Fabrication and Characterization of Highly Sensitive Acetone Chemical Sensor Based on ZnO Nanoballs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological, Structural, Optical and Compositions Properties of ZnO Nanoballs
2.2. Characterization of Acetone Sensor Fabricated Based on ZnO Nanoballs
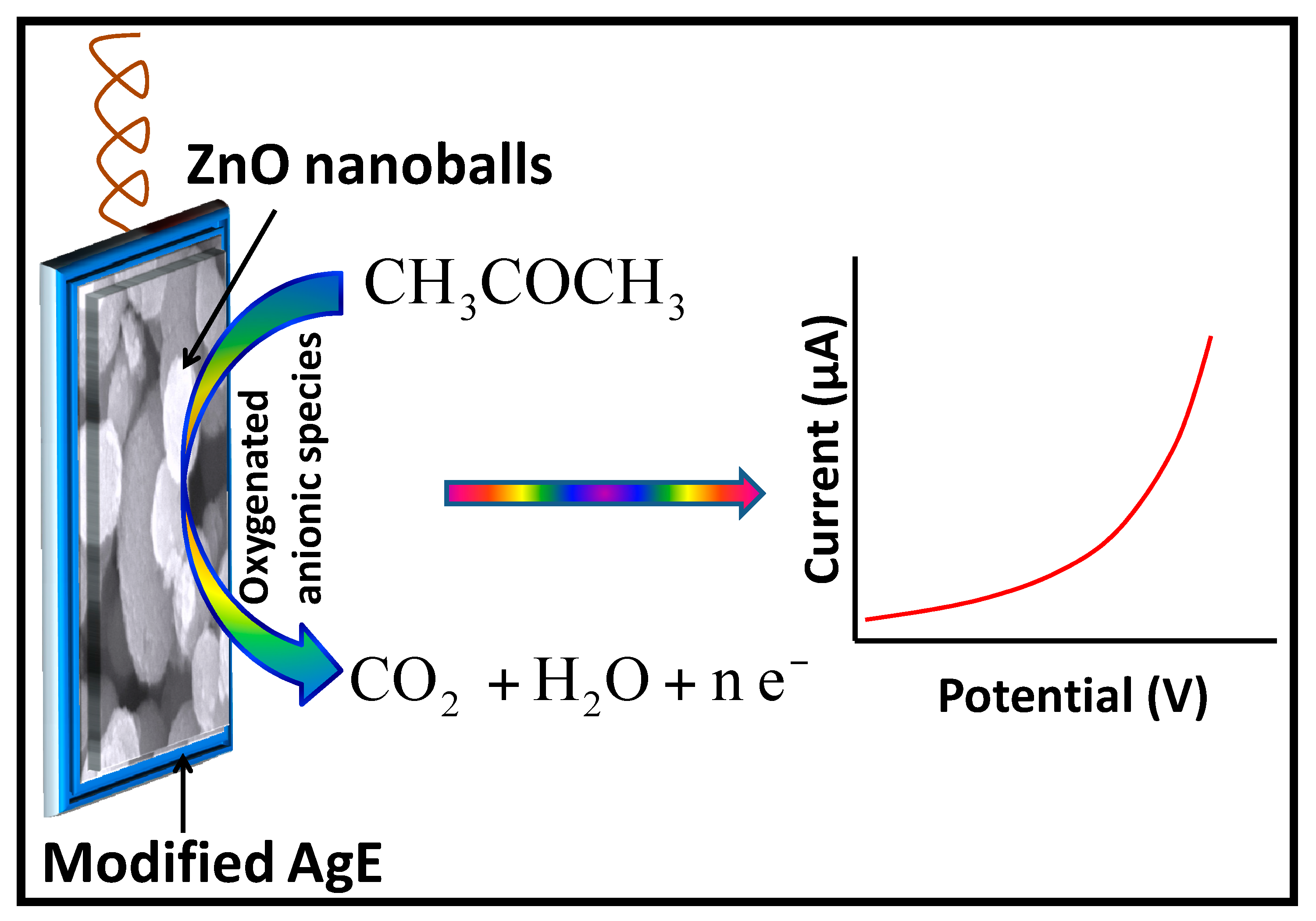
2.3. Proposed Sensing Mechanism
3. Materials and Methods
3.1. Hydrothermal Synthesis of ZnO Nanoballs
3.2. Fabrication of Acetone Sensor Based on ZnO Nanoballs
3.3. Characterization of ZnO Nanoballs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas sensor applications: A review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO—Nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S.; Kumar, A.; Akhtar, M.S. Zinc oxide nanostructure-based dye-sensitized solar cells. J. Mater. Sci. 2017, 52, 4743–4795. [Google Scholar] [CrossRef]
- Chongsri, K.; Sinornate, W.; Boonyarattanakalin, K.; Pecharapa, W. Growth and characterization of Ga/F Co-doped ZnO nanorods/nanodisks via hydrothermal process. J. Nanosci. Nanotechnol. 2016, 16, 12962–12966. [Google Scholar] [CrossRef]
- Park, G.C.; Lee, S.M.; Jeong, S.H.; Choi, J.H.; Lee, C.M.; Seo, T.Y.; Jung, S.-B.; Lim, J.H.; Joo, J. Enhanced photocatalytic activity of ZnO nanorods with tubular facet synthesized by hydrothermal method. J. Nanosci. Nanotechnol. 2016, 16, 11164–11168. [Google Scholar] [CrossRef]
- Nouira, W.; Barhoumi, H.; Maaref, A.; Renault, N.J.; Siadat, M. Tailoring of analytical performances of urea biosensors using nanomaterials. J. Phys. Conf. Ser. 2013, 416, 12010. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, P.; Matras-Postolek, K. Au catalyst decorated silica spheres: Synthesis and high-performance in 4-nitrophenol reduction. J. Nanosci. Nanotechnol. 2016, 16, 5966–5974. [Google Scholar] [CrossRef] [PubMed]
- Hieu, N.M.; Kim, H.; Kim, C.; Hong, S.-K.; Kim, D. A hydrogen sulfide gas sensor based on pd-decorated ZnO nanorods. J. Nanosci. Nanotechnol. 2016, 16, 10351–10355. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Rajkumar, C.; Chen, S.-M.; Lin, K.-Y. Determination of 4-nitrophenol in water by use of a screen-printed carbon electrode modified with chitosan-crafted ZnO nanoneedles. J. Colloid Interface Sci. 2017, 499, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Z.; Zhang, H.; Luo, L.; Yao, S. Sensitive and selective imprinted electrochemical sensor for p-nitrophenol based on ZnO nanoparticles/carbon nanotubes doped chitosan film. Thin Solid Films 2012, 520, 5314–5321. [Google Scholar] [CrossRef]
- Dar, G.N.; Umar, A.; Zaidi, S.A.; Baskoutas, S.; Hwang, S.W.; Abaker, M.; Al-Hajry, A.; Al-Sayari, S.A. Ultra-high sensitive ammonia chemical sensor based on ZnO nanopencils. Talanta 2012, 89, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Q.; Xu, L.; Zeng, W. Gas sensing properties of ZnO/SnO2 nanostructures. J. Nanosci. Nanotechnol. 2015, 15, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Singh, K.; Umar, A.; Chaudhary, G.R.; Singh, S. Ultra-high sensitive hydrazine chemical sensor based on low-temperature grown ZnO nanoparticles. Electrochim. Acta 2012, 69, 128–133. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Z.; Sun, Y.; Wang, Y.; Li, P.; Zhang, W.; Lian, K. Controllable synthesis of branched hierarchical ZnO nanorod arrays for highly sensitive hydrazine detection. Appl. Surf. Sci. 2016, 364, 434–441. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Umar, A.; Kumar, R.; Kim, S.H.; Bumajdad, A.; Baskoutas, S. Sm2O3-doped ZnO beech fern hierarchical structures for nitroaniline chemical sensor. Ceram. Int. 2016, 42, 16505–16511. [Google Scholar] [CrossRef]
- Ahmad, N.; Umar, A.; Kumar, R.; Alam, M. Microwave-assisted synthesis of ZnO doped CeO2 nanoparticles as potential scaffold for highly sensitive nitroaniline chemical sensor. Ceram. Int. 2016, 42, 11562–11567. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Hahn, Y.-B. Highly stable hydrazine chemical sensor based on vertically-aligned ZnO nanorods grown on electrode. J. Colloid Interface Sci. 2017, 494, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-K.; Lee, T.H.; Choi, H.Y.; Lee, T.J. Changing electric resistance of ZnO nano-rods by sulfur compounds for chemical gas sensor. J. Nanosci. Nanotechnol. 2015, 15, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Ameen, S.; Shaheer Akhtar, M.; Shin, H.S. Low temperature grown ZnO nanotubes as smart sensing electrode for the effective detection of ethanolamine chemical. Mater. Lett. 2013, 106, 254–258. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Kumar, R.; Umar, A.; Kim, S.H.; Bumajdad, A.; Ansari, Z.A.; Baskoutas, S. Cauliflower-shaped ZnO nanomaterials for electrochemical sensing and photocatalytic applications. Electrochim. Acta 2016, 222, 463–472. [Google Scholar] [CrossRef]
- Ameen, S.; Park, D.-R.; Akhtar, M.S.; Shin, H.S. Lotus-leaf like ZnO nanostructures based electrode for the fabrication of ethyl acetate chemical sensor. Mater. Lett. 2016, 164, 562–566. [Google Scholar] [CrossRef]
- Jianjiao, Z.; Hongyan, Y.; Erjun, G.; Shaolin, Z.; Liping, W.; Chunyu, Z.; Xin, G.; Jing, C.; Hong, Z. Novel gas sensor based on ZnO nanorod circular arrays for C2H5OH gas detection. J. Nanosci. Nanotechnol. 2015, 15, 2468–2472. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Zhao, A.; Wang, S.; Lv, Z.; Sun, J. Hierarchical triple-shelled porous hollow zinc oxide spheres wrapped in graphene oxide as efficient sensor material for simultaneous electrochemical determination of synthetic antioxidants in vegetable oil. Sens. Actuators B Chem. 2016, 235, 707–716. [Google Scholar] [CrossRef]
- Ya, Y.; Jiang, C.; Li, T.; Liao, J.; Fan, Y.; Wei, Y.; Yan, F.; Xie, L. A zinc oxide nanoflower-based electrochemical sensor for trace detection of sunset yellow. Sensors 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jing, W.; Wu, Q.; Gao, W.; Jiang, Z.; Shi, J.; Cui, Q. Effects of the surface morphologies of ZnO nanotube arrays on the performance of amperometric glucose sensors. Mater. Sci. Semicond. Process. 2016, 56, 137–144. [Google Scholar] [CrossRef]
- Rodrigues, A.; Castegnaro, M.V.; Arguello, J.; Alves, M.C.M.; Morais, J. Development and surface characterization of a glucose biosensor based on a nanocolumnar ZnO film. Appl. Surf. Sci. 2017, 402, 136–141. [Google Scholar] [CrossRef]
- Kitture, R.; Chordiya, K.; Gaware, S.; Ghosh, S.; More, P.A.; Kulkarni, P.; Chopade, B.A.; Kale, S.N. ZnO nanoparticles-red sandalwood conjugate: A promising anti-diabetic agent. J. Nanosci. Nanotechnol. 2015, 15, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Liu, X.; Yan, X.; Song, Y.; Kang, Z.; Luo, N.; Zhang, Y. Multicenter uric acid biosensor based on tetrapod-shaped ZnO nanostructures. J. Nanosci. Nanotechnol. 2012, 12, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Jang, N.K.; Khang, G.; Hahn, Y.B. Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface. Sens. Actuators B Chem. 2015, 206, 146–151. [Google Scholar] [CrossRef]
- Ansari, S.G.; Wahab, R.; Ansari, Z.A.; Kim, Y.S.; Khang, G.; Al-Hajry, A.; Shin, H.S. Effect of nanostructure on the urea sensing properties of sol-gel synthesized ZnO. Sens. Actuators B Chem. 2009, 137, 566–573. [Google Scholar] [CrossRef]
- Ali, S.M.U.; Ibupoto, Z.H.; Salman, S.; Nur, O.; Willander, M.; Danielsson, B. Selective determination of urea using urease immobilized on ZnO nanowires. Sens. Actuators B Chem. 2011, 160, 637–643. [Google Scholar] [CrossRef]
- Liu, H.; Gu, C.; Hou, C.; Yin, Z.; Fan, K.; Zhang, M. Plasma-assisted synthesis of carbon fibers/ZnO core–shell hybrids on carbon fiber templates for detection of ascorbic acid and uric acid. Sens. Actuators B Chem. 2016, 224, 857–862. [Google Scholar] [CrossRef]
- Ghanbari, K.; Moloudi, M. Flower-like ZnO decorated polyaniline/reduced graphene oxide nanocomposites for simultaneous determination of dopamine and uric acid. Anal. Biochem. 2016, 512, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Copa, V.C.; Tuico, A.R.; Mendoza, J.P.; Ferrolino, J.P.R.; Vergara, C.J.T.; Salvador, A.A.; Estacio, E.S.; Somintac, A.S. Development of resistance-based pH sensor using zinc oxide nanorods. J. Nanosci. Nanotechnol. 2016, 16, 6102–6106. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Highly dense ZnO nanowhiskers for the low level detection of p-hydroquinone. Mater. Lett. 2015, 155, 82–86. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Highly sensitive hydrazine chemical sensor fabricated by modified electrode of vertically aligned zinc oxide nanorods. Talanta 2012, 100, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Akhtar, M.S.; Al-Hajry, A.; Al-Assiri, M.S.; Dar, G.N.; Saif Islam, M. Enhanced photocatalytic degradation of harmful dye and phenyl hydrazine chemical sensing using ZnO nanourchins. Chem. Eng. J. 2015, 262, 588–596. [Google Scholar] [CrossRef]
- Zheng, L.; Wan, Y.; Qi, P.; Sun, Y.; Zhang, D.; Yu, L. Lectin functionalized ZnO nanoarrays as a 3D nano-biointerface for bacterial detection. Talanta 2017, 167, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, G.; Umar, A. ZnO nano-mushrooms for photocatalytic degradation of methyl orange. Mater. Lett. 2013, 97, 100–103. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Publishing Co.: Reading, MA, USA, 1978. [Google Scholar]
- Dogar, S.; Kim, S.M.; Kim, S.D. Ultraviolet photonic response of AlGaN/GaN high electron mobility transistor-based sensor with hydrothermal ZnO nanostructures. J. Nanosci. Nanotechnol. 2016, 16, 10175–10181. [Google Scholar] [CrossRef]
- Patterson, A.L. The scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Umar, A.; Alshahrani, A.A.; Algarni, H.; Kumar, R. CuO nanosheets as potential scaffolds for gas sensing applications. Sens. Actuators B Chem. 2017, 250, 24–31. [Google Scholar] [CrossRef]
- Jeong, E.-S.; Kang, M.; Kim, H.-S. Surface acoustic wave propagation properties with ZnO thin film for thermo-electric sensor applications. J. Nanosci. Nanotechnol. 2016, 16, 10219–10224. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Kheel, H.; Lee, W.I.; Lee, C. Enhanced ethanol gas sensing performance of the networked Fe2O3-functionalized ZnO nanowire sensor. J. Nanosci. Nanotechnol. 2016, 16, 8585–8588. [Google Scholar] [CrossRef]
- Umar, A.; Kumar, R.; Akhtar, M.S.; Kumar, G.; Kim, S.H. Growth and properties of well-crystalline cerium oxide (CeO2) nanoflakes for environmental and sensor applications. J. Colloid Interface Sci. 2015, 454, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, M.; Saravanan, S.; Soga, T. Effect of Fe-doping on the structural, morphological and optical properties of ZnO nanoparticles synthesized by solution combustion process. Physica E 2015, 71, 109–116. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, C.; Jiang, P.; Wang, G.; Li, K.; Miao, H. Simple one-pot synthesis of ZnO/Ag heterostructures and the application in visible-light-responsive photocatalysis. RSC Adv. 2014, 4, 7340–7346. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Asiri, A.M.; Alamry, K.A.; Khan, A.A.P.; Khan, A.; Rub, M.A.; Azum, N. Acetone sensor based on solvothermally prepared ZnO doped with Co3O4 nanorods. Microchim. Acta 2013, 180, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Faisal, M.; Rahman, M.M.; Jamal, A. Low-temperature growth of ZnO nanoparticles: Photocatalyst and acetone sensor. Talanta 2011, 85, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Hwang, S.W.; Dar, G.N.; Kim, S.H.; Abaker, M.; Ansari, S.G. Synthesis and characterization of Gd-doped ZnO nanopencils for acetone sensing application. Sci. Adv. Mater. 2015, 7, 1241–1246. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M.; Islam, M.A. Fabrication of selective chemical sensor with ternary ZnO/SnO2/Yb2O3 nanoparticles. Talanta 2017, 170, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Weng, Y.-C.; Chou, T.-C. Acetone sensor using lead foil as working electrode. Sens. Actuators B Chem. 2007, 122, 591–595. [Google Scholar] [CrossRef]
- Chou, T.-C. An amperometric acetone sensor by using an electro-deposited Pb-modified electrode. Z. Naturforsch. B 2006, 61, 560–564. [Google Scholar]
- Rahman, M.M.; Khan, S.B.; Jamal, A.; Faisal, M.; Asiri, A.M. Fabrication of highly sensitive acetone sensor based on sonochemically prepared as-grown Ag2O nanostructures. Chem. Eng. J. 2012, 192, 122–128. [Google Scholar] [CrossRef]
- Saravanan, T.; Raj, S.G.; Chandar, N.R.K.; Jayavel, R. Synthesis, optical and electrochemical properties of Y2O3 nanoparticles prepared by co-precipitation method. J. Nanosci. Nanotechnol. 2015, 15, 4353–4357. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.D.; Shahnawaze Ansari, M.; Salah, N.; Khayyat, S.A.; Khan, Z.H. Zinc oxide-multi walled carbon nanotubes nanocomposites for carbon monoxide gas sensor application. J. Nanosci. Nanotechnol. 2016, 16, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Jung, D.-U.-J.; Hahn, Y.-B. Highly sensitive hydrazine chemical sensor based on ZnO nanorods field-effect transistor. Chem. Commun. 2014, 50, 1890–1893. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, D.; Chang, P.-C.; Tseng, W.-Y.; Lu, J.G. ZnO nanowire field-effect transistor and oxygen sensing property. Appl. Phys. Lett. 2004, 85, 5923–5925. [Google Scholar] [CrossRef]
- Gujarati, T.P.; Ashish, A.G.; Rai, M.; Shaijumon, M.M. Highly ordered vertical arrays of TiO2/ZnO hybrid nanowires: Synthesis and electrochemical characterization. J. Nanosci. Nanotechnol. 2015, 15, 5833–5839. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S. Synthesis and characterisation of SnO2 films obtained by a wet chemical process. Mater. Sci. 2009, 27, 123–129. [Google Scholar]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Hahn, Y.-B. Development of highly-stable binder-free chemical sensor electrodes for p-nitroaniline detection. J. Colloid Interface Sci. 2017, 494, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Chandra, S. Catalyst-free synthesis of ZnO nanowires on oxidized silicon substrate for gas sensing applications. J. Nanosci. Nanotechnol. 2015, 15, 4534–4542. [Google Scholar] [CrossRef] [PubMed]
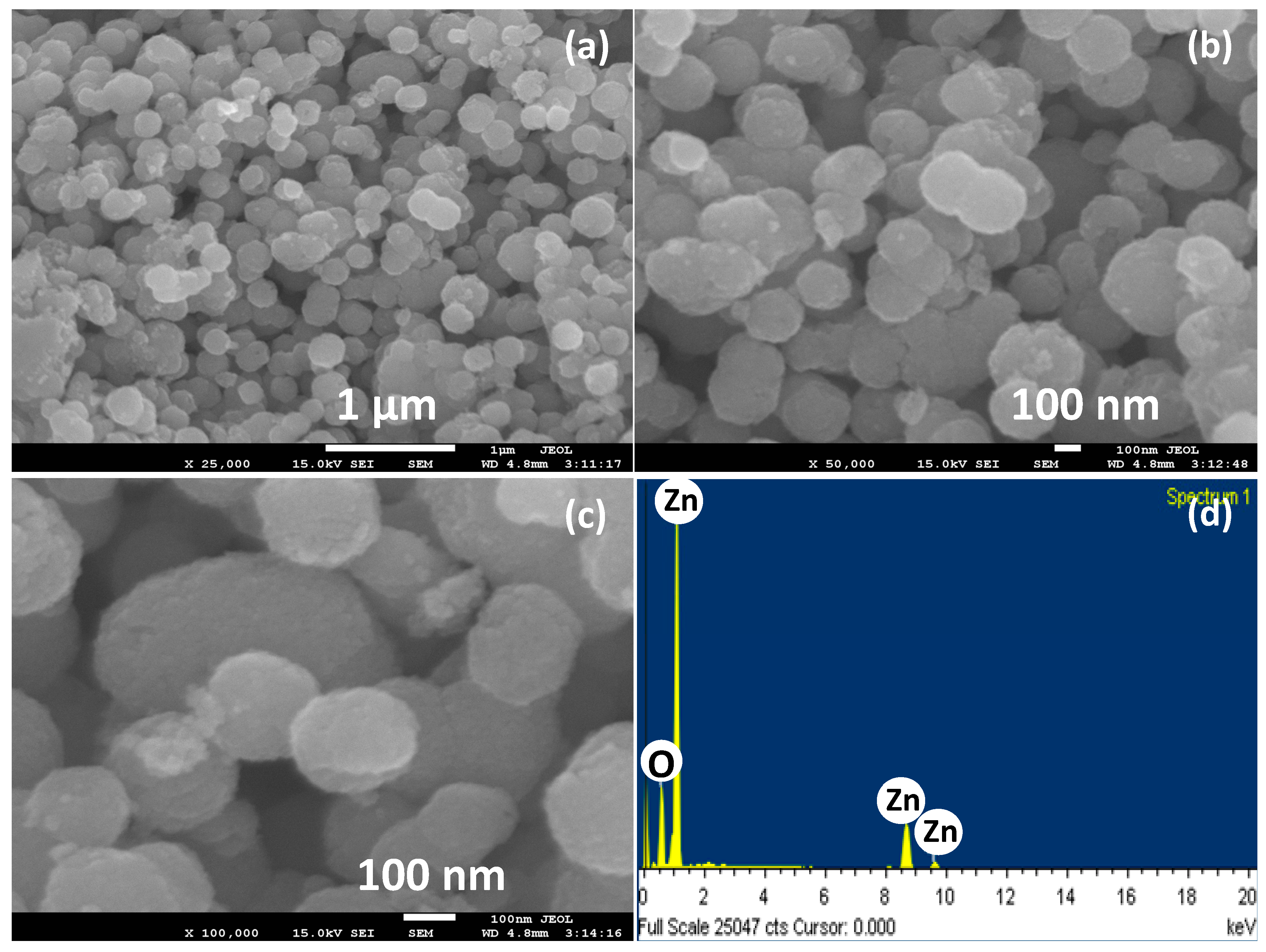


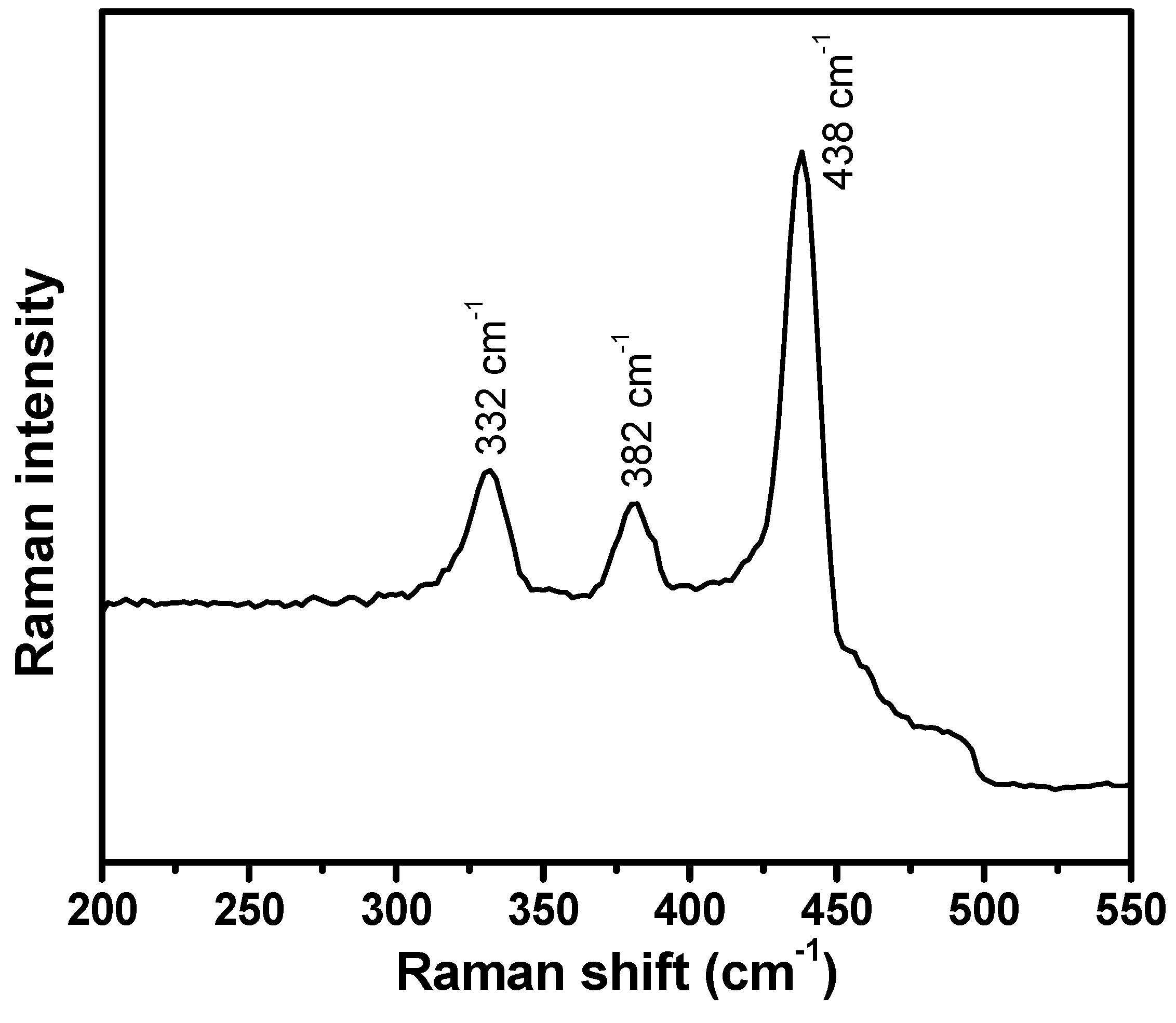

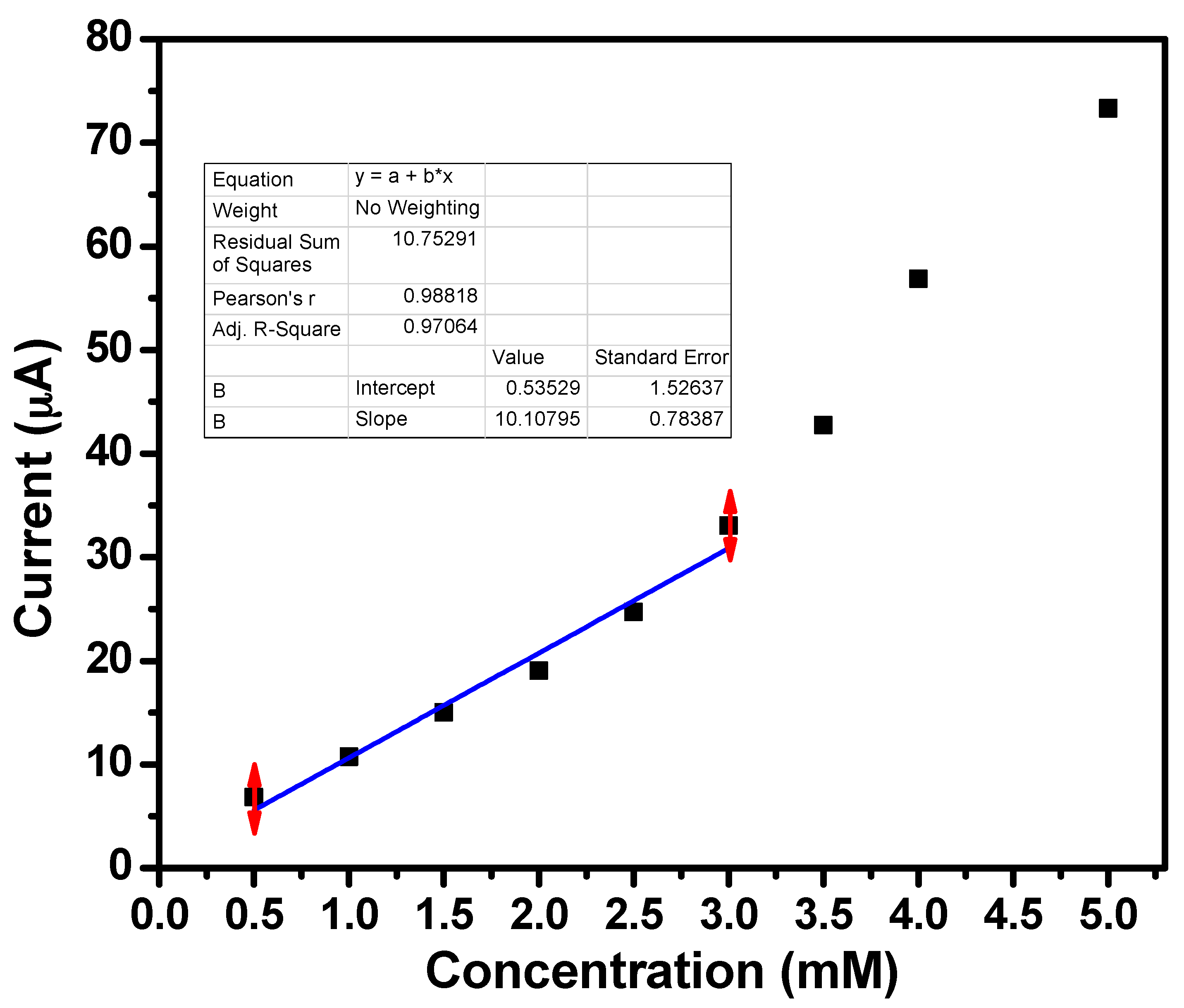
| S.N | (hkl) | 2θ (°) | FWHM (β) | Crystallite Size (nm) |
|---|---|---|---|---|
| 1 | (100) | 31.78 | 0.71936 | 11.36 |
| 2 | (002) | 38.43 | 0.80871 | 10.18 |
| 3 | (101) | 36.23 | 0.83756 | 9.88 |
| Sensor | Method | Sensitivity | LDR | LOD | R2 | Ref. |
|---|---|---|---|---|---|---|
| ZnO-doped Co3O4 Nanorods/AgE | I–V | 3.58 μA·mM−1·cm−2 | 66.8 μM–0.133 mM | 14.7 ± 0.2 μM | 0.9684 | [50] |
| ZnO NPs/GCE | I–V | 0.14065 μA·mM−1·cm−2 | 0.13 mM–0.13 M | 0.068 ± 0.01 mM | - | [51] |
| Gd-ZnO-Nanopencils/AgE | I–V | 208 ± 62 μA·mM−1·cm−2 | 750 μM–100 mM | 0.7 mM | 0.885 | [52] |
| ZnO/SnO2/Yb2O3/GCE | I–V | 17.09 μA·mM−1·cm−2 | 0.34 nM–3.4 mM | 0.05 ± 0.002 nM | 0.9394 | [53] |
| Lead foil electrode | Amperometric | 2.07 μA·cm−2·ppm−1 | 50–250 ppm | 50 ppm | 0.998 | [54] |
| Electro-deposited Pb electrode | Amperometric | 4.16 μA·cm−2·ppm−1 | 100–400 ppm | - | 0.99 | [55] |
| Ag2O microflower/GCE | I–V | 1.699 μA·mM−1·cm−2 | 0.13 μM–0.67 M | 0.11 μM | 0.9462 | [56] |
| ZnO nanoballs/AgE | I–V | 472.33 μA·mM−1·cm−2 | 0.5 mM–3.0 mM | 0.5 mM | 0.9706 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Hong, C.; Yao, Y.; Ibrahim, A.M.; Xu, L.; Kumar, R.; Talballa, S.M.; Kim, S.H.; Umar, A. Fabrication and Characterization of Highly Sensitive Acetone Chemical Sensor Based on ZnO Nanoballs. Materials 2017, 10, 799. https://doi.org/10.3390/ma10070799
Zhou Q, Hong C, Yao Y, Ibrahim AM, Xu L, Kumar R, Talballa SM, Kim SH, Umar A. Fabrication and Characterization of Highly Sensitive Acetone Chemical Sensor Based on ZnO Nanoballs. Materials. 2017; 10(7):799. https://doi.org/10.3390/ma10070799
Chicago/Turabian StyleZhou, Qu, ChangXiang Hong, Yao Yao, Ahmed Mohamed Ibrahim, Lingna Xu, Rajesh Kumar, Sumaia Mohamed Talballa, S. H. Kim, and Ahmad Umar. 2017. "Fabrication and Characterization of Highly Sensitive Acetone Chemical Sensor Based on ZnO Nanoballs" Materials 10, no. 7: 799. https://doi.org/10.3390/ma10070799




