A Study of Structure and Magnetic Properties of Low Purity Fe-Co-Based Metallic Glasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
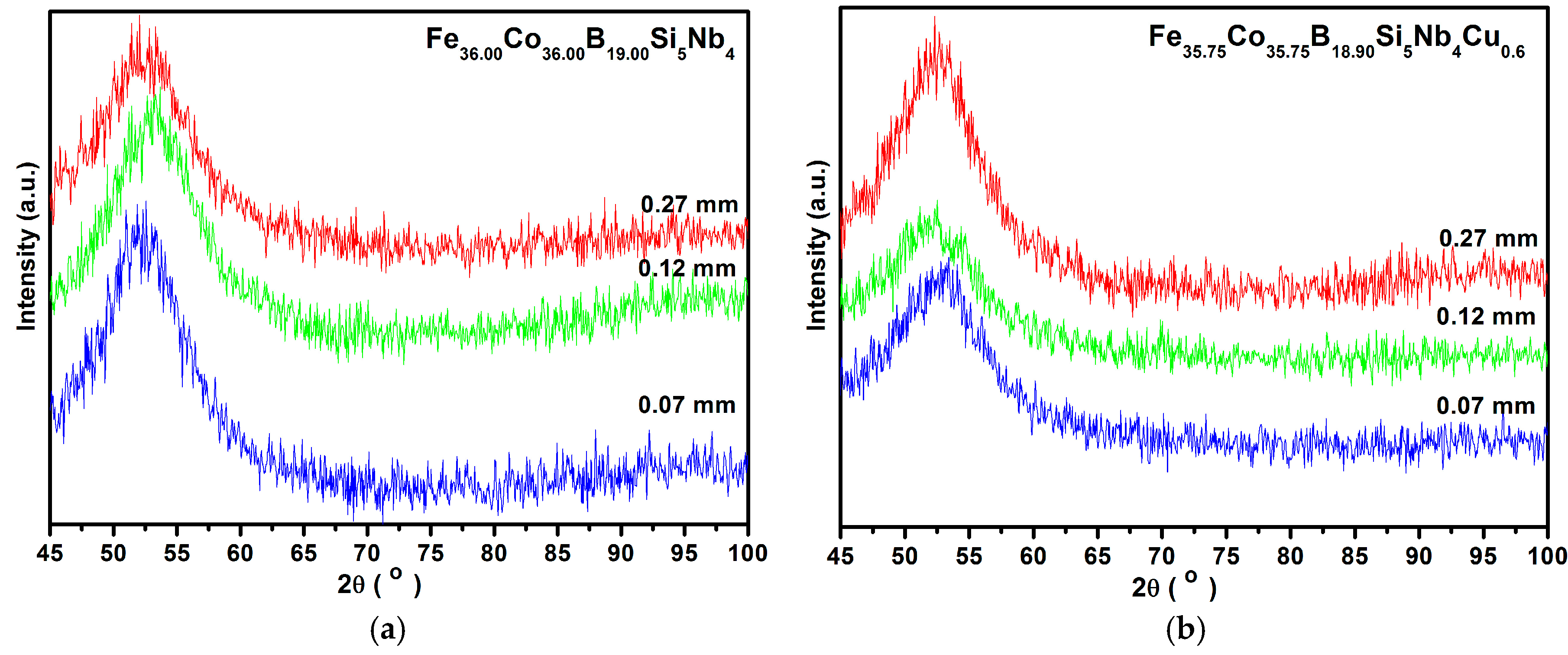
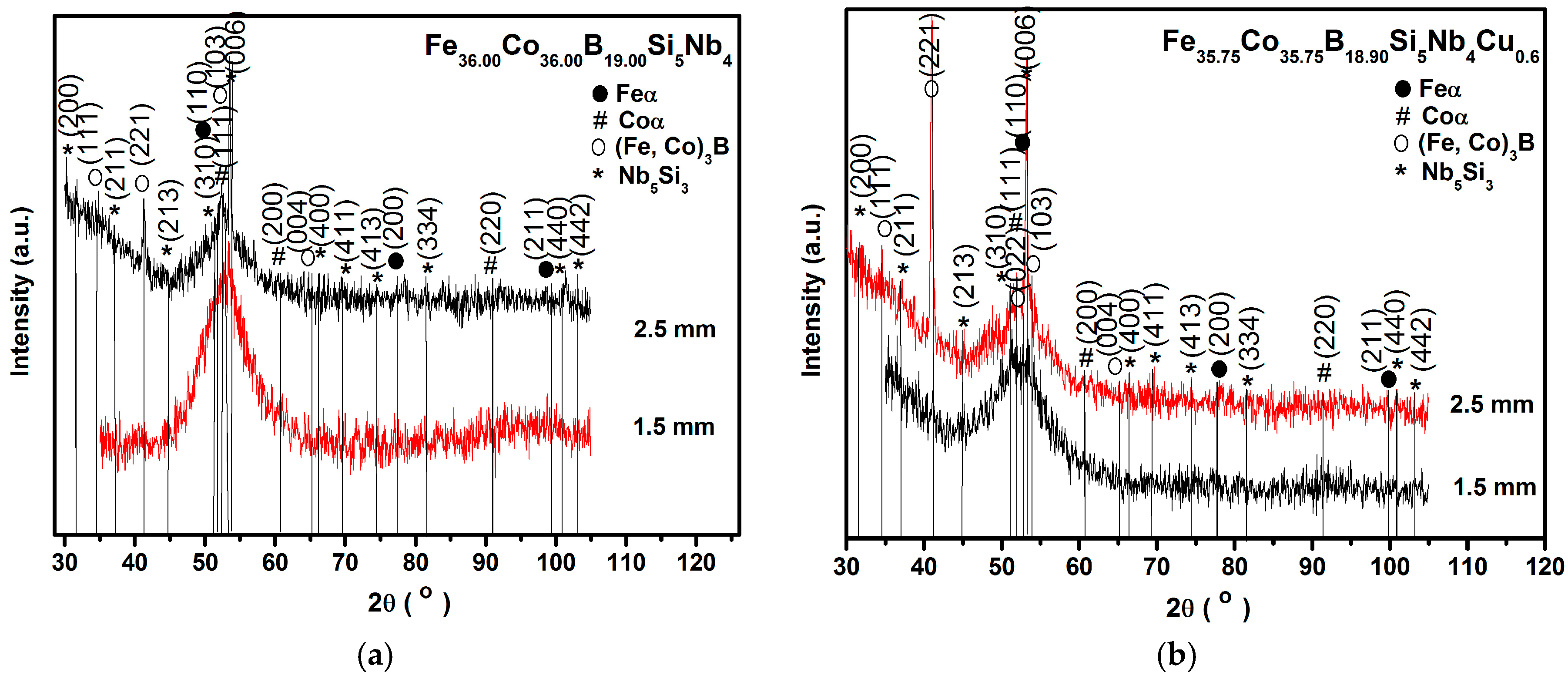
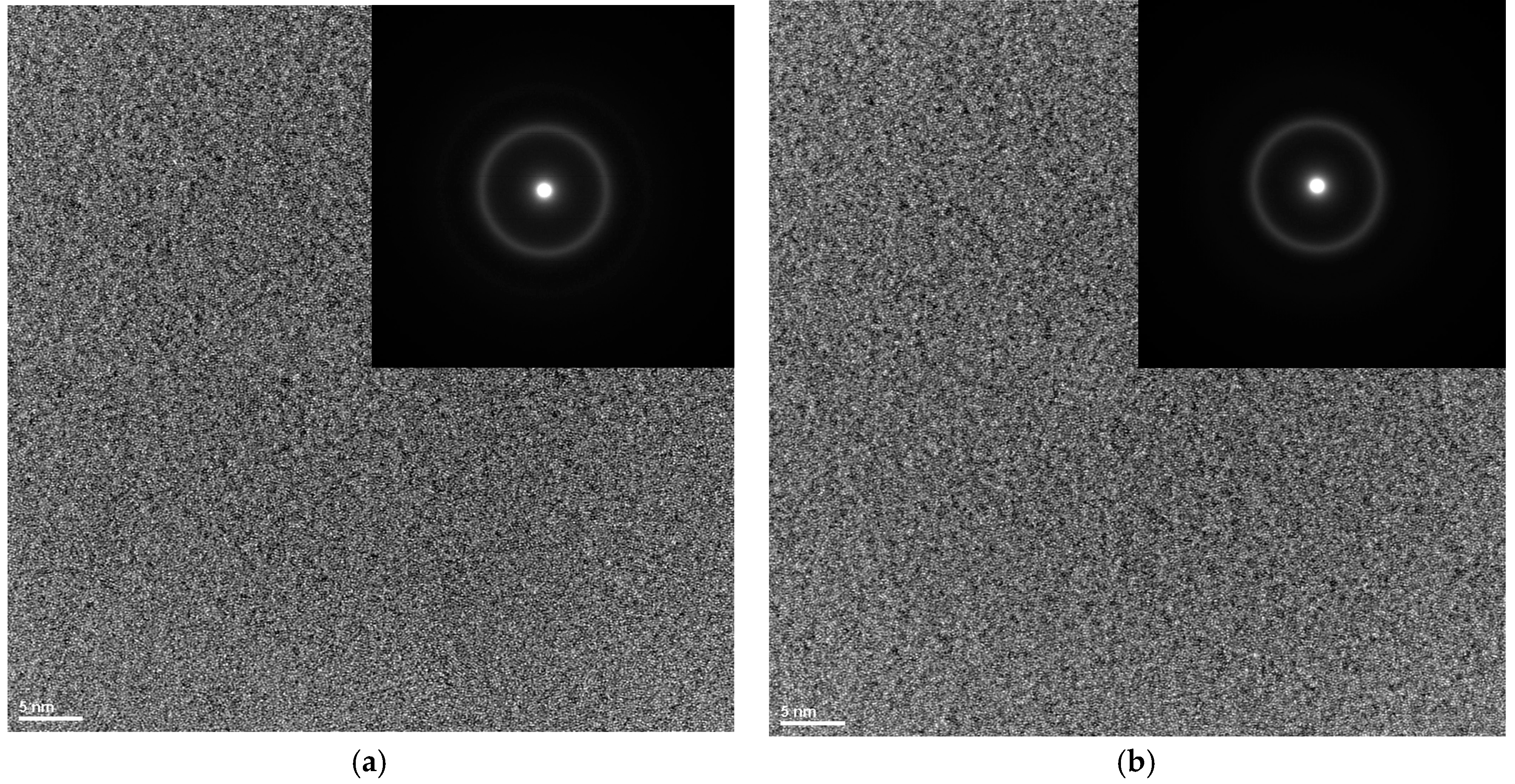
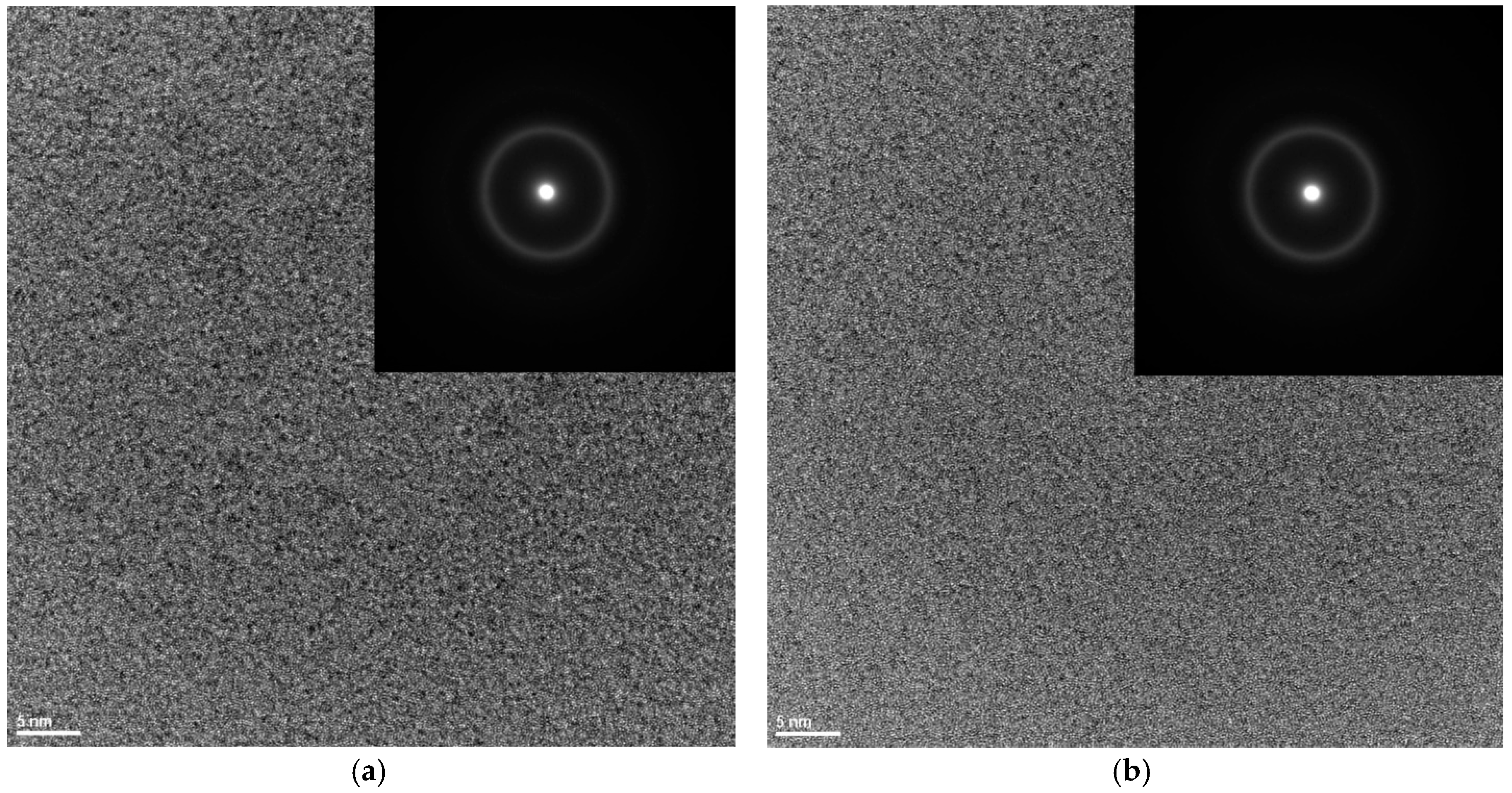
3. Results and Discussion
4. Conclusions
- Charge materials in the form of ferroalloys allow production of conventional and bulk metallic glasses with good soft magnetic properties, thus ensuring the reduction of manufacturing costs.
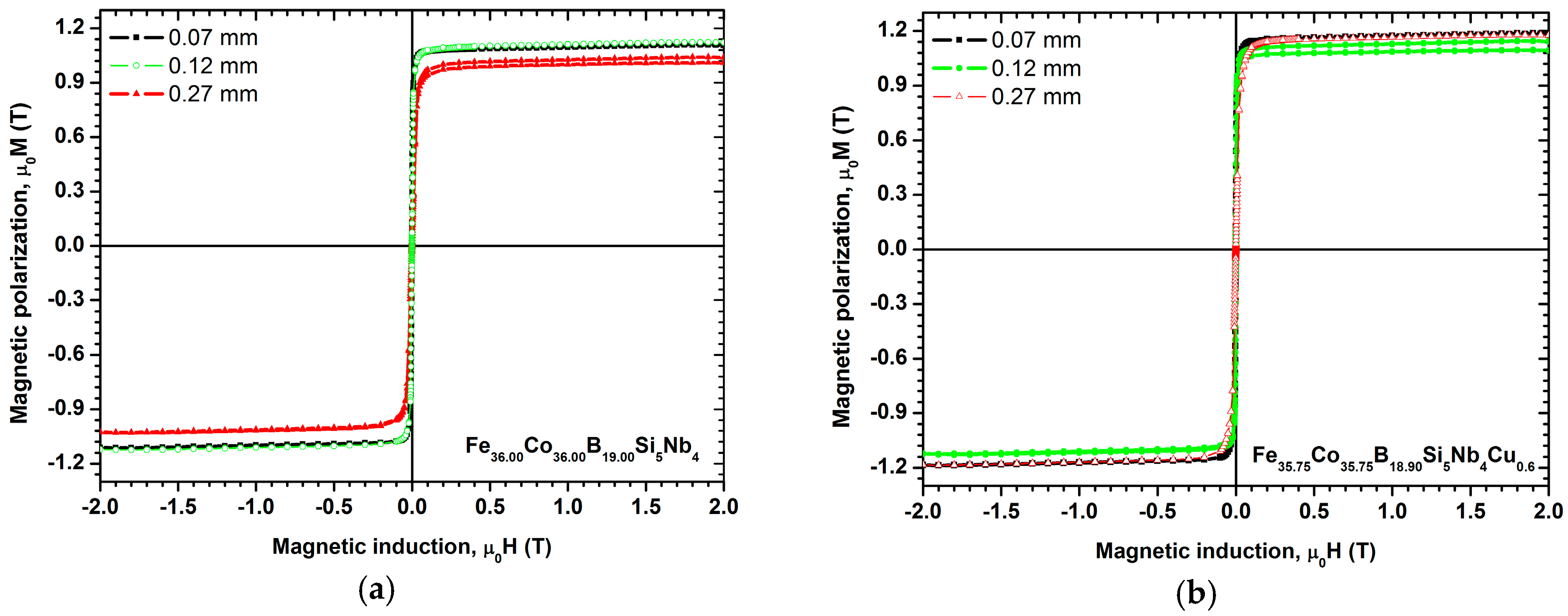
- Produced materials with amorphous structures, which are confirmed by direct methods, such as XRD, TEM, SEM-methods, and indirect methods, such as magnetic properties investigations, meet the most important requirements for soft magnetic materials at room temperature and show low coercive force Hc and high saturation magnetic polarization Js. The highest saturation magnetic polarization Js = 1.18 T and relative initial magnetic permeability are demonstrated by the Fe35.75Co35.75B18.90Si5Nb4Cu0.6 alloy in the form of ribbons with the thicknesses of 0.07 and 0.27 mm. For alloy rods containing copper, the saturation magnetic polarization Js was from 1.03 to 1.18 T, which is similar to that of the base Fe36.00Co36.00B19.00Si5Nb4 alloy—Js was from 0.97 to 1.17 T. It is determined by the higher excess volume concentration for test specimens in the form of ribbons than for those in the form of rods, which is caused by the application of a higher cooling rate of ribbons.
- The casting process parameters affect the share of microvoids in the alloy, and consequently the obtained magnetic properties. Ribbons with lower thickness show higher magnetic permeability relaxation intensity after demagnetization (Δμ/μ) than those with higher thickness. The magnetic permeability relaxation intensity after demagnetization is proportional to the concentration of microvoids (excess volume) in magnetic metallic glasses. Cu addition in the Fe-Co-Nb-Si-B alloy makes the magnetic permeability relaxation intensity increase after demagnetization (Δμ/μ) as a result of the presence of free volumes and Cu clusters in the amorphous structure of the alloy, even before the actual crystallization process has started.
- Copper addition improves the soft magnetic properties of the alloy, by decreasing the coercive force and increasing the relative initial magnetic permeability—μi. Lower coercive force is caused by structural relaxation, which allows the reduction in stress present in the amorphous matrix, or by the presence of Cu clusters in the alloys containing Cu. The presence of impurities in the alloy hinders the movement of domain walls, thus resulting in a high coercive force—Hc.
- The highest coercive force is observed in rods with the diameter of 2.5 mm. The deterioration in magnetic softness is connected with the appearance of boride phases in the structure of the alloy, which are characterized by strong magnetocrystalline anisotropy.
Acknowledgments
Conflicts of Interest
References
- Suryanarayana, C.; Inoue, A.C. Bulk Metallic Glasses; CRC Press/Taylor & Francis Group : Boca Raton, FL, USA/London, UK, 2011. [Google Scholar]
- Jia, Y.; Zeng, S.; Shan, S.; Zhang, L.; Fan, C.; Zhang, B.; Zhan, Z.; Liu, R.; Wang, W. Effect of copper addition on the glass forming ability of a Fe-Co based alloy. J. Alloys Compd. 2007, 440, 113–116. [Google Scholar] [CrossRef]
- Li, R.; Stoica, M.; Eckert, J. Effect of minor Cu addition on phase evolution and magnetic properties of {[(Fe0.5Co0.5)0.75Si0.05B0.20]0.96Nb0.04}100−xCux alloys. J. Phys. 2009, 144. [Google Scholar] [CrossRef]
- Stoica, M.; Li, R.W.; Roth, S.; Yavari, A.R. [(Fe0.5Co0.5)0.75B0.20Si0.05]96Nb4 metallic glasses with small Cu additions. Metall. Mater. Trans. A 2011, 42, 1476–1480. [Google Scholar] [CrossRef]
- Salimon, A.I.; Ashby, M.F.; Bréchet, Y.; Greer, A.L. Bulk metallic glasses: What are they good for? Mater. Sci. Eng. A 2004, 375, 385–388. [Google Scholar] [CrossRef]
- Axinte, E. Metallic glasses from “alchemy” to pure science: Present and future of design, processing and applications of glassy metals. Mater. Des. 2012, 35, 518–556. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, Lighter, and More Energy Efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, C.; Inoue, A. Iron-based bulk metallic glasses. Int. Mater. Rev. 2013, 58, 131–166. [Google Scholar] [CrossRef]
- Kulik, T.; Ferenc, J.; Kolano-Burian, A.; Liang, X.B.; Kowalczyk, M. Magnetically soft nanomaterials for high-temperature applications. J. Alloys Compd. 2007, 434, 623–627. [Google Scholar] [CrossRef]
- Stokłosa, Z.; Kwapulinski, P.; Rasek, J.; Haneczok, G.; Kubisztal, M. Magnetic properties and loss separation in Fe76−xAgxNb2Si13B9 amorphous alloys. Mater. Sci. Eng. B 2015, 196, 1–6. [Google Scholar] [CrossRef]
- Pawlik, P.; Pawlik, K.; Davies, H.A.; Kaszuwara, W.; Wysłocki, J.J. The influence of heat treatment on the microstructure and magnetic properties of (Fe,Co)-Zr-(Pr,Dy)–B- nanocomposite alloys. J. Magn. Magn. Mater. 2007, 316, e124–e127. [Google Scholar] [CrossRef]
- Nabiałek, M.G.; Szota, M.; Dospiał, M.J. Effect of Co on the microstructure, magnetic properties and thermal stability of bulk Fe73−xCoxNb5Y3B19 (where x = 0 or 10) amorphous alloys. J. Alloys Compd. 2012, 526, 68–73. [Google Scholar] [CrossRef]
- Shen, B.L.; Inoue, A. Superhigh strength and good soft-magnetic properties of (Fe,Co)-B-Si-Nb bulk glassy alloys with high glass-forming ability. Appl. Phys. Lett. 2004, 85, 4911–4913. [Google Scholar] [CrossRef]
- Müller, M.; Mattern, N.; Illgen, L. The influence of different Cu/Nb contents on the structure and on the magnetic properties in nanocrystalline FeBSi base alloys. J. Magn. Magn. Mater. 1992, 112, 263–268. [Google Scholar] [CrossRef]
- Szewieczek, D.; Lesz, S. The structure and selected physical properties of the nanocrystalline Fe92.4Hf4.2B3.4 alloy. J. Mater. Process. Technol. 2004, 157, 771–775. [Google Scholar] [CrossRef]
- Jia, P.; Wang, E.; Han, K. The effects of a high magnetic field on the annealing of [(Fe0.5Co0.5)0.75B0.2Si0.05]96Nb4 bulk metallic glass. Materials 2016, 9, 899. [Google Scholar] [CrossRef]
- Makino, A.; Kubota, T.; Chang, C.; Makabe, M.; Inoue, A. FeSiBP bulk metallic glasses with high magnetization and excellent magnetic softness. J. Magn. Magn. Mater. 2008, 320, 2499–2503. [Google Scholar] [CrossRef]
- Inoue, A. Bulk amorphous alloys. In Amorphous and Nanocrystalline Materials: Preparation, Properties and Applications; Inoue, A., Hashimoto, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–48. [Google Scholar]
- Tiberto, P.; Piccin, R.; Lupu, N.; Chiriac, H.; Baricco, M. Magnetic properties of Fe–Co–based bulk metallic glasses. J. Alloys Compd. 2009, 483, 608–612. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- May, J.E.; de Oliveira, M.F.; Afonso, C.R.M.; Sa´ Lisboa, R.D.; Kuri, S.E. Amorphous phase partitioning in Fe–Co–based metallic glass alloys. J. Non-Cryst. Solids 2004, 348, 250–257. [Google Scholar] [CrossRef]
- Zhai, F.; Pineda, E.; Duarte, M.J.; Crespo, D. Role of Nb in glass formation of Fe-Cr-Mo-C-B-Nb BMGs. J. Alloys Compd. 2014, 604, 157–163. [Google Scholar] [CrossRef]
- Al-Hallaj, S.; Kiszynski, K. Renewable Energy Sources and Energy Conversion Devices, Green Energy and Technology; Springer: London, UK, 2011. [Google Scholar]
- Dobrzański, L.A.; Drygała, A. Influence of laser processing on polycrystalline silicon surface. Mater. Sci. Forum 2012, 706, 829–834. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. Role of minor alloying additions in formation of bulk metallic glasses: A Review. J. Mater. Sci. 2004, 39, 3965–3974. [Google Scholar] [CrossRef]
- Herzer, G. Nanocrystalline soft magnetic alloys. In Handbook of Magnetic Materials; Buschow, K.H.J., Ed.; Elsevier Science BV: Amsterdam, The Netherlands, 1997; pp. 417–461. [Google Scholar]
- Kulik, T.; Horubała, T.; Matyja, H. Flash annealing nanocrystallization of Fe–Si–B–based glasses. Mater. Sci. Eng. A 1992, 157, 107–112. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Oguma, S.; Yamauchi, K. New Fe–based soft magnetic alloys composed of ultrafine grain structure. J. Appl. Phys. 1988, 64, 6044–6046. [Google Scholar] [CrossRef]
- Jung, H.Y.; Stoica, M.; Yi, S.; Kim, D.H.; Eckert, J. Crystallization kinetics of Fe76.5−xC6.0Si3.3B5.5P8.7Cux (x = 0, 0.5, and 1 at. pct) bulk amorphous alloy. Metall. Mater. Trans. A 2015, 46, 2415–2421. [Google Scholar] [CrossRef]
- Youssef, H.A.; El-Hofy, H.A.; Ahmed, M.H. Manufacturing Technology: Materials, Processes, and Equipment; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA/London, UK, 2011; Volume 83, pp. 749–765. [Google Scholar]
- Drygała, A.; Dobrzański, L.A.; Szindler, M.; Szindler, M.M.; Prokopiuk vel Prokopowicz, M.; Jonda, E. Influence of laser texturization surface and atomic layer deposition on optical properties of polycrystalline silicon. Int. J. Hydrogen Energy 2016, 41, 7563–7567. [Google Scholar] [CrossRef]
- Singh, D.K. Fundamentals of Manufacturing Engineering, 3rd ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA/London, UK, 2014; pp. 501–616. [Google Scholar]
- Li, H.X.; Wang, S.L.; Yi, S.; Jiao, Z.B.; Wu, Y.; Lu, Z.P. Glass formation and magnetic properties of Fe-C-Si-B-P-(Cr-Al-Co) bulk metallic glasses fabricated using industrial raw materials. J. Magn. Magn. Mater. 2009, 321, 2833–2837. [Google Scholar] [CrossRef]
- Haibo, L.; Qiang, L.; Jijun, Z.; Yaqiang, D.; Chuntao, C.; Hongxiang, L.; Jaeheon, K.; Seonghoon, Y. Preparation of quasi-ternary Fe-P-C bulk metallic glass using industrial raw materials with the help of fluxing technique. Adv. Eng. Mater. 2015, 17, 1045–1050. [Google Scholar]
- Wang, H.B.; Ma, L.X.; Li, L.; Zhang, B. Fabrication of Fe–based bulk metallic glasses from low-purity industrial raw materials. J. Alloys Compd. 2015, 629, 1–4. [Google Scholar] [CrossRef]
- Cai, Y.; Ling, H.; Jiang, T. Effect of industrial raw materials on the glass forming ability, Magnetic and mechanical properties of Fe–based bulk metallic glasses. Metall. Mater. Trans. B 2015, 46, 2484–2489. [Google Scholar] [CrossRef]
- Lesz, S. Structure and Properties of Conventional and Bulk Metallic Glasses Prepared from the Ferroalloys; Silesian University Press: Gliwice, Poland, 2016. [Google Scholar]
- Hanawalt, J.D.; Rinn, H.W. Identification of crystalline materials: Classification and use of X-ray diffraction patterns. Powder Diffr. 1986, 1, 2–6. [Google Scholar] [CrossRef]
- Hanawalt, J.D. Manual Search/Match Methods for Powder Diffraction in 1986. Powder Diffr. 1986, 1, 7–13. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy. A Textbook for Materials Science; Springer Science: Huntsville, AL, USA, 2009. [Google Scholar]
- Stokłosa, Z.; Rasek, J.; Kwapuliński, P.; Badura, G.; Haneczok, G.; Pająk, L.; Lelątko, J.; Kolano-Burian, A. Magnetic, electrical and plastic properties of Fe76Nb2Si13B9, Fe75Ag1Nb2Si13B9 and Fe75Cu1Nb2Si13B9 amorphous alloys. J. Alloys Compd. 2011, 509, 050–9054. [Google Scholar] [CrossRef]
- Lesz, S.; Kwapuliński, P.; Nabiałek, M.; Zackiewicz, P.; Hawelek, L. Thermal stability, crystallization and magnetic properties of Fe-Co-based metallic glasses. J. Therm. Anal. Calorim. 2016, 125, 1143–1149. [Google Scholar] [CrossRef]
- Schuh, C.A.; Hufnagel, T.C.; Ramamurty, U. Mechanical behavior of amorphous alloys. Acta Mater. 2007, 55, 4067–4109. [Google Scholar] [CrossRef]
- Torrens-Serra, J.; Bruna, P.; Roth, S.; Rodriguez-Viejo, J.; Clavaguera-Mora, M.T. Effect of minor Co additions on the crystallization and magnetic properties of Fe(Co)NbBCu alloys. J. Alloys Compd. 2010, 496, 202–207. [Google Scholar] [CrossRef]
- Stoica, M.; Li, R.; Yavari, A.R.; Vaughan, G.; Eckert, J.; Steenberge, N.; Romera, D.R. Thermal stability and magnetic properties of FeCoBSiNb bulk metallic glasses. J. Alloys Compd. 2010, 504S, 123–128. [Google Scholar] [CrossRef]
- Stoica, M.; Ramasamy, P.; Kaban, I.; Scudino, S.; Nicoara, M.; Vaughan, G.B.M.; Wright, J.; Kumar, R.; Eckert, J. Structure evolution of soft magnetic (Fe36Co36B19.2Si4.8Nb4)100−xCux bulk glassy alloys. Acta Mater. 2015, 95, 335–342. [Google Scholar] [CrossRef]
- Lesz, S.; Babilas, R.; Nabiałek, M.; Szota, M.; Dośpiał, M.; Nowosielski, R. The characterization of structure, thermal stability and magnetic properties of Fe-Co-B-Si-Nb bulk amorphous and nanocrystalline alloys. J. Alloys Compd. 2011, 509, S197–S201. [Google Scholar] [CrossRef]
- Lesz, S. Effect of cooling rates on the structure, density and micro-indentation behavior of the Fe, Co-based bulk metallic glass. Mater. Charact. 2017, 124, 97–106. [Google Scholar] [CrossRef]
- Leamy, H.J.; Chen, H.S.; Wang, T.T. Plastic flow and fracture of metallic glass. Metall. Trans. 1972, 3, 699–708. [Google Scholar] [CrossRef]
- Szewieczek, D.; Tyrlik-Held, J.; Lesz, S. Changes of mechanical properties and fracture morphology of amorphous tapes involved by heat treatment. J. Mater. Process. Technol. 2001, 109, 190–195. [Google Scholar] [CrossRef]
- Alam, T.; Borkar, T.; Joshi, S.S.; Katakam, S.; Chen, X.; Dahotre, N.B.; Ramanujan, R.V.; Banerjee, R. Influence of niobium on laser de-vitrification of Fe-Si-B based amorphous magnetic alloys. J. Non-Cryst. Solids 2015, 428, 75–81. [Google Scholar] [CrossRef]
- Hono, K.; Zhang, Y.; Inoue, A.; Sakurai, T. Atom probe studies of nanocrystalline microstructural evolution in some amorphous alloys. Mater. Trans. JIM 1995, 36, 909–917. [Google Scholar] [CrossRef]
- Nabiałek, M. Influence of the quenching rate on the structure and magnetic properties of the Fe–based amorphous alloy. Arch. Metall. Mater. 2016, 61, 439–444. [Google Scholar] [CrossRef]
- Sun, W.S.; Kulik, T.; Liang, X.B.; Ferenc, J. Thermal stability and magnetic properties of Co-Fe-Hf-Ti-Mo-B bulk metallic glass. Intermetallics 2006, 14, 1066–1068. [Google Scholar] [CrossRef]
- Lesz, S.; Babilas, R.; Nowosielski, R. Influence of copper addition on glass forming ability, thermal stability, structure and magnetic properties of Fe-Co-based BMGs. Solid State Phenom. 2013, 203–204, 296–301. [Google Scholar] [CrossRef]
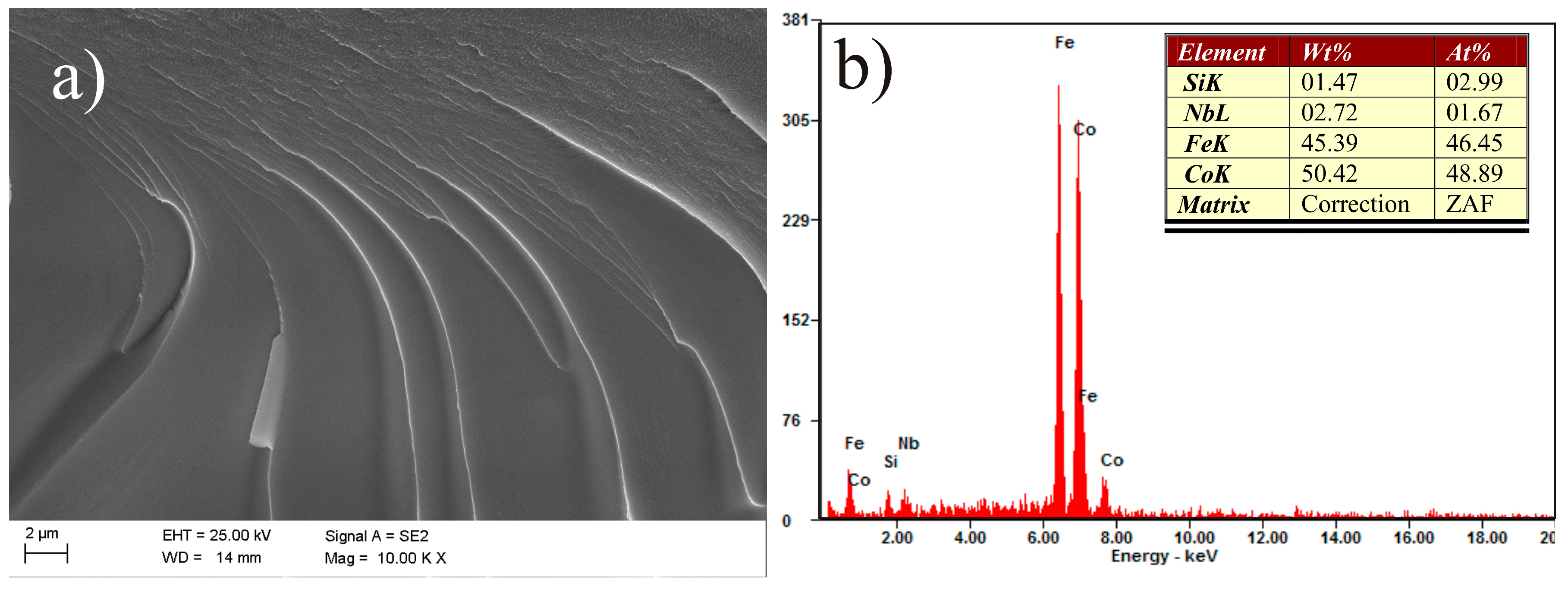



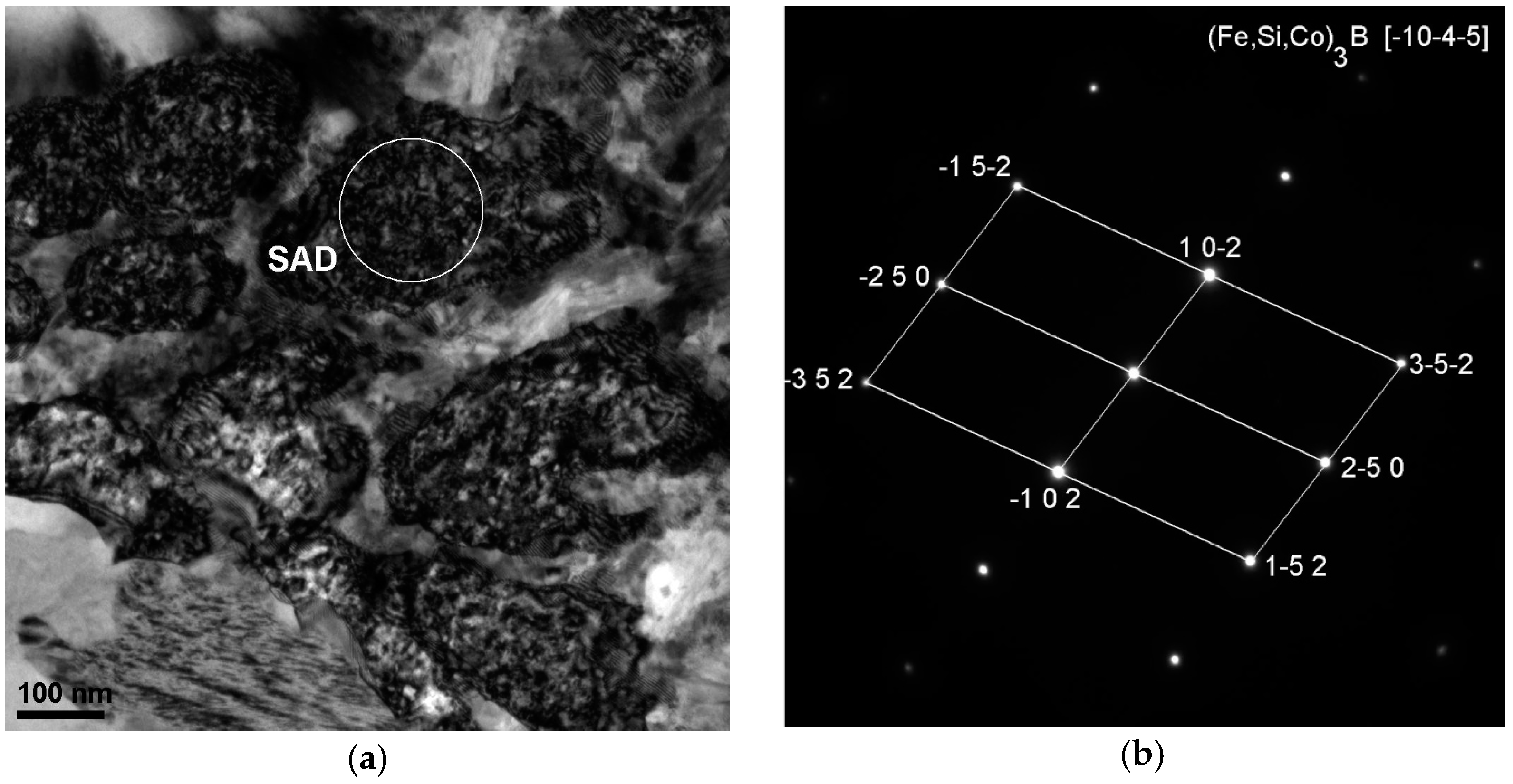

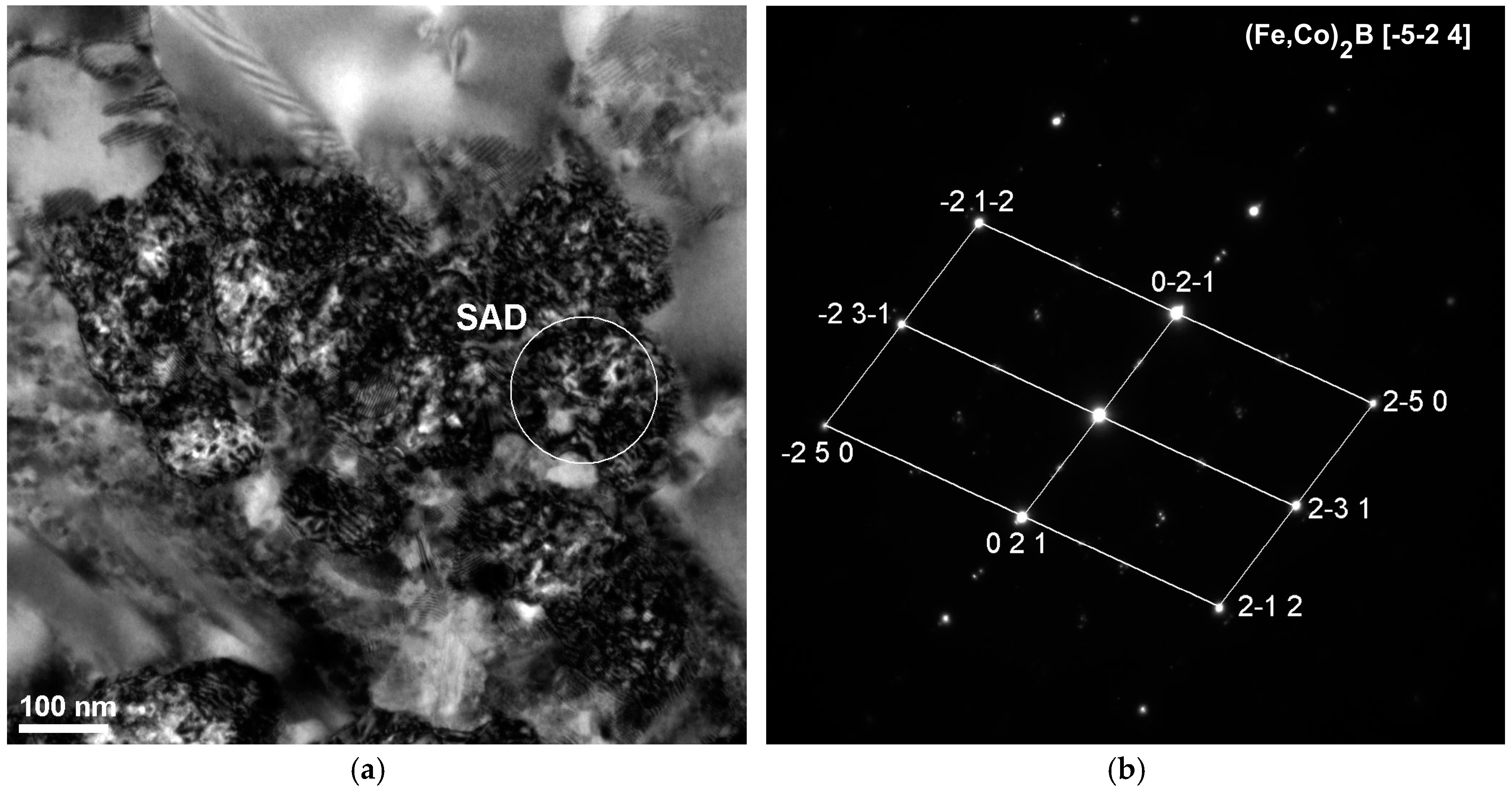
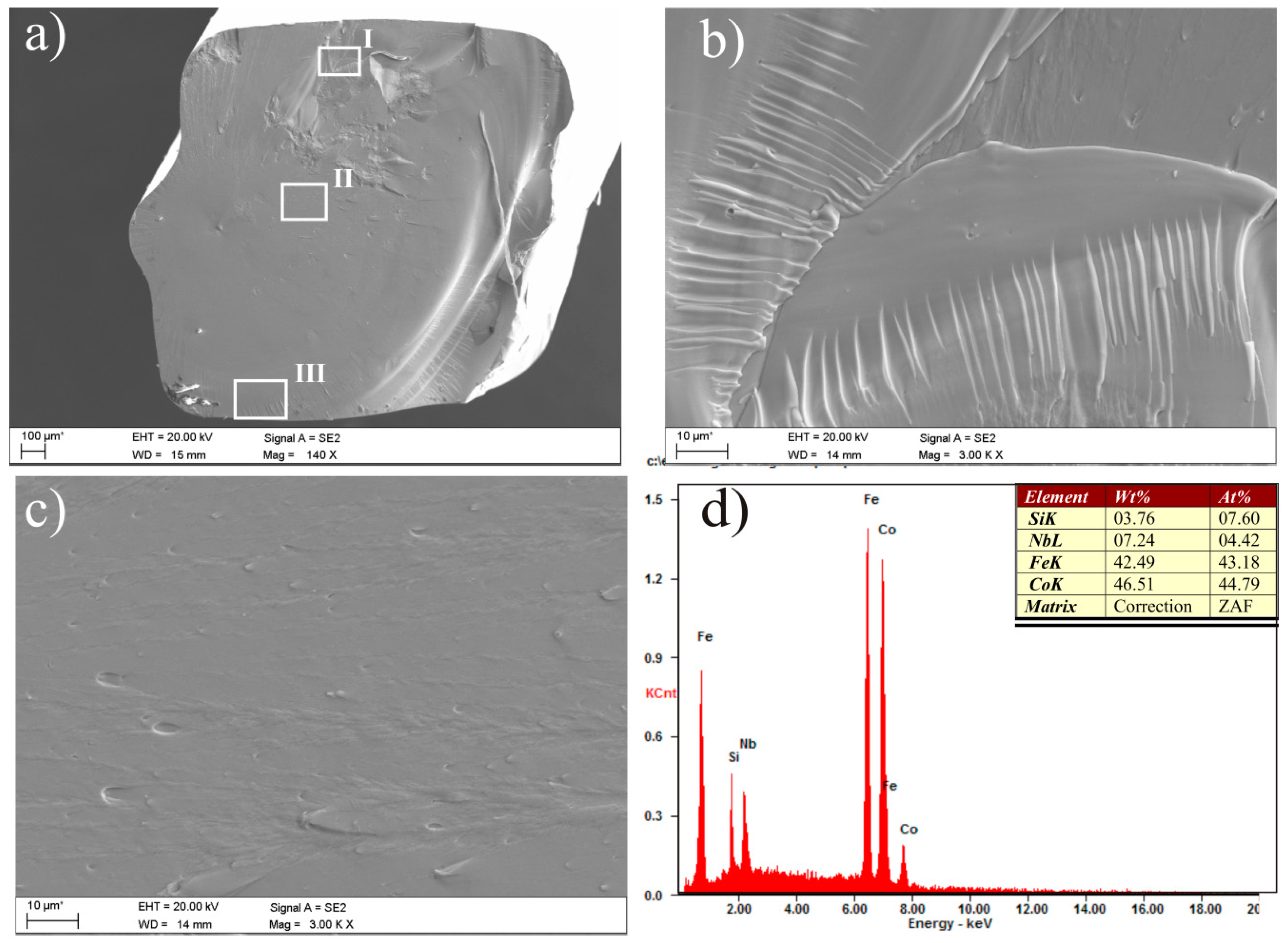
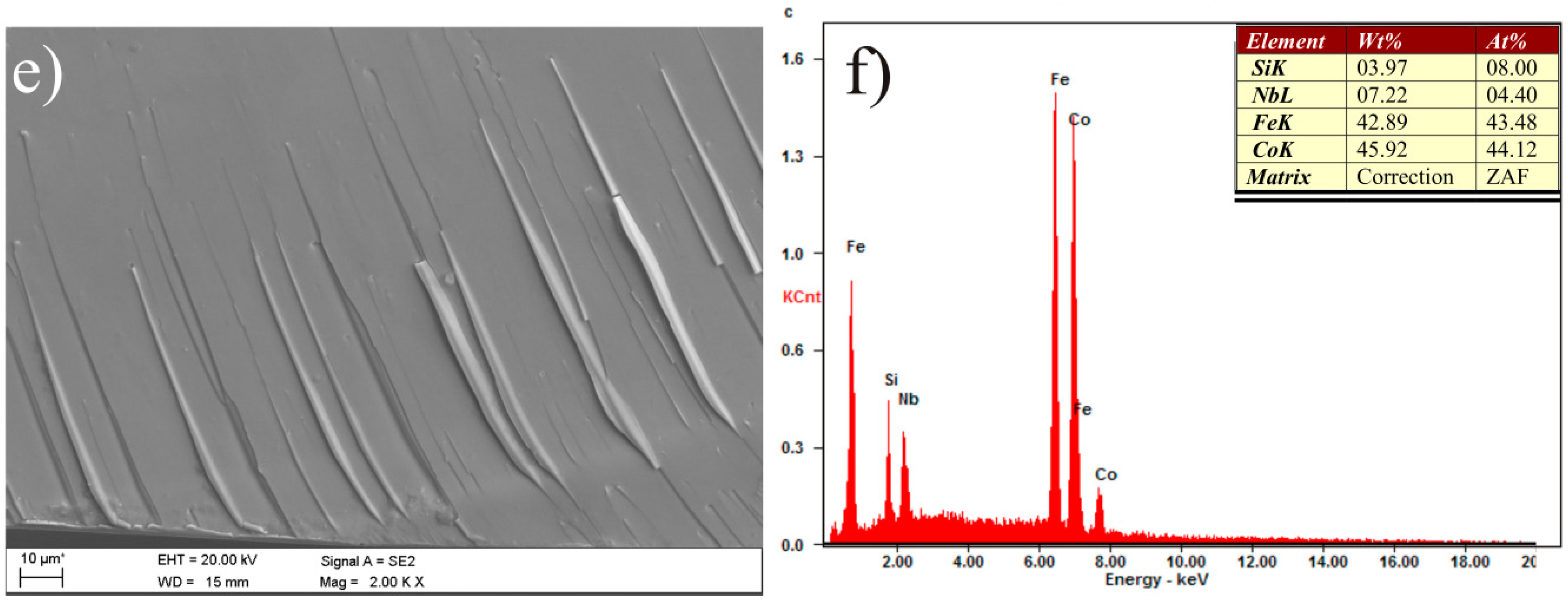
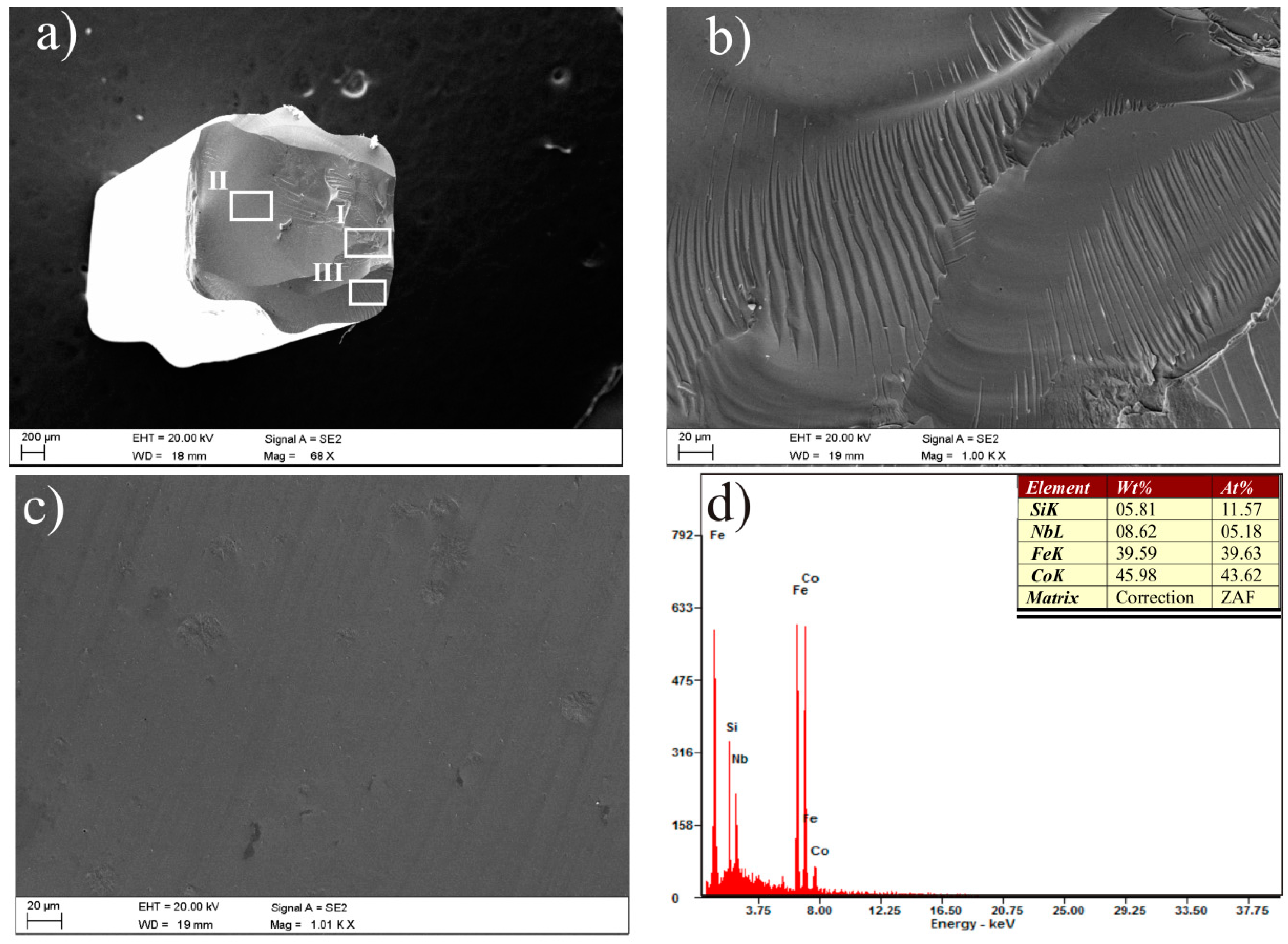
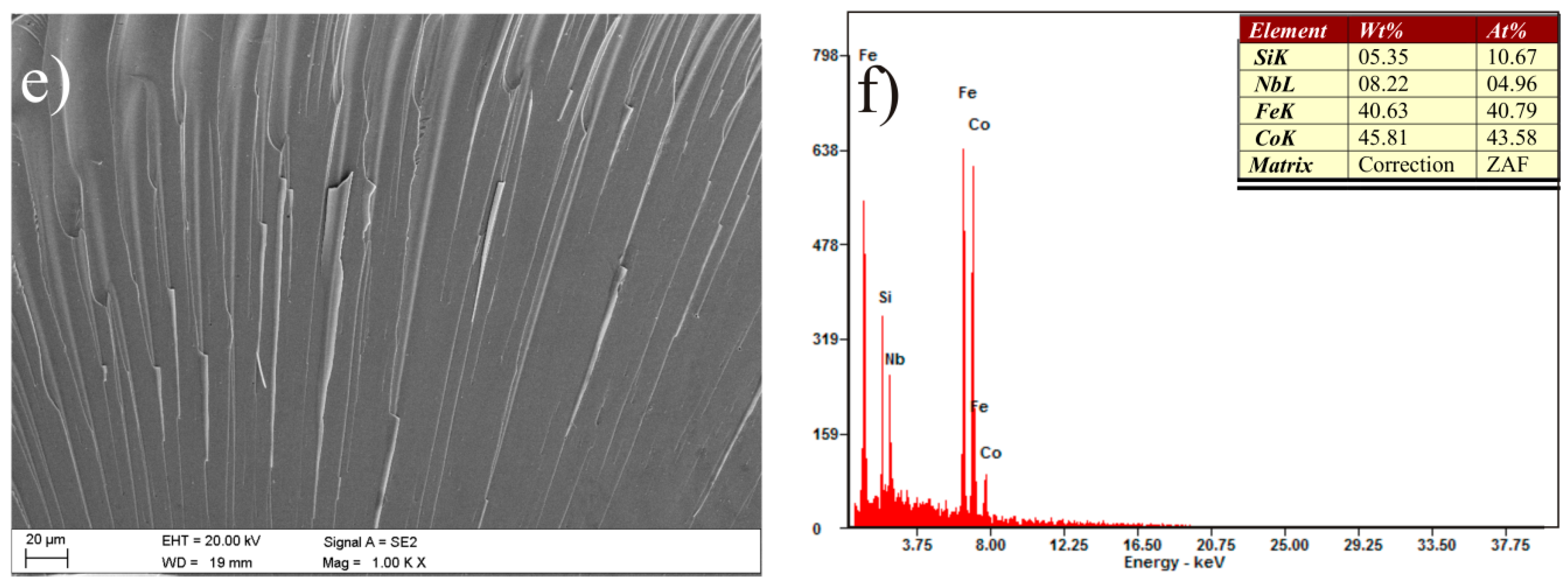

| Alloy | Thickness 1/ | - | Magnetic | Properties | - |
|---|---|---|---|---|---|
| - | Diameters 2 (mm) | μi | Δµ/µ (%) | Hc (A/m) | Js (T) |
| Fe36.00Co36.00B19.00Si5Nb4 | 0.07 1 | 3000 | 5.0 | 65.2 | 1.11 |
| Fe36.00Co36.00B19.00Si5Nb4 | 0.12 1 | 1567 | 3.6 | 41.4 | 1.12 |
| Fe36.00Co36.00B19.00Si5Nb4 | 0.27 1 | 410 | 3.0 | 30.7 | 1.04 |
| Fe36.00Co36.00B19.00Si5Nb4 | 1.5 2 | - | - | 219.0 | 0.97 |
| Fe36.00Co36.00B19.00Si5Nb4 | 2.5 2 | - | - | 6069.4 | 1.17 |
| Fe35.75Co35.75B18.90Si5Nb4Cu0.6 | 0.07 1 | 3620 | 11.0 | 53.5 | 1.18 |
| Fe35.75Co35.75B18.90Si5Nb4Cu0.6 | 0.12 1 | 1980 | 10.0 | 20.5 | 1.14 |
| Fe35.75Co35.75B18.90Si5Nb4Cu0.6 | 0.27 1 | 820 | 8.0 | 12.5 | 1.18 |
| Fe35.75Co35.75B18.90Si5Nb4Cu0.6 | 1.5 2 | - | - | 163.0 | 1.03 |
| Fe35.75Co35.75B18.90Si5Nb4Cu0.6 | 2.5 2 | - | - | 13771.8 | 1.18 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesz, S. A Study of Structure and Magnetic Properties of Low Purity Fe-Co-Based Metallic Glasses. Materials 2017, 10, 625. https://doi.org/10.3390/ma10060625
Lesz S. A Study of Structure and Magnetic Properties of Low Purity Fe-Co-Based Metallic Glasses. Materials. 2017; 10(6):625. https://doi.org/10.3390/ma10060625
Chicago/Turabian StyleLesz, Sabina. 2017. "A Study of Structure and Magnetic Properties of Low Purity Fe-Co-Based Metallic Glasses" Materials 10, no. 6: 625. https://doi.org/10.3390/ma10060625






