Modeling the Non-Equilibrium Process of the Chemical Adsorption of Ammonia on GaN(0001) Reconstructed Surfaces Based on Steepest-Entropy-Ascent Quantum Thermodynamics
Abstract
:1. Introduction
2. Theory and Model
2.1. SEAQT Equation of Motion
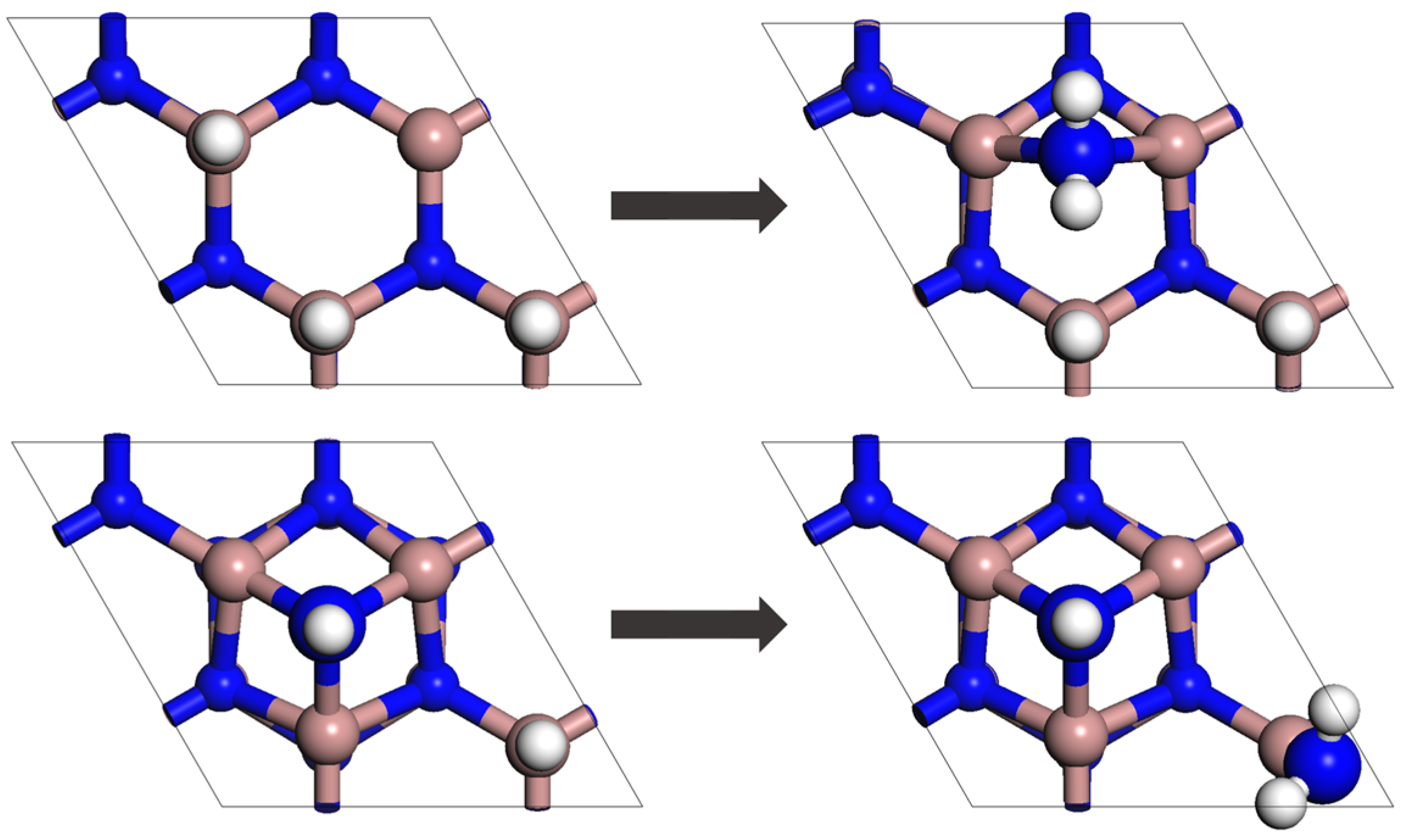
2.2. System and Energy Eigenstructure
2.3. Initial State and Model Parameters
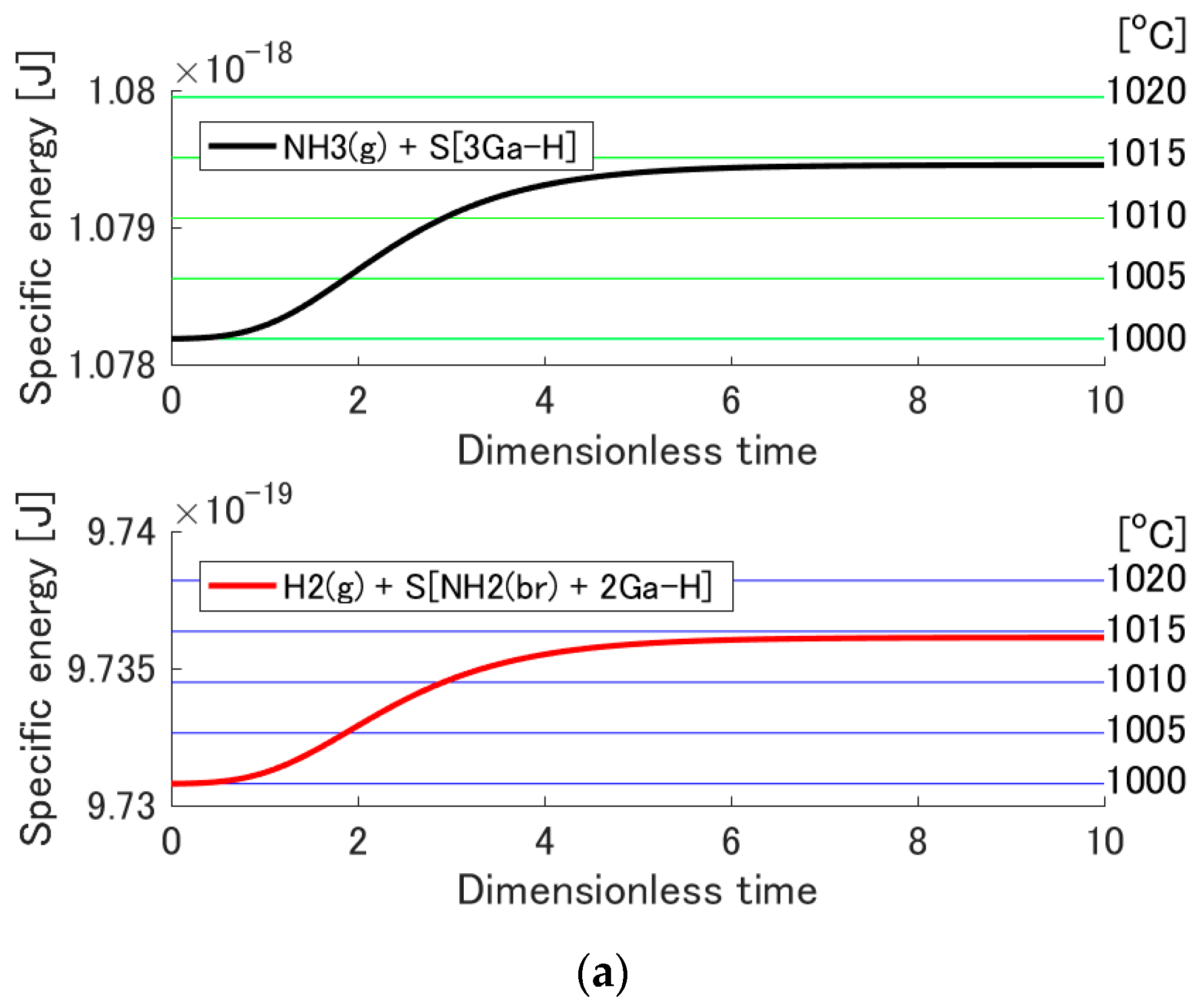
3. Results and Discussion
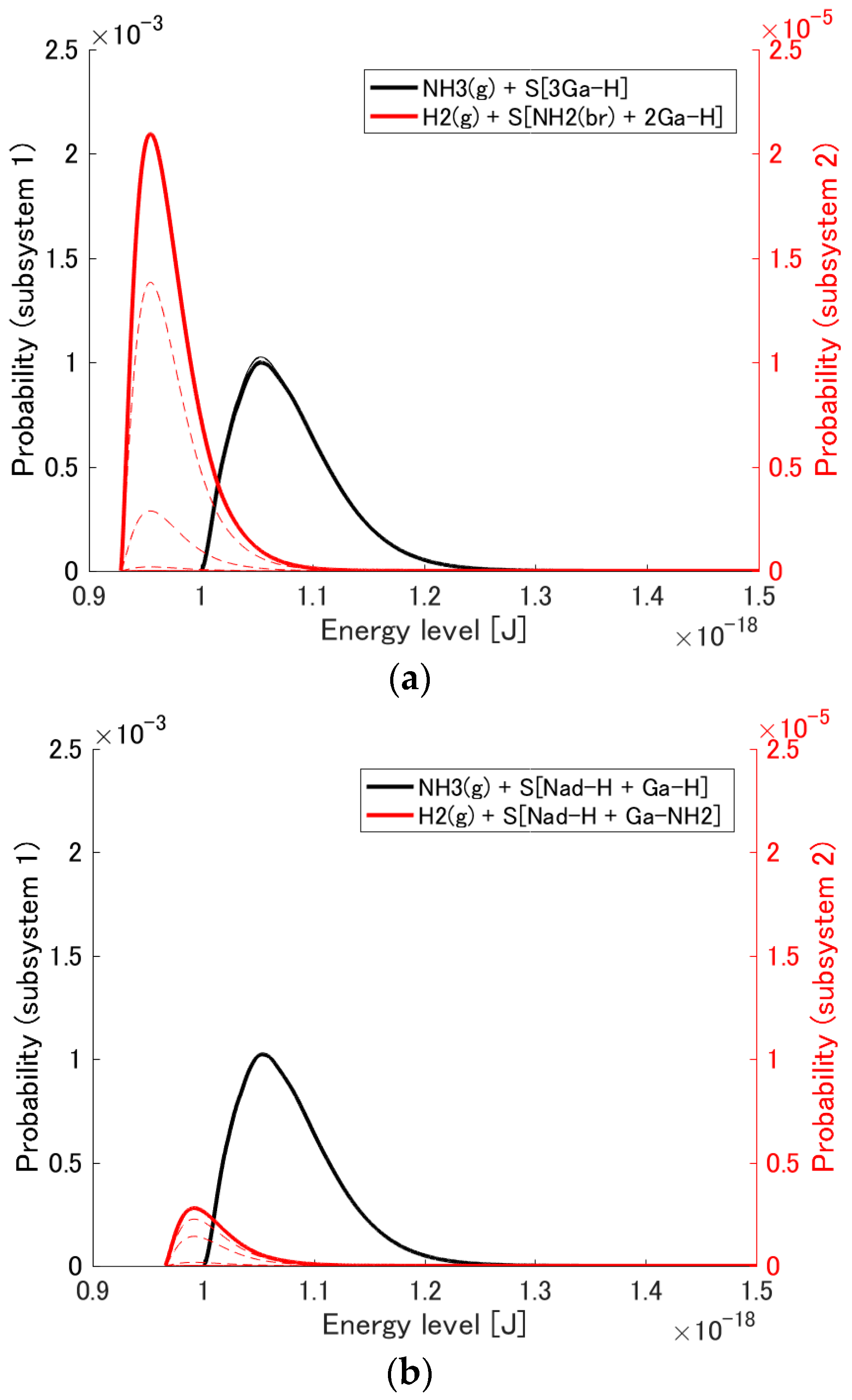
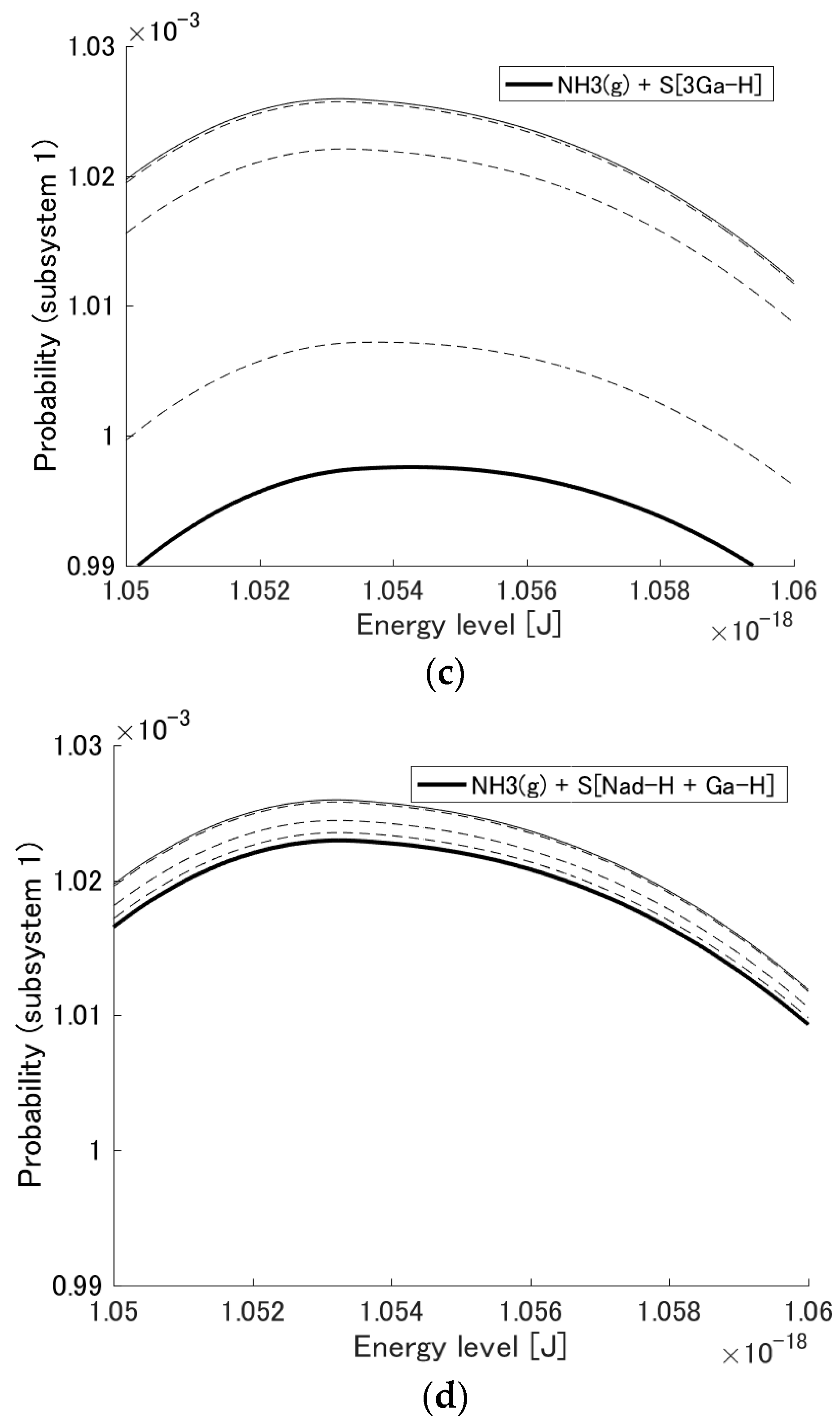
3.1. Probability Distribution Among Energy Eigenlevels
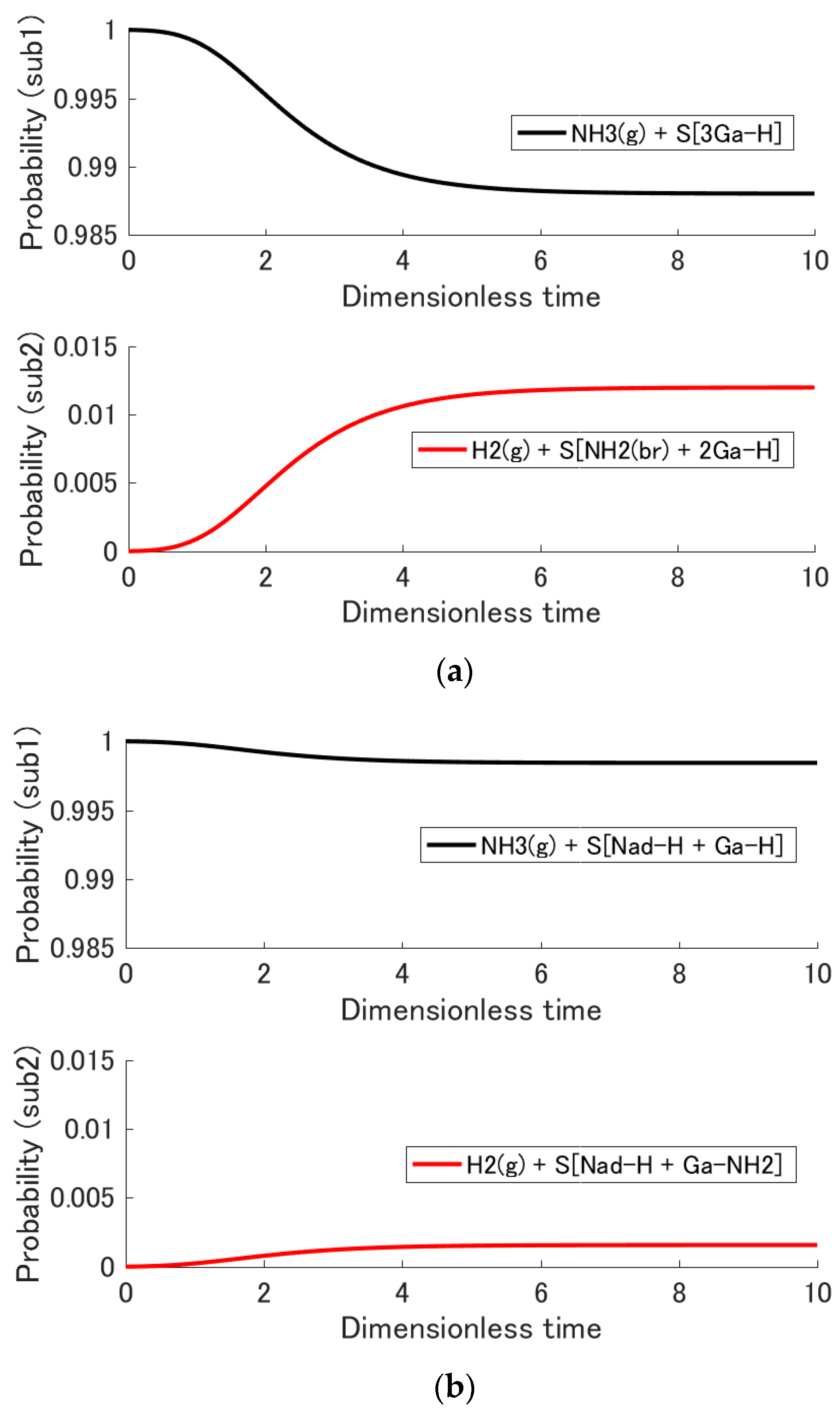
3.2. Adsorption Probability
3.3. Temperature Increase by Adsorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akasaki, I. Nobel Lecture: Fascinated journeys into blue light. Rev. Mod. Phys. 2015, 87, 1119–1131. [Google Scholar] [CrossRef]
- Amano, H. Nobel Lecture: Growth of GaN on sapphire via low-temperature deposited buffer layer and realization of p-type GaN by Mg doping followed by low-energy electron beam irradiation. Rev. Mod. Phys. 2015, 87, 1133–1138. [Google Scholar] [CrossRef]
- Nakamura, S. Nobel Lecture: Background story of the invention of efficient blue InGaN light emitting diodes. Rev. Mod. Phys. 2015, 87, 1139–1151. [Google Scholar] [CrossRef]
- Kushimoto, M.; Tanikawa, T.; Honda, Y.; Amano, H. Optically pumped lasing properties of (101) InGaN/GaN stripe multiquantum wells with ridge cavity structure on patterned (001) Si substrates. Appl. Phys. Express 2015, 8, 022702. [Google Scholar] [CrossRef]
- Shojiki, K.; Tanikawa, T.; Choi, J.H.; Kuboya, S.; Hanada, T.; Katayama, R.; Matsuoka, T. Red to blue wavelength emission of N-polar (000) InGaN light-emitting diodes grown by metalorganic vapor phase epitaxy. Appl. Phys. Express 2015, 8, 061005. [Google Scholar] [CrossRef]
- Ichikawa, S.; Iwata, Y.; Funato, M.; Nagata, S.; Kawakami, Y. High quality semipolar (102) AlGaN/AlN quantum wells with remarkably enhanced optical transition probabilities. Appl. Phys. Lett. 2014, 104, 252102. [Google Scholar] [CrossRef]
- Okumura, H. Present Status and Future Prospect of Widegap Semiconductor High-Power Devices. Jpn. J. Appl. Phys. 2006, 45, 7565–7586. [Google Scholar] [CrossRef]
- Kachi, T. Recent progress of GaN power devices for automotive applications. Jpn. J. Appl. Phys. 2014, 53, 100210. [Google Scholar] [CrossRef]
- Amano, H. Progress and Prospect of the Growth of Wide-Band-Gap Group III Nitrides: Development of the Growth Method for Single-Crystal Bulk GaN. Jpn. J. Appl. Phys. 2013, 52, 050001. [Google Scholar] [CrossRef]
- Imade, M.; Imanishi, M.; Todoroki, Y.; Imabayashi, H.; Matsuo, D.; Murakami, K.; Takazawa, H.; Kitamoto, A.; Maruyama, M.; Yoshimura, M.; et al. Fabrication of low-curvature 2 in. GaN wafers by Na-flux coalescence growth technique. Appl. Phys. Express 2014, 7, 035503. [Google Scholar] [CrossRef]
- Däweritz, L.; Hey, R. Reconstruction and defect structure of vicinal GaAs(001) and AlxGa1−xAs(001) surfaces during MBE growth. Surf. Sci. 1990, 236, 15–22. [Google Scholar] [CrossRef]
- Kangawa, Y.; Ito, T.; Taguchi, A.; Shiraishi, K.; Ohachi, T. A new theoretical approach to adsorption-desorption behavior of Ga on GaAs surfaces. Surf. Sci. 2001, 493, 178–181. [Google Scholar] [CrossRef]
- Kangawa, Y.; Ito, T.; Hiraoka, Y.S.; Taguchi, A.; Shiraishi, K.; Ohachi, T. Theoretical approach to influence of As2 pressure on GaAs growth kinetics. Surf. Sci. 2002, 507, 285–289. [Google Scholar] [CrossRef]
- Kangawa, Y.; Akiyama, T.; Ito, T.; Shiraishi, K.; Nakayama, T. Surface Stability and Growth Kinetics of Compound Semiconductors: An Ab Initio-Based Approach. Materials 2013, 6, 3309–3360. [Google Scholar] [CrossRef]
- Northrup, J.E.; Di Felice, R.; Neugebauer, J. Energetics of H and NH2 on GaN (100) and implications for the origin of nanopipe defects. Phys. Rev. B 1997, 56, R4325–R4328. [Google Scholar] [CrossRef]
- Northrup, J.E.; Neugebauer, J. Strong affinity of hydrogen for the GaN (000-1) surface: Implications for molecular beam epitaxy and metalorganic chemical vapor deposition. Appl. Phys. Lett. 2004, 85, 3429–3431. [Google Scholar] [CrossRef]
- Van de Walle, C.G.; Neugebauer, J. First-Principles Surface Phase Diagram for Hydrogen on GaN Surfaces. Phys. Rev. Lett. 2002, 88, 066103. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, C.G.; Neugebauer, J. Structure and energetics of nitride surfaces under MOCVD growth conditions. J. Cryst. Growth 2003, 248, 8–13. [Google Scholar] [CrossRef]
- Akiyama, T.; Ammi, D.; Nakamura, K.; Ito, T. Surface reconstruction and magnesium incorporation on semipolar GaN (101) surfaces. Phys. Rev. B 2010, 81, 245317. [Google Scholar] [CrossRef]
- Akiyama, T.; Yamashita, T.; Nakamura, K.; Ito, T. Stability of hydrogen on nonpolar and semipolar nitride surfaces: Role of surface orientation. J. Cryst. Growth 2011, 318, 79–83. [Google Scholar] [CrossRef]
- Beretta, G.P.; Gyftopoulos, E.P.; Park, J.L.; Hatsopoulos, G.N. Quantum Thermodynamics. A New Equation of Motion for a Single Constituent of Matter. Nuovo Cimento B 1984, 82, 169–191. [Google Scholar] [CrossRef]
- Beretta, G.P.; Gyftopoulos, E.P.; Park, J.L. Quantum Thermodynamics. A New Equation of Motion for a General Quantum System. Nuovo Cimento B 1985, 87, 77–97. [Google Scholar] [CrossRef]
- Beretta, G.P. Nonlinear model dynamics for closed-system, constrained, maximal-entropy-generation relaxation by energy redistribution. Phys. Rev. E 2006, 73, 026113. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.P. Nonlinear quantum evolution equations to model irreversible adiabatic relaxation with maximal entropy production and other nonunitary processes. Rep. Math. Phys. 2009, 64, 139–168. [Google Scholar] [CrossRef]
- Beretta, G.P. Steepest entropy ascent model for far-nonequilibrium thermodynamics: Unified implementation of the maximum entropy production principle. Phys. Rev. E 2014, 90, 042113. [Google Scholar] [CrossRef] [PubMed]
- Montefusco, A.; Consonni, F.; Beretta, G.P. Essential equivalence of the general equation for the nonequilibrium reversible-irreversible coupling (GENERIC) and steepest-entropy-ascent models of dissipation for nonequilibrium thermodynamics. Phys. Rev. E 2015, 91, 042138. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; von Spakovsky, M.R. Comparison of the non-equilibrium predictions of Intrinsic Quantum Thermodynamics at the atomistic level with experimental evidence. J. Phys. Conf. Ser. 2012, 380, 012015. [Google Scholar] [CrossRef]
- Cano-Andrade, S.; Beretta, G.P.; von Spakovsky, M.R. Steepest-entropy-ascent quantum thermodynamic modeling of decoherence in two different microscopic composite systems. Phys. Rev. A 2015, 91, 013848. [Google Scholar] [CrossRef]
- Cano-Andrade, S.; von Spakovsky, M.R.; Beretta, G.P. Steepest-Entropy-Ascent Quantum Thermodynamic Non-Equilibrium Modeling of Decoherence of a Composite System of Two Interacting Spin-1/2 Systems. In Heat Transfer and Thermal Engineering, Proceedings of ASME 2013 International Mechanical Engineering Congress and Exposition, San Diego, CA, USA, 15 November 2013; Paper No. IMECE2013-63596; ASME: New York, NY, USA, 2013; pp. V08BT09A043:1–V08BT09A043:8. [Google Scholar] [CrossRef]
- Beretta, G.P.; Al-Abbasi, O.; von Spakovsky, M.R. Steepest-entropy-ascent nonequilibrium quantum thermodynamic framework to model chemical reaction rates at an atomistic level. Phys. Rev. E 2017, 95, 042139. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; von Spakovsky, M.R. Steepest-entropy-ascent quantum thermodynamic modeling of the relaxation process of isolated chemically reactive systems using density of states and the concept of hypoequilibrium state. Phys. Rev. E 2016, 93, 012137. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; von Spakovsky, M.R. Generalized thermodynamic relations for a system experiencing heat and mass diffusion in the far-from-equilibrium realm based on steepest entropy ascent. Phys. Rev. E 2016, 94, 032117. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; von Spakovsky, M.R. Modeling the nonequilibrium effects in a nonquasi-equilibrium thermodynamic cycle based on steepest entropy ascent and an isothermal-isobaric ensemble. Energy 2016, 115, 498–512. [Google Scholar] [CrossRef]
- Li, G.; von Spakovsky, M.R. Steepest-Entropy-Ascent Quantum Thermodynamic Modeling of the Far-From-Equilibrium Interactions between Nonequilibrium Systems of Indistinguishable Particle Ensembles. to be submitted for publication. arXiv 2016, arXiv:1601.02703. [Google Scholar]
- Li, G.; Al-Abbasi, O.; von Spakovsky, M.R. Atomistic-level non-equilibrium model for chemically reactive systems based on steepest-entropy-ascent quantum thermodynamics. J. Phys. Conf. Ser. 2014, 538, 012013. [Google Scholar] [CrossRef]
- Li, G.; von Spakovsky, M.R. Study of Nonequilibrium Size and Concentration Effects on the Heat and Mass Diffusion of Indistinguishable Particles using Steepest-Entropy-Ascent Quantum Thermodynamics. J. Heat Transf. 2017, 139, 122003. [Google Scholar] [CrossRef]
- Li, G.; von Spakovsky, M.R. Study of the Transient Behavior and Microstructure Degradation of a SOFC Cathode Using an Oxygen Reduction Model Based on Steepest-Entropy-Ascent Quantum Thermodynamics. In Energy, Proceedings of ASME 2015 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13 November 2015; Paper No. IMECE2015-53726; ASME: New York, NY, USA, 2015; pp. V06BT07A016:1–V06BT07A016:12. [Google Scholar] [CrossRef]
- Li, G.; von Spakovsky, M.R.; Shen, C.; Lu, C. Multiscale Transient and Steady State Study of the Influence of Microstructure Degradation and Chromium Oxide Poisoning on Solid Oxide Fuel Cell Cathode Performance. J. Non-Equilib. Thermodyn. under review.
- Li, G.; von Spakovsky, M.R. Application of Steepest-Entropy-Ascent Quantum Thermodynamics to Predicting Heat and Mass Diffusion From the Atomistic Up to the Macroscopic Level. In Energy, Proceedings of ASME 2015 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13 November 2015; Paper No. IMECE2015-53581; ASME: New York, NY, USA, 2015; pp. V06BT07A015:1–V06BT07A015:10. [Google Scholar] [CrossRef]
- Von Spakovsky, M.R.; Gemmer, J. Some Trends in Quantum Thermodynamics. Entropy 2014, 16, 3434–3470. [Google Scholar] [CrossRef]
- Kim, I.; von Spakovsky, M.R. Ab initio relaxation times and time-dependent Hamiltonians within the steepest-entropy-ascent quantum thermodynamic framework. Phys. Rev. E 2017, 96, 022129. [Google Scholar] [CrossRef]
- Gyftopoulos, E.P.; Ҫubukҫu, E. Entropy: Thermodynamic definition and quantum expression. Phys. Rev. E 1997, 55, 3851–3858. [Google Scholar] [CrossRef]
- Zanchini, E.; Beretta, G.P. Recent Progress in the Definition of Thermodynamic Entropy. Entropy 2014, 16, 1547–1570. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K. A New Slab Model Approach for Electronic Structure Calculation of Polar Semiconductor Surface. J. Phys. Soc. Jpn 1990, 59, 3455–3458. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Mesrine, M.; Grandjean, N.; Massies, J. Efficiency of NH3 as nitrogen source for GaN molecular beam epitaxy. Appl. Phys. Lett. 1998, 72, 350–352. [Google Scholar] [CrossRef]
- Karpov, S.Y.; Prokofyev, V.G.; Yakovlev, E.V.; Talalaev, R.A.; Makarov, Y.N. Novel approach to simulation of group-III nitrides growth by MOVPE. MRS Internet J. Nitride Semicond. Res. 1999, 4, e4. [Google Scholar] [CrossRef]
- Karpov, S.Y.; Bord, O.V.; Talalaev, R.A.; Makarov, Y.N. Gallium droplet formation during MOVPE and thermal annealing of GaN. Mater. Sci. Eng. B 2001, 82, 22–24. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusaba, A.; Li, G.; Von Spakovsky, M.R.; Kangawa, Y.; Kakimoto, K. Modeling the Non-Equilibrium Process of the Chemical Adsorption of Ammonia on GaN(0001) Reconstructed Surfaces Based on Steepest-Entropy-Ascent Quantum Thermodynamics. Materials 2017, 10, 948. https://doi.org/10.3390/ma10080948
Kusaba A, Li G, Von Spakovsky MR, Kangawa Y, Kakimoto K. Modeling the Non-Equilibrium Process of the Chemical Adsorption of Ammonia on GaN(0001) Reconstructed Surfaces Based on Steepest-Entropy-Ascent Quantum Thermodynamics. Materials. 2017; 10(8):948. https://doi.org/10.3390/ma10080948
Chicago/Turabian StyleKusaba, Akira, Guanchen Li, Michael R. Von Spakovsky, Yoshihiro Kangawa, and Koichi Kakimoto. 2017. "Modeling the Non-Equilibrium Process of the Chemical Adsorption of Ammonia on GaN(0001) Reconstructed Surfaces Based on Steepest-Entropy-Ascent Quantum Thermodynamics" Materials 10, no. 8: 948. https://doi.org/10.3390/ma10080948





