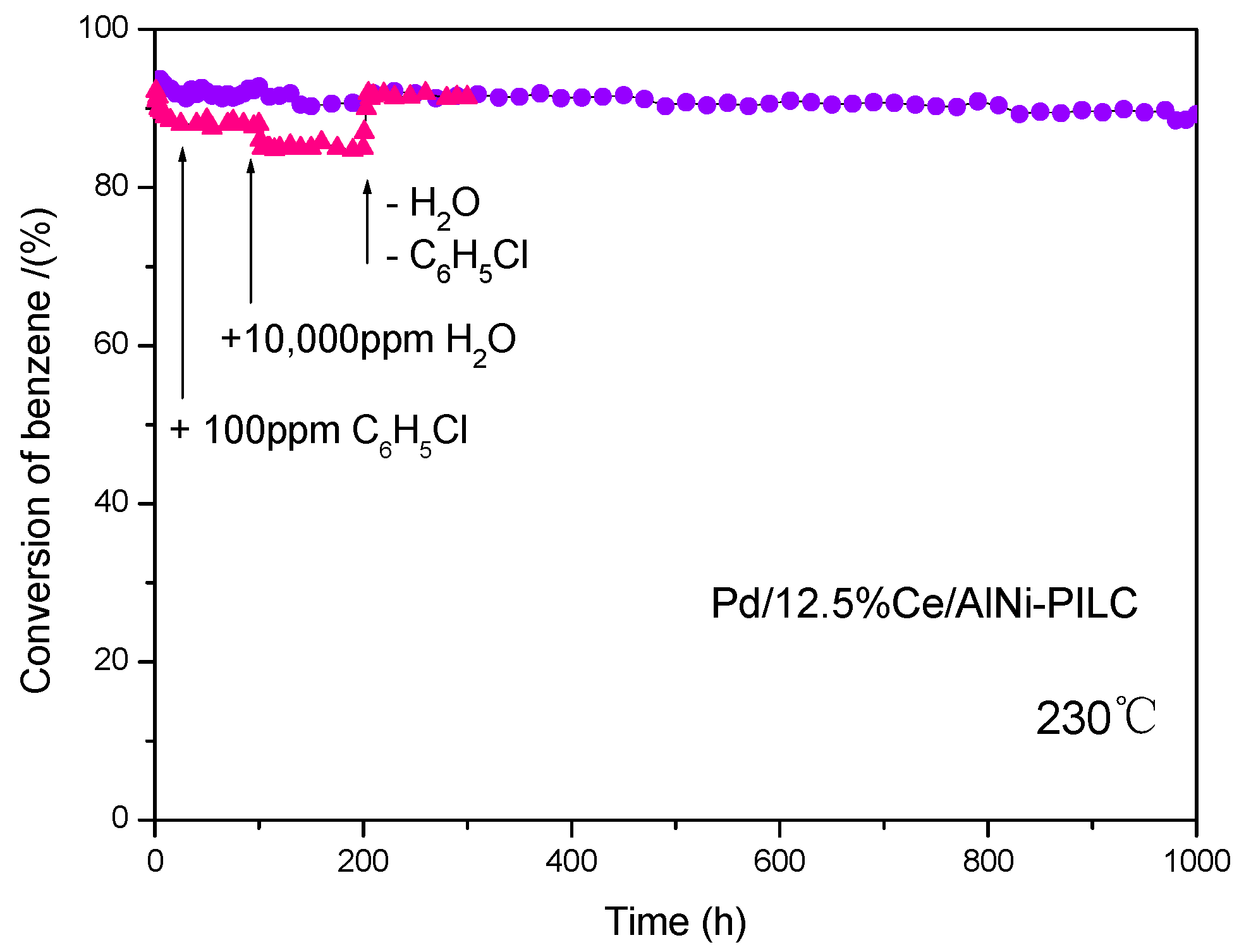
3.1. Catalytic Performance and Stability Test
3.2. Effects of Loading Pd Content and Benzene Concentration
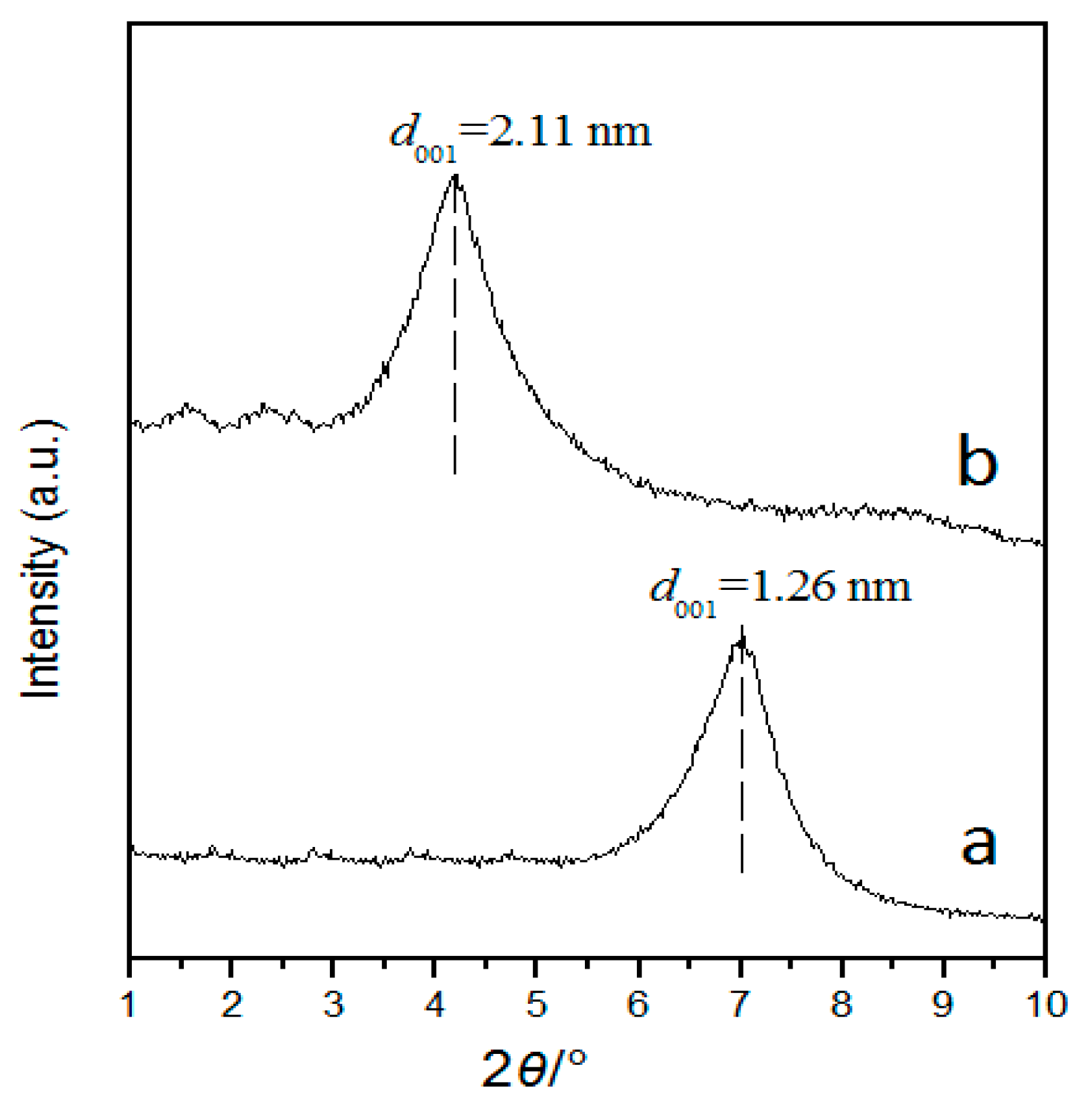
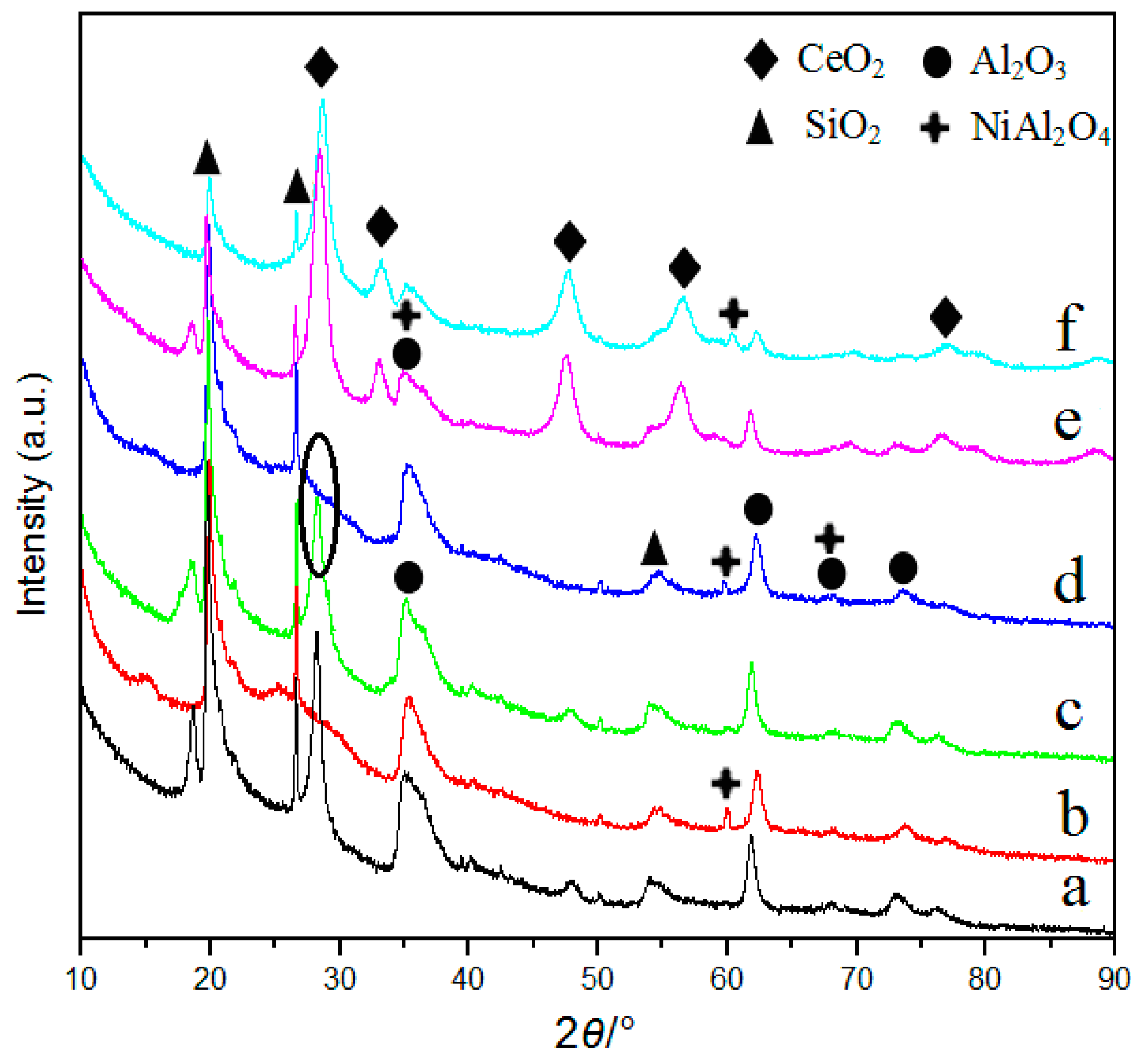
3.3. XRD and N2 Adsorption Analysis
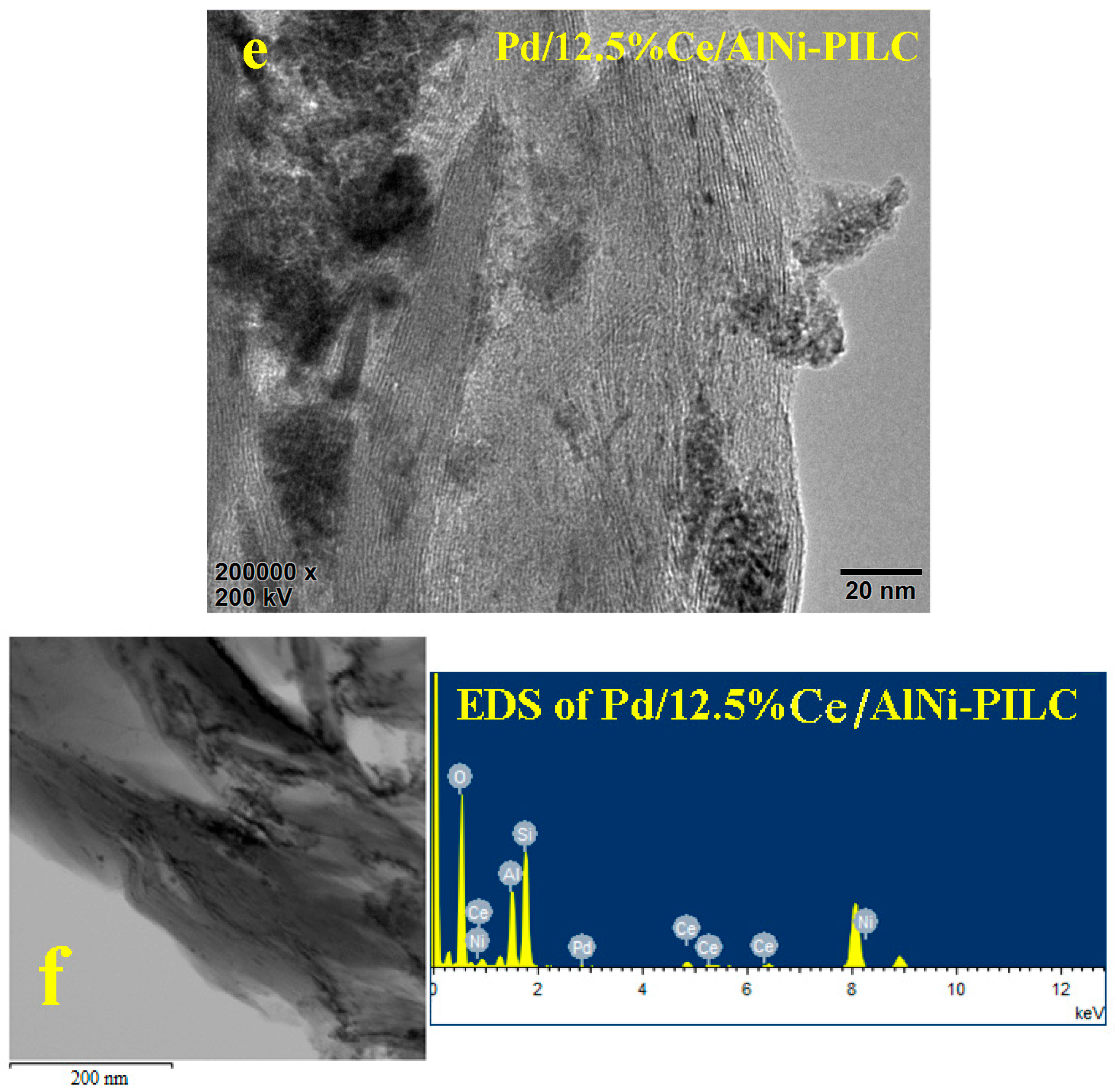
3.4. HRTEM Analysis
3.5. ICP-OES Analysis
3.6. TPD Analysis
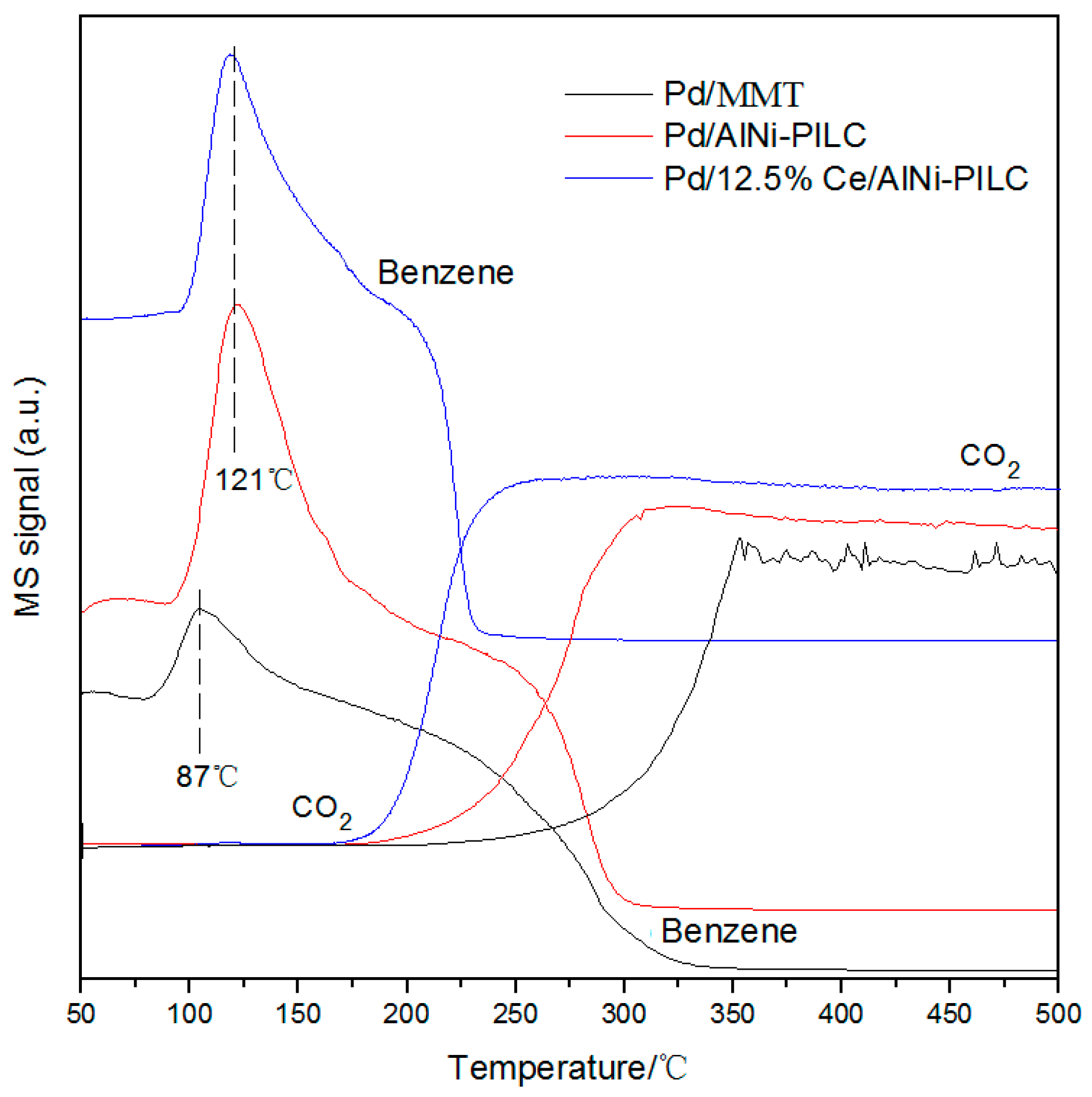
3.7. Benzene-TPSR Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lindgren, T. A case of indoor air pollution of ammonia emitted from concrete in a newly built office in Beijing. Build. Environ. 2010, 45, 596–600. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Jiang, Z.; Shang guan, W.F. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Yang, P.; Yang, S.S.; Shi, Z.N.; Tao, F.; Guo, X.L.; Zhou, R.X. Accelerating effect of ZrO2 doping on catalytic performance and thermal stability of CeO2–CrOx mixed oxide for 1,2-dichloroethane elimination. Chem. Eng. J. 2016, 285, 544–553. [Google Scholar] [CrossRef]
- Aznárez, A.; Korili, S.A.; Gil, A. The promoting effect of cerium on the characteristics and catalytic performance of palladium supported on alumina pillared clays for the combustion of propene. Appl. Catal. A Gen. 2014, 474, 95–99. [Google Scholar] [CrossRef]
- Castaño, M.H.; Molina, R.; Moreno, S. Cooperative effect of the Co-Mn mixed oxides for the catalytic oxidation of VOCs: Influence of the synthesis method. Appl. Catal. A Gen. 2015, 492, 48–59. [Google Scholar] [CrossRef]
- Ding, M.L.; Zuo, S.F.; Qi, C.Z. Preparation and characterization of novel composite AlCr-pillared clays and preliminary investigation for benzene adsorption. Appl. Clay Sci. 2015, 115, 9–16. [Google Scholar] [CrossRef]
- Yang, P.; Xue, X.M.; Meng, Z.H.; Zhou, R.X. Enhanced catalytic activity and stability of Ce doping on Cr supported HZSM-5 catalysts for deep oxidation of chlorinated volatile organic compounds. Chem. Eng. J. 2013, 234, 203–210. [Google Scholar] [CrossRef]
- Yang, H.G.; Deng, J.G.; Xie, S.H.; Jiang, Y.; Dai, H.X.; Au, C.T. Au/MnOx/3DOM SiO2: Highly active catalysts for toluene oxidation. Appl. Catal. A Gen. 2015, 507, 139–148. [Google Scholar] [CrossRef]
- Zuo, S.F.; Du, Y.J.; Liu, F.J.; Han, D.; Qi, C.Z. Influence of ceria promoter on shell-powder-supported Pd catalyst for the complete oxidation of benzene. Appl. Catal. A Gen. 2013, 451, 65–70. [Google Scholar] [CrossRef]
- Yang, P.; Zuo, S.F.; Shi, Z.N.; Tao, F.; Zhou, R.X. Elimination of 1,2-dichloroethane over (Ce, Cr)xO2/MOy catalysts (M = Ti, V, Nb, Mo, W and La). Appl. Catal. B Environ. 2016, 191, 53–61. [Google Scholar] [CrossRef]
- Yang, P.; Yang, S.S.; Shi, Z.N.; Meng, Z.H.; Zhou, R.X. Deep oxidation of chlorinated VOCs over CeO2-based transition metal mixed oxide catalysts. Appl. Catal. B Environ. 2015, 162, 227–235. [Google Scholar] [CrossRef]
- Zhao, S.; Li, K.; Jiang, S.; Li, J. Pd-Co based spinal oxides derived from Pd nanoparticles immobilized on layered double hydroxides for toluene combustion. Appl. Catal. B Environ. 2016, 181, 236–248. [Google Scholar] [CrossRef]
- Li, P.; He, C.; Cheng, J.; Ma, C.Y.; Dou, B.J.; Hao, Z.P. Catalytic oxidation of toluene over Pd/Co3AlO catalysts derived from hydrotalcite-like compounds: Effects of preparation methods. Appl. Catal. B Environ. 2011, 101, 570–579. [Google Scholar] [CrossRef]
- Deng, J.; He, S.; Xie, S.; Yang, H.; Liu, Y.; Guo, G.; Dai, H. Ultralow Loading of Silver Nanoparticles on Mn2O3 Nanowires Derived with Molten Salts: A High-Efficiency Catalyst for the Oxidative Removal of Toluene. Environ. Sci. Technol. 2015, 49, 11089–11095. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.J.; Zuo, S.F.; Wang, C.; Li, J.T.; Xiao, F.S.; Qi, C.Z. Pd/transition metal oxides functionalized ZSM-5 single crystals with b-axis aligned mesopores: Efficient and long-lived catalysts for benzene combustion. Appl. Catal. B Environ. 2014, 148–149, 106–113. [Google Scholar] [CrossRef]
- Xing, T.; Wan, H.Q.; Shao, Y.; Han, Y.X.; Xu, Z.Y.; Zheng, S.R. Catalytic combustion of benzene over γ-alumina supported chromium oxide catalysts. Appl. Catal. A Gen. 2013, 468, 269–275. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Issa, G.; Blasco, T.; Dimitrov, M.; Popova, M.; Hernández, S.; Kovacheva, D.; Atanasova, G.; Nieto, J.L. Catalytic VOCs elimination over copper and cerium oxide modified mesoporous SBA-15 silica. Appl. Catal. A Gen. 2013, 453, 1–12. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Mahdi, R.S.; Aejung, A.; Mohammadreza, S. Gadolinium Triflate Immobilized on Magnetic Nanocomposites as Recyclable Lewis Acid Catalyst for Acetylation of Phenols. Nanosci. Nanotechnol. Lett. 2014, 6, 309–313. [Google Scholar]
- Kim, A.; Rafiaei, S.M.; Abolhosseini, S.; Shokouhimehr, M. Palladium Nanocatalysts Confined in Mesoporous Silica for Heterogeneous Reduction of Nitroaromatics. Energy Environ. Focus 2015, 4, 18–23. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Shin, K.Y.; Lee, J.S.; Hackett, M.J.; Jun, S.W.; Oh, M.H.; Jang, J.; Hyeon, T. Magnetically recyclable core—Shell nanocatalysts for efficient heterogeneous oxidation of alcohols. J. Mater. Chem. A 2014, 2, 7593–7599. [Google Scholar] [CrossRef]
- Zhao, W.; Cheng, J.; Wang, L.; Chu, J.L.; Qu, J.K.; Liu, Y.H.; Li, S.H.; Zhang, H.; Wang, J.C.; Hao, Z.P.; et al. Catalytic combustion of chlorobenzene on the Ln modified Co/HMS. Appl. Catal. B Environ. 2012, 127, 246–254. [Google Scholar] [CrossRef]
- Sedjame, H.J.; Fontaine, C.; Lafaye, G.; Barbier, J., Jr. On the promoting effect of the addition of ceria to platinum based alumina catalysts for VOCs oxidation. Appl. Catal. B Environ. 2014, 144, 233–242. [Google Scholar] [CrossRef]
- Ferreira, D.; Zanchet, R.; Rinaldi, U.; Schuchardt, S. Effect of the CeO2 content on the surface and structural properties of CeO2–Al2O3 mixed oxides prepared by sol-gel method. Appl. Catal. A Gen. 2010, 388, 45–56. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Otto, K. Characterization of Pt/γ-alumina catalysts containing ceria. J. Catal. 1989, 115, 16–23. [Google Scholar] [CrossRef]
- Yermakov, Y.I.; Kuznetsov, B.N. Supported metallic catalysts prepared by decomposition of surface ornagometallic complexes. J. Mol. Catal. 1980, 9, 13–40. [Google Scholar] [CrossRef]
- Ballini, R.; Fiorini, D.; Victoria, G.M.; Palmier, A. Michael addition of α-nitro ketones to conjugated enones under solventless conditions using silica. Green Chem. 2003, 5, 475–476. [Google Scholar] [CrossRef]
- Rani, R.V.; Srinivas, N.; Kisham, R.M.; Kulkarni, S.J.; Raghavan, K.V. Zeolite-catalyzed cyclocondensation reaction for the selective synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Green Chem. 2001, 3, 305–306. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Patil, K.Y.; Jana, S.K. Acylation of aromatic alcohols and phenols over InCl3/montmorillonite K-10 catalysts. J. Chem. Sci. 2004, 116, 175–177. [Google Scholar] [CrossRef]
- Tong, D.S.; Zhou, C.H.; Lu, Y.; Yu, H.; Zhang, G.F.; Yu, W.H. Adsorption of Acid Red G dye on octadecyl trimethylammonium montmorillonite. Appl. Clay Sci. 2010, 50, 427–431. [Google Scholar] [CrossRef]
- Molina, C.B.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. A comparison of Al-Fe and Zr-Fe pillared clays for catalytic wet peroxide oxidation. Chem. Eng. J. 2006, 118, 29–35. [Google Scholar] [CrossRef]
- Babu, B.H.; Lee, M.; Hwang, D.W.; Kim, Y.; Chae, H.J. An integrated process for production of jet-fuel range olefins from ethylene using Ni-Al-SBA-15 and Amberlyst-35 catalysts. Appl. Catal. A Gen. 2017, 530, 48–55. [Google Scholar] [CrossRef]
- Tanaka, M.; Itadani, A.; Kuroda, Y.; Iwamoto, M. Effect of Pore Size and Nickel Content of Ni-MCM-41 on Catalytic Activity for Ethene Dimerization and Local Structures of Nickel Ions. J. Phys. Chem. C 2012, 116, 5664–5672. [Google Scholar] [CrossRef]
- Lallemand, M.; Rusu, O.A.; Dumitriu, E.; Finiels, A.; Fajula, F.; Hulea, V. NiMCM-36 and NiMCM-22 catalysts for the ethylene oligomerization: Effect of zeolite texture and nickel cations/acid sites ratio. Appl. Catal. A Gen. 2008, 338, 37–43. [Google Scholar] [CrossRef]
- Martínez, A.; Arribas, M.A.; Concepción, P.; Moussa, S. New bifunctional Ni-H-Beta catalysts for the heterogeneous oligomerization of ethylene. Appl. Catal. A Gen. 2013, 467, 509–518. [Google Scholar] [CrossRef]
- Lin, S.; Shi, L.; Zhang, H.; Zhang, N.; Yi, X.; Zheng, A.; Li, X. Tuning the pore structure of plug-containing Al-SBA-15 by post-treatment and its selectivity for C16 olefin in ethylene oligomerization. Microporous Mesoporous Mater. 2014, 184, 151–161. [Google Scholar] [CrossRef]
- Andrei, R.D.; Popa, M.I.; Fajula, F.; Hulea, V. Heterogeneous oligomerization of ethylene over highly active and stable Ni-Al-SBA-15 mesoporous catalysts. J. Catal. 2015, 323, 76–84. [Google Scholar] [CrossRef]
- Aznarez, A.; Delaigle, R.; Eloy, P.; Gaigneaux, E.M.; Korili, S.A.; Gil, A. Catalysts based on pillared clays for the oxidation of chlorobenzene. Catal. Today 2015, 246, 15–27. [Google Scholar] [CrossRef]
- Zuo, S.F.; Zhou, R.X. Influence of synthesis condition on pore structure of Al pillared clays and supported Pd catalysts for deep oxidation of benzene. Microporous Mesoporous Mater. 2008, 113, 472–480. [Google Scholar] [CrossRef]
- Yang, P.; Li, J.R.; Zuo, S.F. Promoting effects of Ce and Pt addition on the destructive performances of V2O5/γ-Al2O3 for catalytic combustion of benzene. Appl. Catal. A Gen. 2017, 542, 38–46. [Google Scholar] [CrossRef]
- Li, J.R.; Zuo, S.F.; Qi, C.Z. Preparation and high performance of rare earth modified Co/USY for benzene catalytic combustion. Catal. Commun. 2017, 91, 30–33. [Google Scholar] [CrossRef]
- Zou, X.L.; Rou, Z.B.; Song, S.Q.; Ji, H.B. Enhanced menthane combustion performance over NiAl2O4-interface promoted Pd/γ-Al2O3. J. Catal. 2016, 338, 192–201. [Google Scholar] [CrossRef]
- Tang, W.X.; Deng, Y.Z.; Chen, Y.F. Promoting effect of acid treatment on Pd-Ni/SBA-15 catalyst for complete oxidation of gaseous benzene. Catal. Commun. 2017, 89, 86–90. [Google Scholar] [CrossRef]
- Shim, W.G.; Kim, S.C. Heterogeneous adsorption and catalytic oxidation of benzene, toluene and xylene over spent and chemically regenerated platinum catalyst supported on activated carbon. Appl. Surf. Sci. 2010, 256, 5566–5571. [Google Scholar] [CrossRef]
- Bottazzi, G.; Martinez, M.; Costa, M.; Anunziata, O.; Beltramone, A. Inhibition of the hydrogenation of tetralin by nitrogen and sulfur compounds over Ir/SBA-16. Appl. Catal. A Gen. 2011, 404, 30–38. [Google Scholar] [CrossRef]
- Zhao, Q.; Xu, Y.; Li, Y.; Jiang, T.; Li, C.; Yin, H. Effect of the Si/Ce molar ratio on the textural properties of rare earth element cerium incorporated mesoporous molecular sieves obtained room temperature. Appl. Surf. Sci. 2009, 255, 9425–9429. [Google Scholar] [CrossRef]
- Somanathan, T.; Pandurangan, A.; Sathiyamoorthy, D. Catalytic influence of mesoporous Co-MCM-41 molecular sieves for the synthesis of SWNTs via CVD method. J. Mol. Catal. A Chem. 2006, 256, 193–199. [Google Scholar] [CrossRef]
- Bing, J.S.; Li, L.S.; Lan, B.Y.; Liao, G.Z.; Zeng, J.Y.; Zhang, Q.Y.; Li, X.K. Synthesis of cerium-doped MCM-41 for ozonation of p-chlorobenzoic acid in aqueous solution. Appl. Catal. B Environ. 2012, 115–116, 16–24. [Google Scholar] [CrossRef]




| Samples | SBET a (m2/g) | Ames b (m2/g) | Vp c (cm3/g) | Vmic d (cm3/g) |
|---|---|---|---|---|
| MMT | 24.6 | 20.1$ | 0.054 | 0.0018 |
| AlNi-PILC | 374.8 | 86.1 | 0.212 | 0.1336 |
| Pd/MMT | 20 | 17.9 | 0.05 | 0.0007 |
| Pd/AlNi-PILC | 298.4 | 61.4 | 0.17 | 0.1101 |
| Pd/12.5% Ce/AlNi-PILC | 230.5 | 56.8 | 0.131 | 0.0804 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zuo, S.; Yang, P.; Qi, C. Study of CeO2 Modified AlNi Mixed Pillared Clays Supported Palladium Catalysts for Benzene Adsorption/Desorption-Catalytic Combustion. Materials 2017, 10, 949. https://doi.org/10.3390/ma10080949
Li J, Zuo S, Yang P, Qi C. Study of CeO2 Modified AlNi Mixed Pillared Clays Supported Palladium Catalysts for Benzene Adsorption/Desorption-Catalytic Combustion. Materials. 2017; 10(8):949. https://doi.org/10.3390/ma10080949
Chicago/Turabian StyleLi, Jingrong, Shufeng Zuo, Peng Yang, and Chenze Qi. 2017. "Study of CeO2 Modified AlNi Mixed Pillared Clays Supported Palladium Catalysts for Benzene Adsorption/Desorption-Catalytic Combustion" Materials 10, no. 8: 949. https://doi.org/10.3390/ma10080949