Flower-Like CuO/ZnO Hybrid Hierarchical Nanostructures Grown on Copper Substrate: Glycothermal Synthesis, Characterization, Hydrophobic and Anticorrosion Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
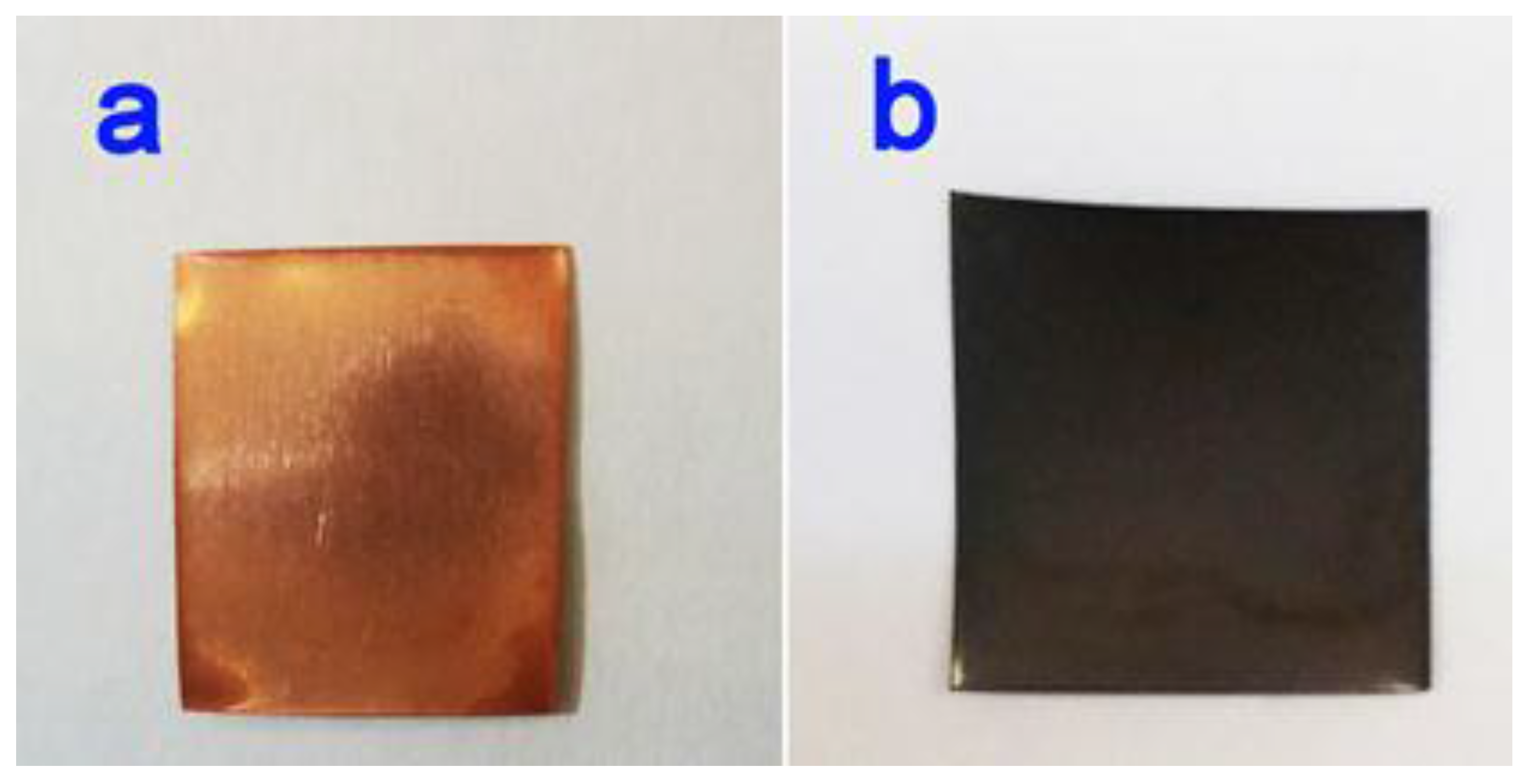
2.2. Synthesis of Nanoscale CuO Arrays on the Cu Substrate
2.3. Preparation of 3D CuO-ZnO Hybrid Hierarchical Structures
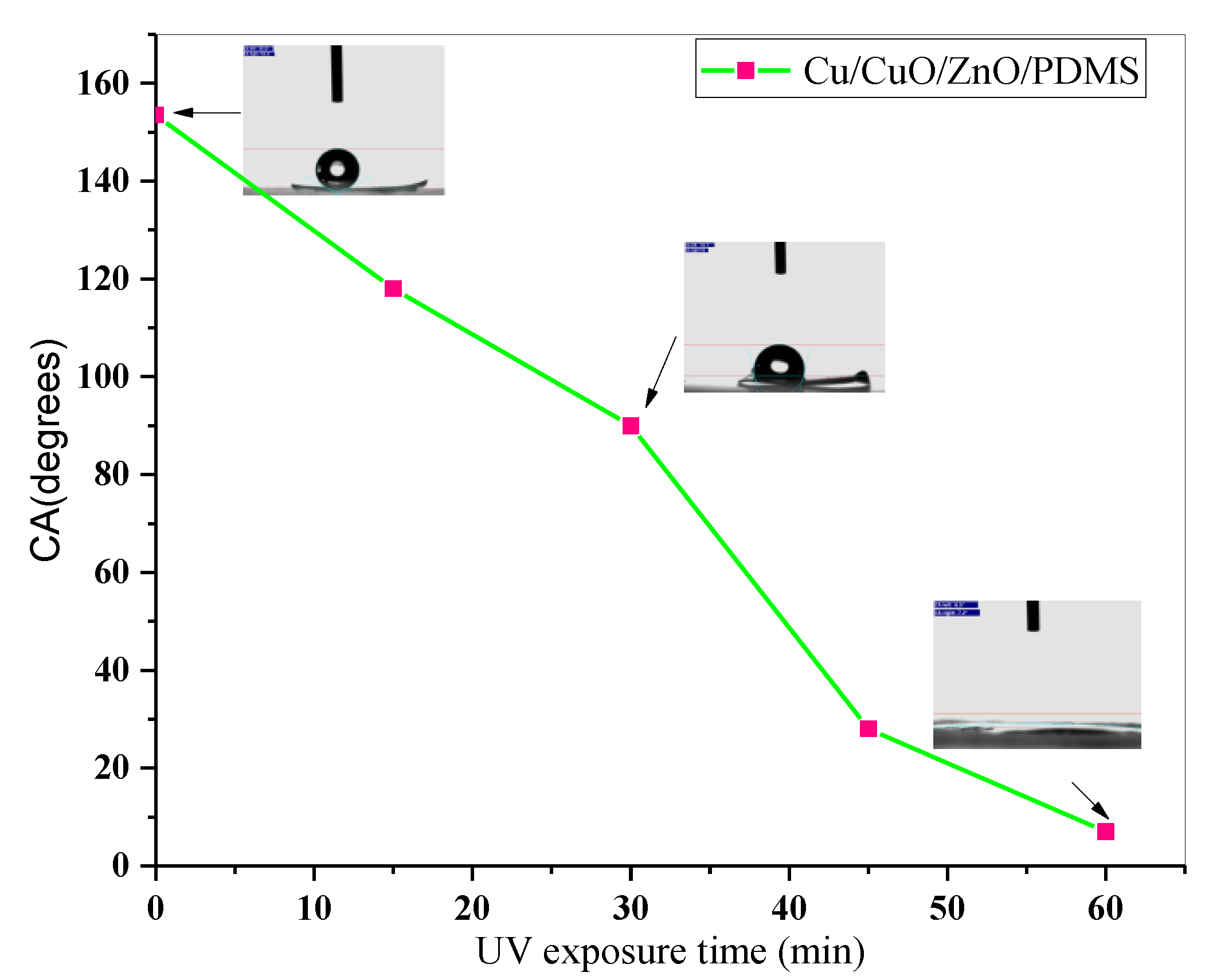
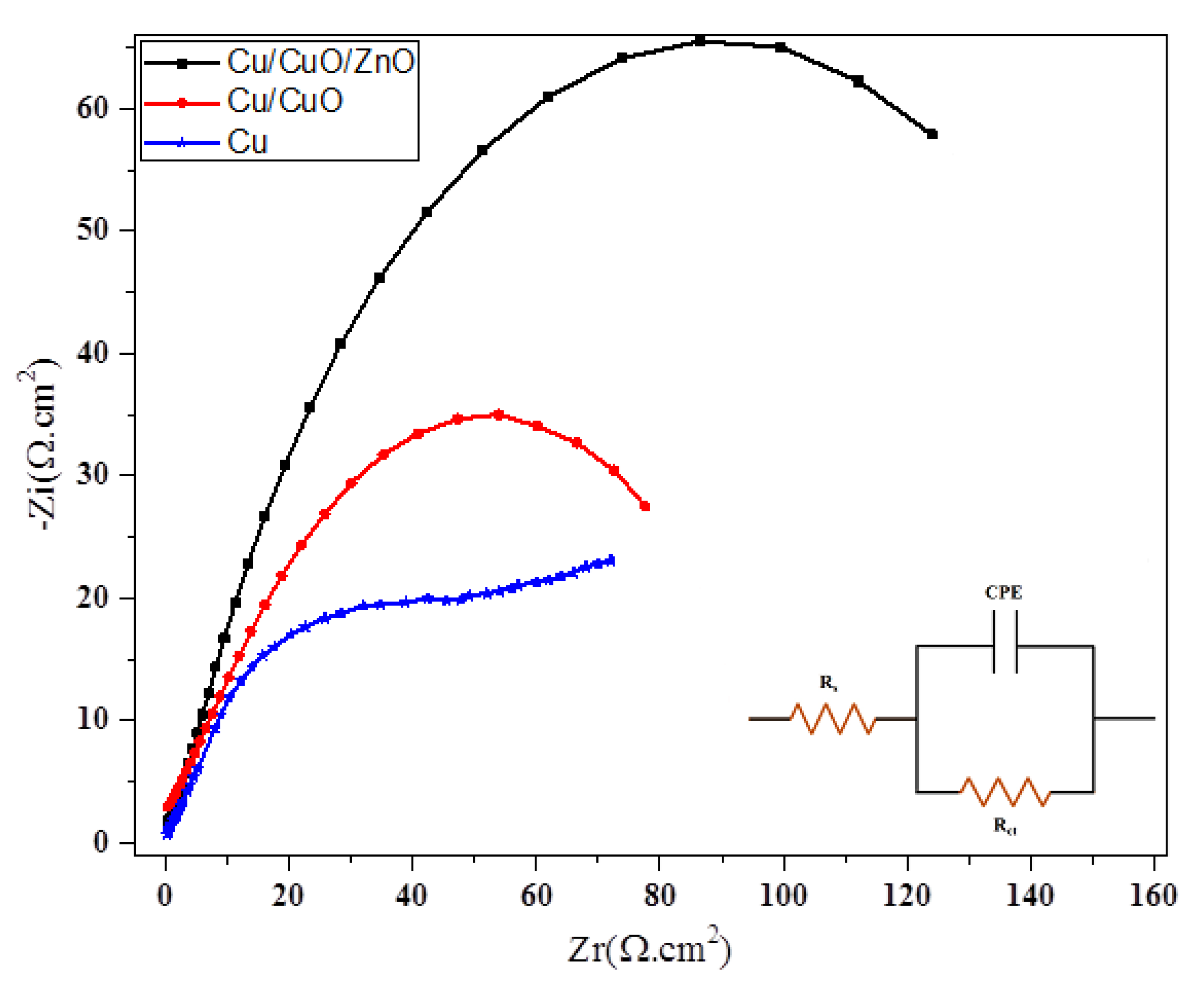
2.4. Anticorrosion Behaviour of the Superhydrophobic CuO-ZnO Film
3. Results and Discussion
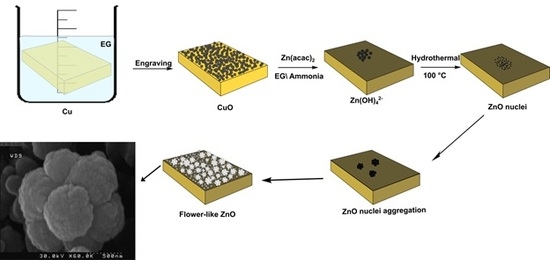
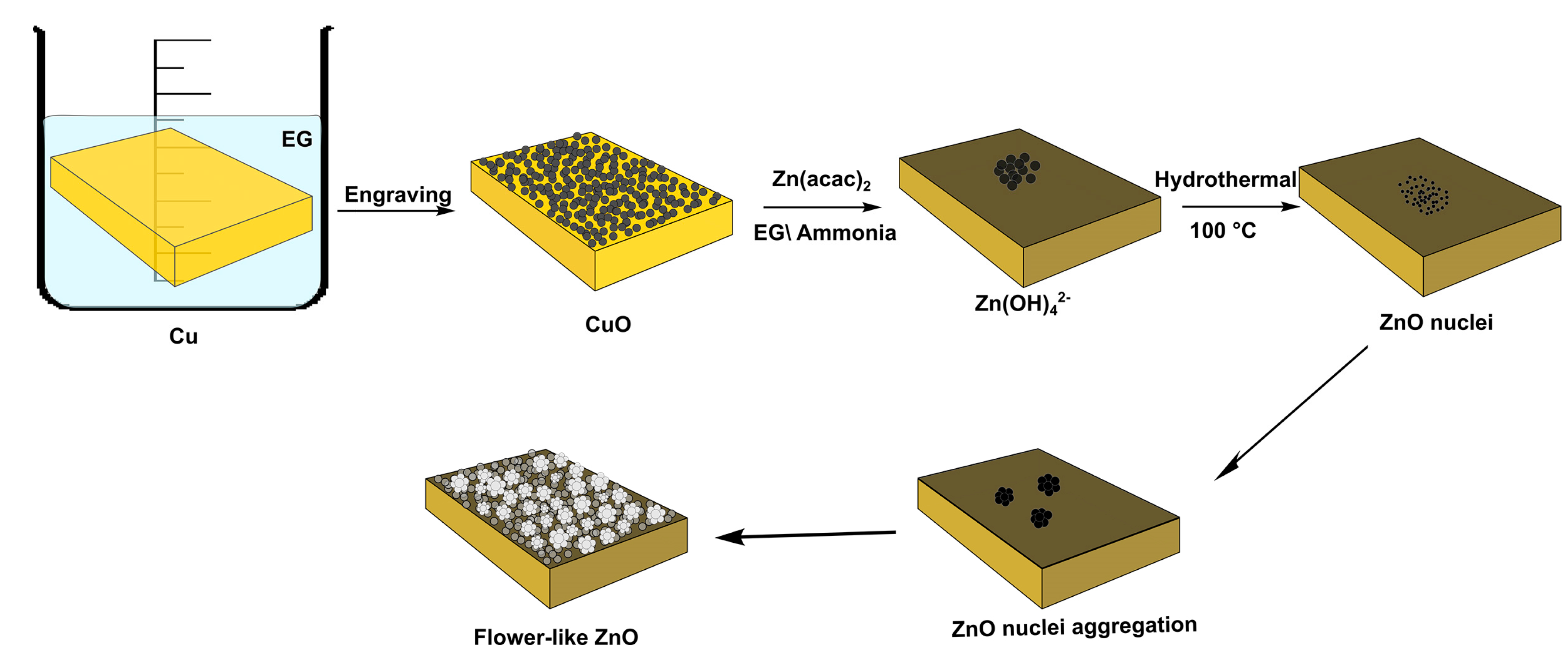
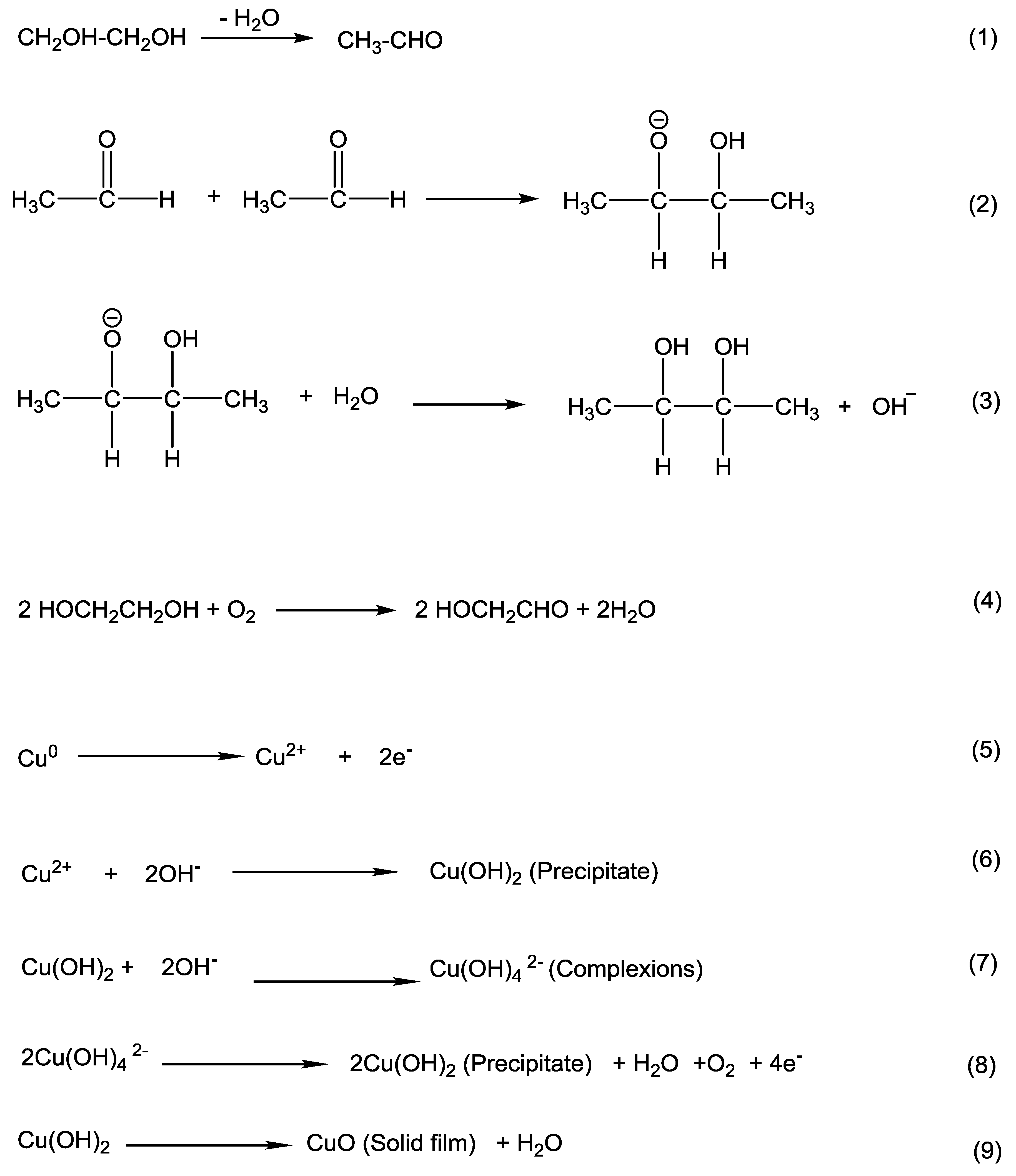
3.1. The Mechanism of CuO Formation
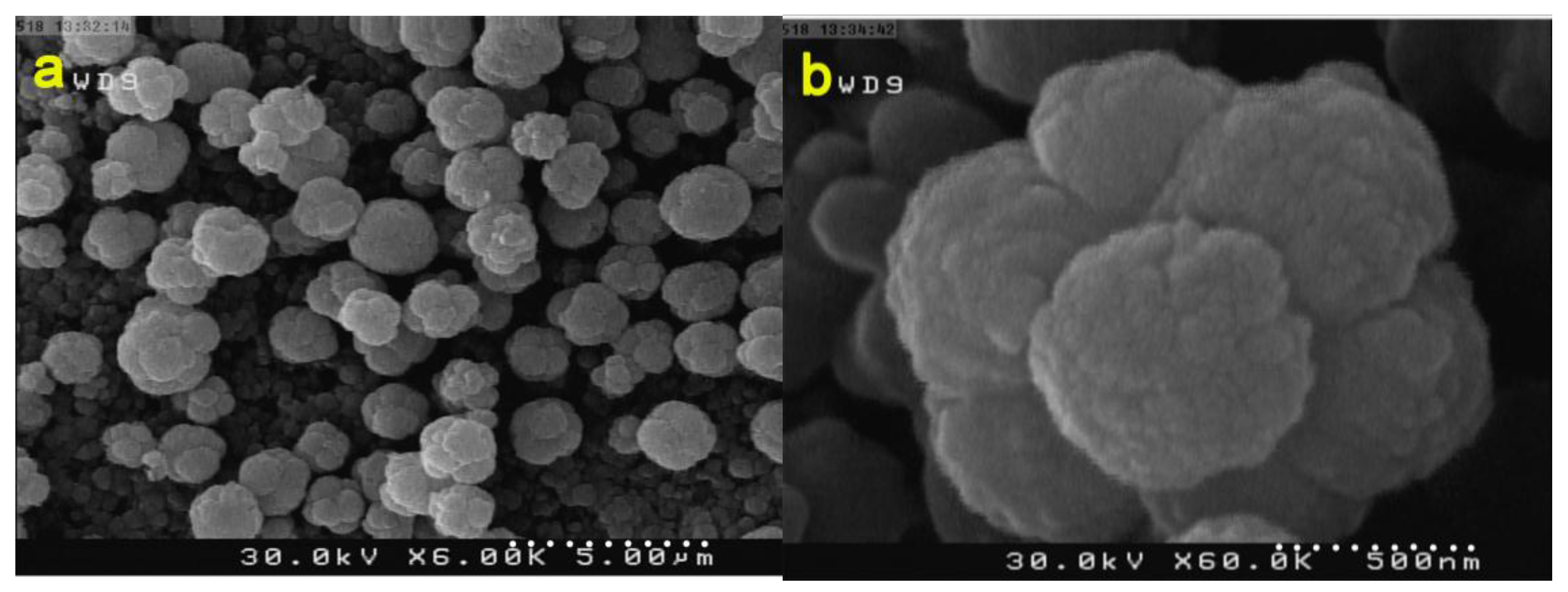
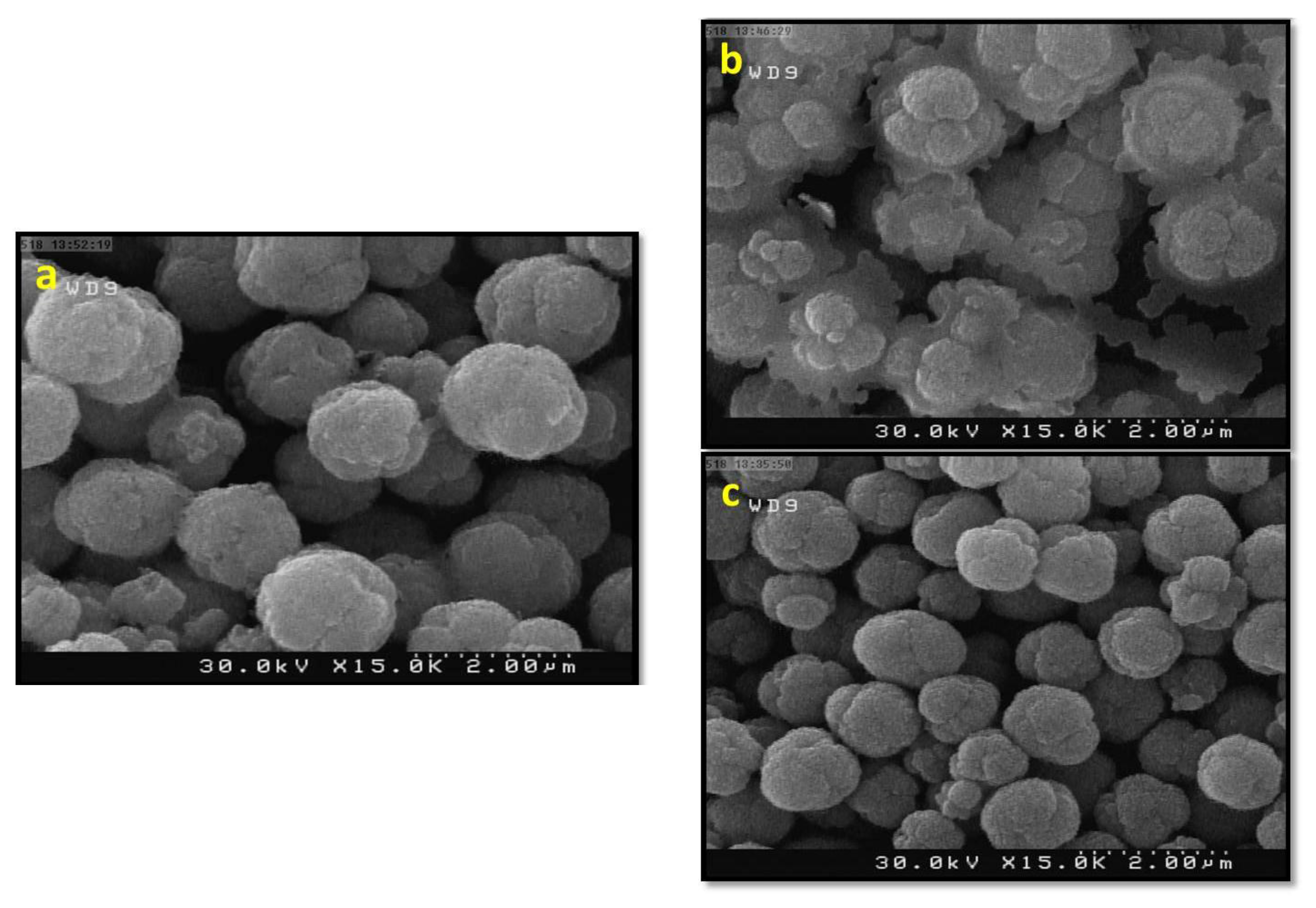
3.2. Studying the Morphology and Growth Mechanism of the Flower Like ZnO Products
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Núñez, L.; Reguera, E.; Corvo, F.; González, E.; Vazquez, C. Corrosion of copper in seawater and its aerosols in a tropical island. Corros. Sci. 2005, 47, 461–484. [Google Scholar] [CrossRef]
- Wu, H.; Xue, M.; Ou, J.; Wang, F.; Li, W. Effect of annealing temperature on surface morphology and work function of ZnO nanorod arrays. J. Alloys Compd. 2013, 565, 85–89. [Google Scholar] [CrossRef]
- Lu, C.; Qi, L.; Yang, J.; Zhang, D.; Wu, N.; Ma, J. Simple template-free solution route for the controlled synthesis of Cu(OH)2 and CuO nanostructures. J. Phys. Chem. B 2004, 108, 17825–17831. [Google Scholar]
- Xu, J.; Xue, D. Fabrication of malachite with a hierarchical sphere-like architecture. J. Phys. Chem. B 2005, 109, 17157–17161. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, X.; Gu, X.; Su, H. CuO–ZnO heterometallic hollow spheres: Morphology and defect structure. J. Solid State Chem. 2012, 186, 76–80. [Google Scholar] [CrossRef]
- Zhang, R.; Fan, L.; Fang, Y.; Yang, S. Electrochemical route to the preparation of highly dispersed composites of ZnO/carbon nanotubes with significantly enhanced electrochemiluminescence from ZnO. J. Mater. Chem. 2008, 18, 4964–4970. [Google Scholar] [CrossRef]
- Rahman, M.M.; Jamal, A.; Khan, S.B.; Faisal, M. CuO codoped ZnO based nanostructured materials for sensitive chemical sensor applications. ACS Appl. Mater. Interfaces 2011, 3, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shin, P.; Cao, L.; Gao, D. Preferential growth of long zno nanowire array and its application in dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 125–129. [Google Scholar] [CrossRef]
- Norton, D.P.; Heo, Y.W.; Ivill, M.P.; Ip, K.; Pearton, S.J.; Chisholm, M.F.; Steiner, T. ZnO: Growth, doping & processing. Mater. Today 2004, 7, 34–40. [Google Scholar]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [PubMed]
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale kirkendall effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sow, C.H.; Yu, T.; Zhao, Q.; Li, P.; Shen, Z.; Yu, D.; Thong, J.T.L. Co-synthesis of ZnO–CuO nanostructures by directly heating brass in air. Adv. Funct. Mater. 2006, 16, 2415–2422. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, P.; Li, B. CuO/ZnO core/shell heterostructure nanowire arrays: Synthesis, optical property, and energy application. Chem. Commun. 2010, 46, 6768–6770. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Cheng, H.-W.; Chen, S.-M.; Wang, S.-F. Preparation and characterization of copper nanoparticles/zinc oxide composite modified electrode and its application to glucose sensing. Mater. Sci. Eng. C 2010, 30, 86–91. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.; Li, J.; Liu, J.-H.; Huang, X.-J. ZnO/CuO hetero-hierarchical nanotrees array: Hydrothermal preparation and self-cleaning properties. Langmuir 2011, 27, 6193–6200. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Yong, K. Fabrication of CuO-ZnO nanowires on a stainless steel mesh for highly efficient photocatalytic applications. Chem. Commun. 2011, 47, 2643–2645. [Google Scholar] [CrossRef] [PubMed]
- Shaislamov, U.; Krishnamoorthy, K.; Kim, S.J.; Chun, W.; Lee, H.-J. Facile fabrication and photoelectrochemical properties of a CuO nanorod photocathode with a ZnO nanobranch protective layer. RSC Adv. 2016, 6, 103049–103056. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Zhang, H.; An, T.; Yang, H.; Tang, Z.; Cai, W.; Zhao, H. Vapor-phase hydrothermal transformation of HTiOF3 intermediates into {001} faceted anatase single-crystalline nanosheets. Small 2012, 8, 3664–3673. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettability and surface free energy of graphene films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Yuan, S.; Liang, B.; Li, G.; Pehkonen, S.O.; Zhang, T. Superhydrophobic CuO nanoneedle-covered copper surfaces for anticorrosion. J. Mater. Chem. A 2015, 3, 4374–4388. [Google Scholar] [CrossRef]
- Beshkar, F.; Zinatloo-Ajabshir, S.; Salavati-Niasari, M. Simple morphology-controlled fabrication of nickel chromite nanostructures via a novel route. Chem. Eng. J. 2015, 279, 605–614. [Google Scholar] [CrossRef]
- Mousavi, Z.; Soofivand, F.; Esmaeili-Zare, M.; Salavati-Niasari, M.; Bagheri, S. ZnCr2O4 Nanoparticles: facile synthesis, characterization, and photocatalytic properties. Sci. Rep. 2016, 6, 20071. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Kapoor, S.; Dave, D.P.; Mukherjee, T. Synthesis of Pt, Pd, Pt/Ag and Pd/Ag nanoparticles by microwave-polyol method. J. Chem. Sci. 2005, 117, 311–316. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Wiley, B.J.; Kim, M.; Formo, E.V.; Xia, Y. On the polyol synthesis of silver nanostructures: Glycolaldehyde as a reducing agent. Nano Lett. 2008, 8, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.-D. Fabrication of CuO nanoplatelets for highly sensitive enzyme-free determination of glucose. Electrochim. Acta 2011, 56, 7510–7516. [Google Scholar] [CrossRef]
- Jana, S.; Das, S.; Das, N.S.; Chattopadhyay, K.K. CuO nanostructures on copper foil by a simple wet chemical route at room temperature. Mater. Res. Bull. 2010, 45, 693–698. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Chen, Y.-C.; Lin, Y.-G. Spontaneous formation of CuO nanosheets on Cu foil for H2O2 detection. Appl. Surf. Sci. 2015, 354, 85–89. [Google Scholar] [CrossRef]
- Ekthammathat, N.; Thongtem, T.; Thongtem, S. Antimicrobial activities of CuO films deposited on Cu foils by solution chemistry. Appl. Surf. Sci. 2013, 277, 211–217. [Google Scholar] [CrossRef]
- Fan, G.; Li, F. Effect of sodium borohydride on growth process of controlled flower-like nanostructured Cu2O/CuO films and their hydrophobic property. Chem. Eng. J. 2011, 167, 388–396. [Google Scholar] [CrossRef]
- Shen, H.; Eisenberg, A. Morphological phase diagram for a ternary system of block copolymer PS310-b-PAA52/Dioxane/H2O. J. Phys. Chem. B 1999, 103, 9473–9487. [Google Scholar] [CrossRef]
- Burke, S.E.; Eisenberg, A. Effect of Sodium Dodecyl sulfate on the morphology of polystyrene-b-poly(acrylic acid) aggregates in dioxane−water Mixtures. Langmuir 2001, 17, 8341–8347. [Google Scholar] [CrossRef]
- Hui, Z.; Deren, Y.; Xiangyang, M.; Yujie, J.; Jin, X.; Duanlin, Q. Synthesis of flower-like ZnO nanostructures by an organic-free hydrothermal process. Nanotechnology 2004, 15, 622. [Google Scholar]
- Sun, Y.; Hu, J.; Wang, N.; Zou, R.; Wu, J.; Song, Y.; Chen, H.; Chen, H.; Chen, Z. Controllable hydrothermal synthesis, growth mechanism, and properties of ZnO three-dimensional structures. New J. Chem. 2010, 34, 732–737. [Google Scholar] [CrossRef]
- Yamabi, S.; Imai, H. Growth conditions for wurtzite zinc oxide films in aqueous solutions. J. Mater. Chem. 2002, 12, 3773–3778. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Li, Y.; Sulieman, K.M.; He, X.; Sun, F. Hierarchical nanostructures of cupric oxide on a copper substrate: Controllable morphology and wettability. J. Mater. Chem. 2006, 16, 4427–4434. [Google Scholar] [CrossRef]
- Wu, X.; Shi, G. Production and characterization of stable superhydrophobic surfaces based on copper hydroxide nanoneedles mimicking the legs of water striders. J. Phys. Chem. B 2006, 110, 11247–11252. [Google Scholar] [CrossRef] [PubMed]

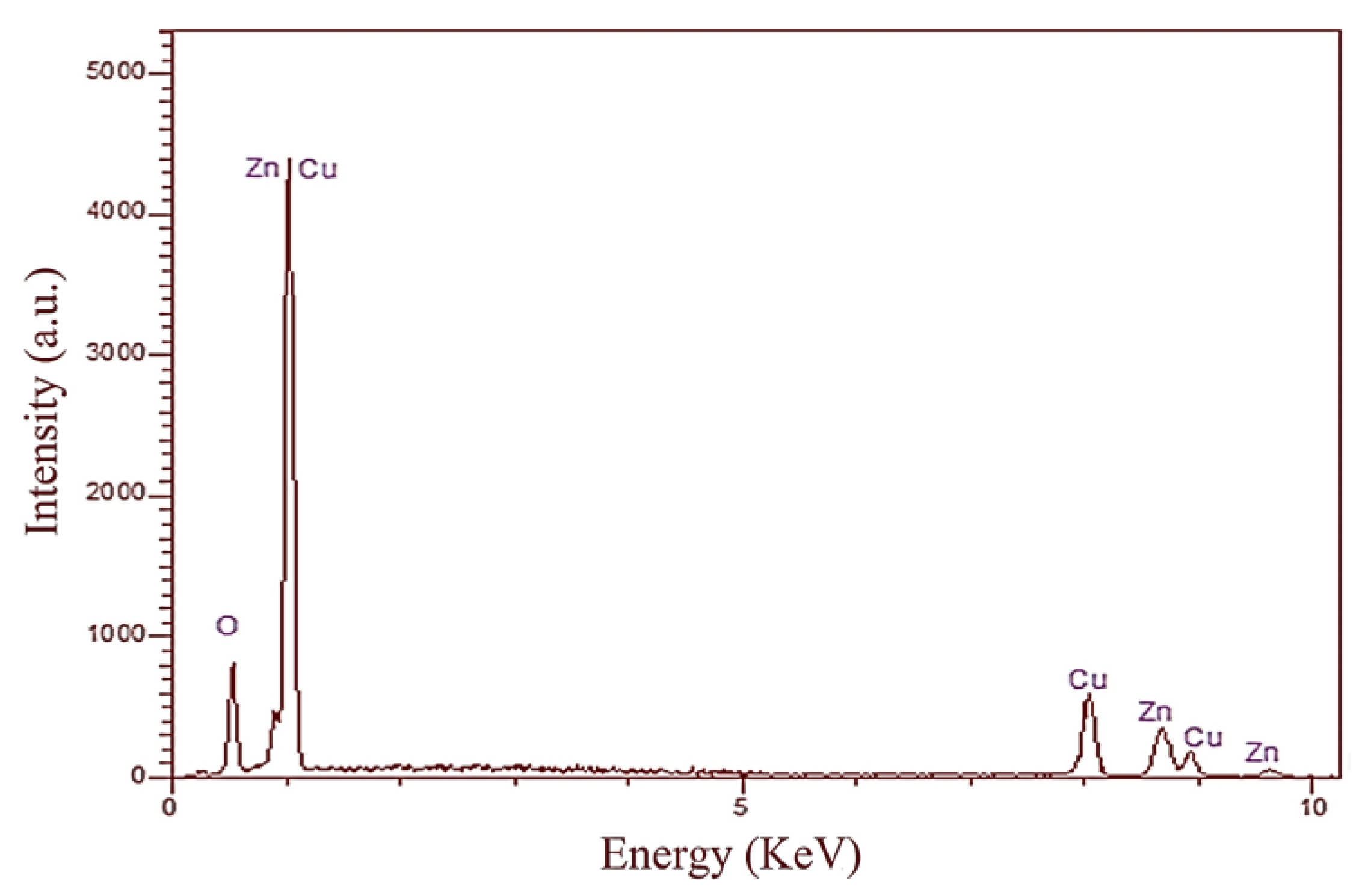




| Sample | Stabilizer or Surfactant | Temperature (°C) | Time (h) | Morphology |
|---|---|---|---|---|
| C0 | - | 80 | 120 | Rough surface with nanoscale particles |
| C1 | PEG 6000 | 80 | 120 | Coalesced particles and bulk structures |
| C2 | SDS | 80 | 120 | Sponge-like |
| C3 | CTAB | 80 | 120 | Agglomerated and impacted structures |
| Z0 | - | 100 | 8 | Symmetry flower-like microstructures |
| Z1 | PEG 6000 | 100 | 8 | Symmetry cabbage-like microstructures |
| Z2 | SDS | 100 | 8 | Asymmetry microstructures |
| Z3 | CTAB | 100 | 8 | Non-sized cabbage-like microstructures |
| Sample | Rs (Ω·cm2) | Rct (Ω·cm2) | C (μF·cm2) | η% |
|---|---|---|---|---|
| Cu | 14 ± 4% | 81.3 ± 4% | 79.2 ± 6% | - |
| Cu/CuO | 16.62 ± 4% | 146.8 ± 4% | 69.7 ± 7% | 44.61 |
| Cu/CuO/ZnO | 23.58 ± 10% | 650 ± 5% | 27.4 ± 7% | 87.49 |
| Cu/CuO/ZnO/SA | 35.9 ± 9% | 3220 ± 10% | 7.06 ± 8% | 97.47 |
| Cu/CuO/ZnO/PDMS | 651 ± 8% | 40,500 ± 9% | 3.61 ± 7% | 99.79 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beshkar, F.; Khojasteh, H.; Salavati-Niasari, M. Flower-Like CuO/ZnO Hybrid Hierarchical Nanostructures Grown on Copper Substrate: Glycothermal Synthesis, Characterization, Hydrophobic and Anticorrosion Properties. Materials 2017, 10, 697. https://doi.org/10.3390/ma10070697
Beshkar F, Khojasteh H, Salavati-Niasari M. Flower-Like CuO/ZnO Hybrid Hierarchical Nanostructures Grown on Copper Substrate: Glycothermal Synthesis, Characterization, Hydrophobic and Anticorrosion Properties. Materials. 2017; 10(7):697. https://doi.org/10.3390/ma10070697
Chicago/Turabian StyleBeshkar, Farshad, Hossein Khojasteh, and Masoud Salavati-Niasari. 2017. "Flower-Like CuO/ZnO Hybrid Hierarchical Nanostructures Grown on Copper Substrate: Glycothermal Synthesis, Characterization, Hydrophobic and Anticorrosion Properties" Materials 10, no. 7: 697. https://doi.org/10.3390/ma10070697
APA StyleBeshkar, F., Khojasteh, H., & Salavati-Niasari, M. (2017). Flower-Like CuO/ZnO Hybrid Hierarchical Nanostructures Grown on Copper Substrate: Glycothermal Synthesis, Characterization, Hydrophobic and Anticorrosion Properties. Materials, 10(7), 697. https://doi.org/10.3390/ma10070697