Comparison of Two Xenograft Materials Used in Sinus Lift Procedures: Material Characterization and In Vivo Behavior
Abstract
:1. Introduction
2. Results
2.1. Graft Implants Characterization
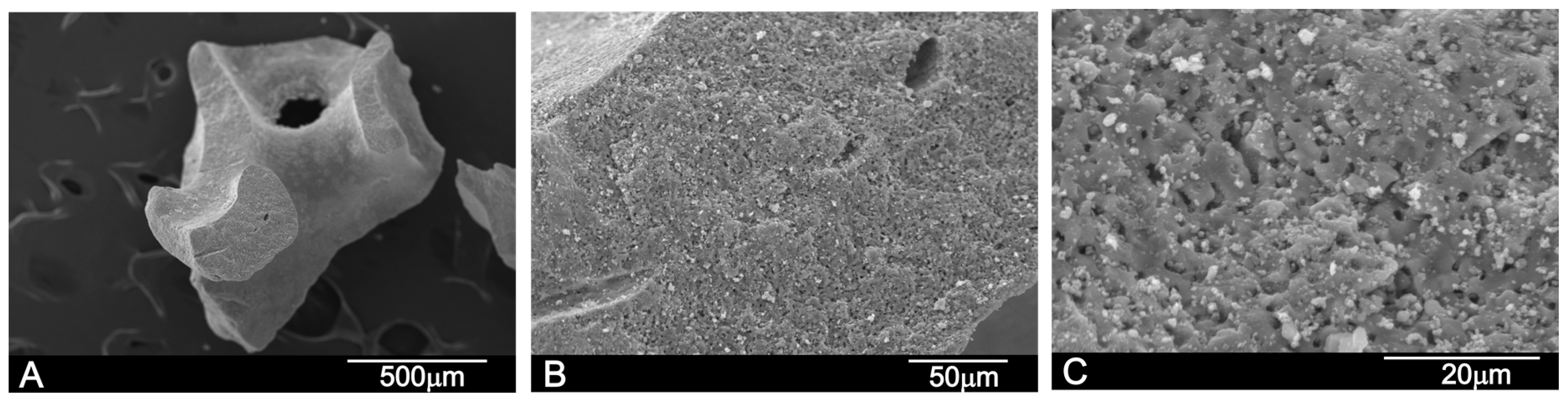
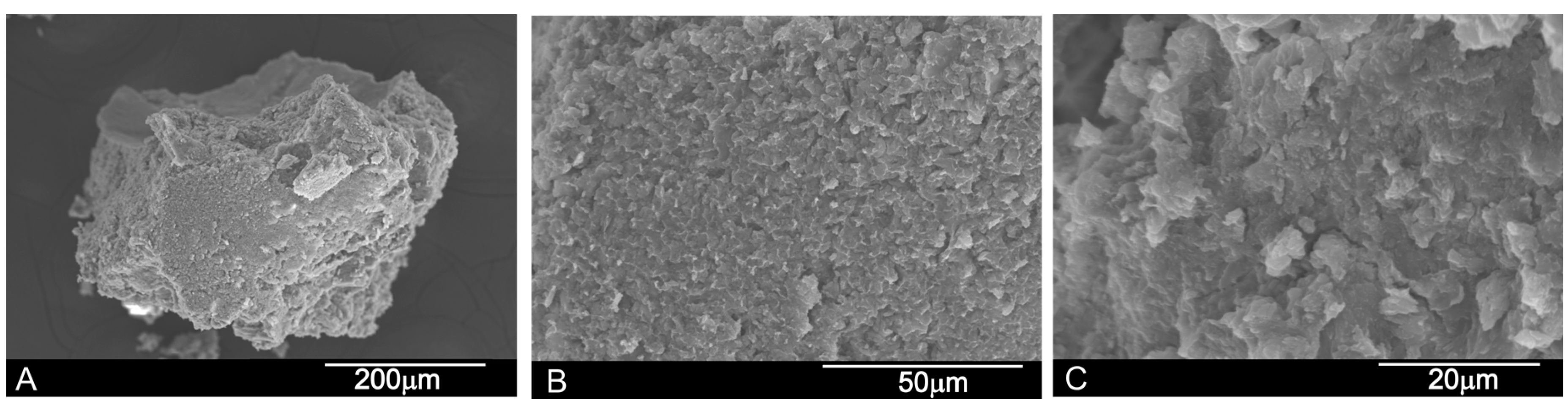
2.1.1. Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDX) Analysis
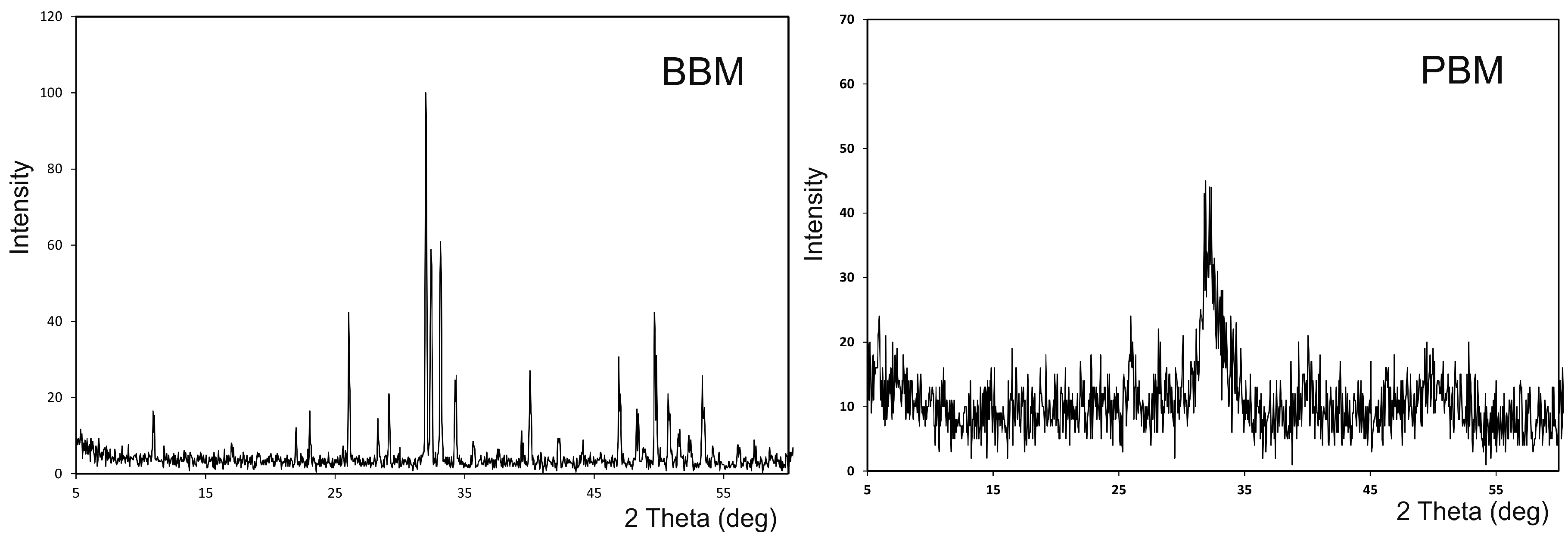
2.1.2. X-ray Diffraction Analysis (XRD)
2.1.3. Mercury Intrusion Porosimetry (MIP) Analysis
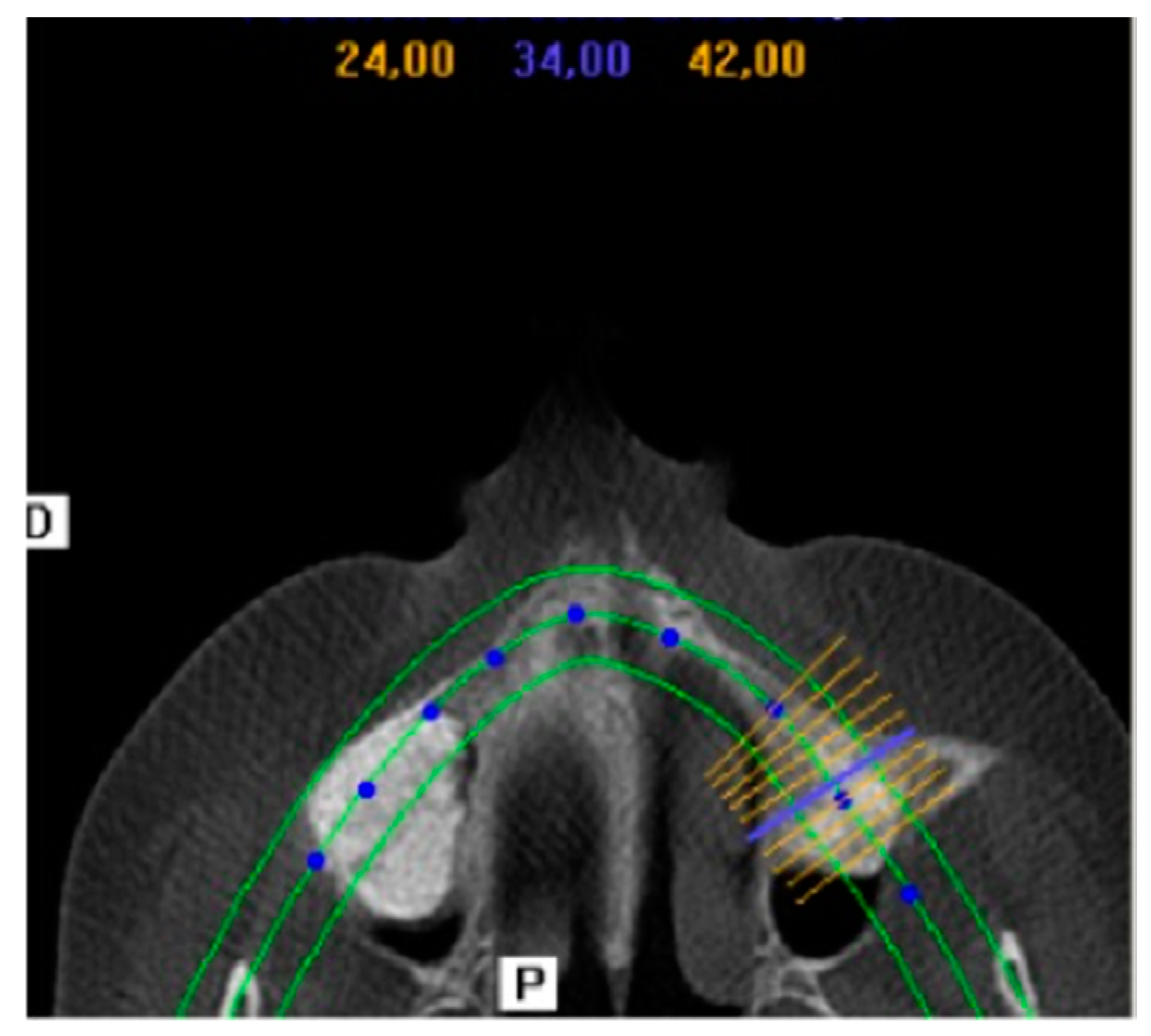
2.2. Clinical and Radiographic Results
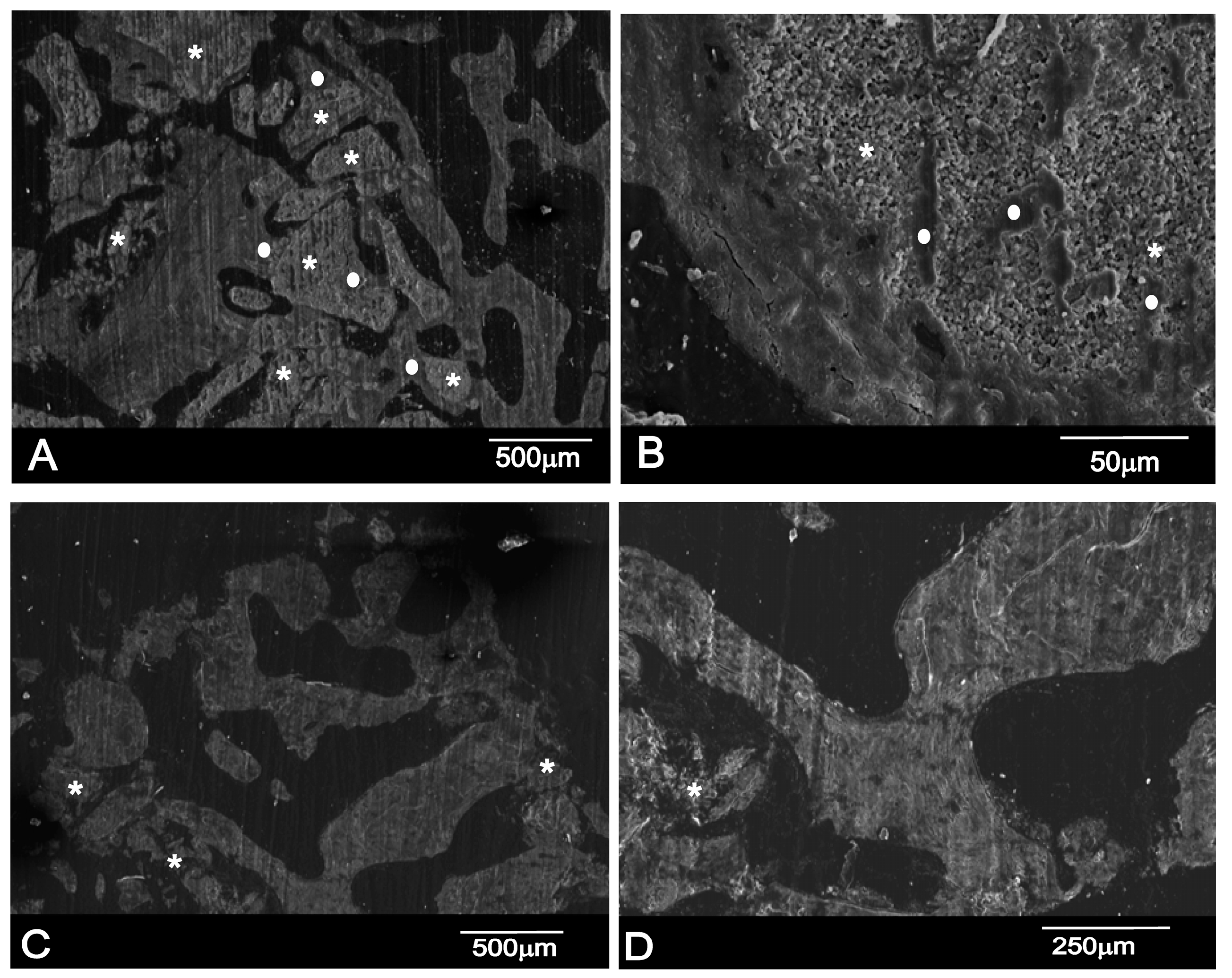
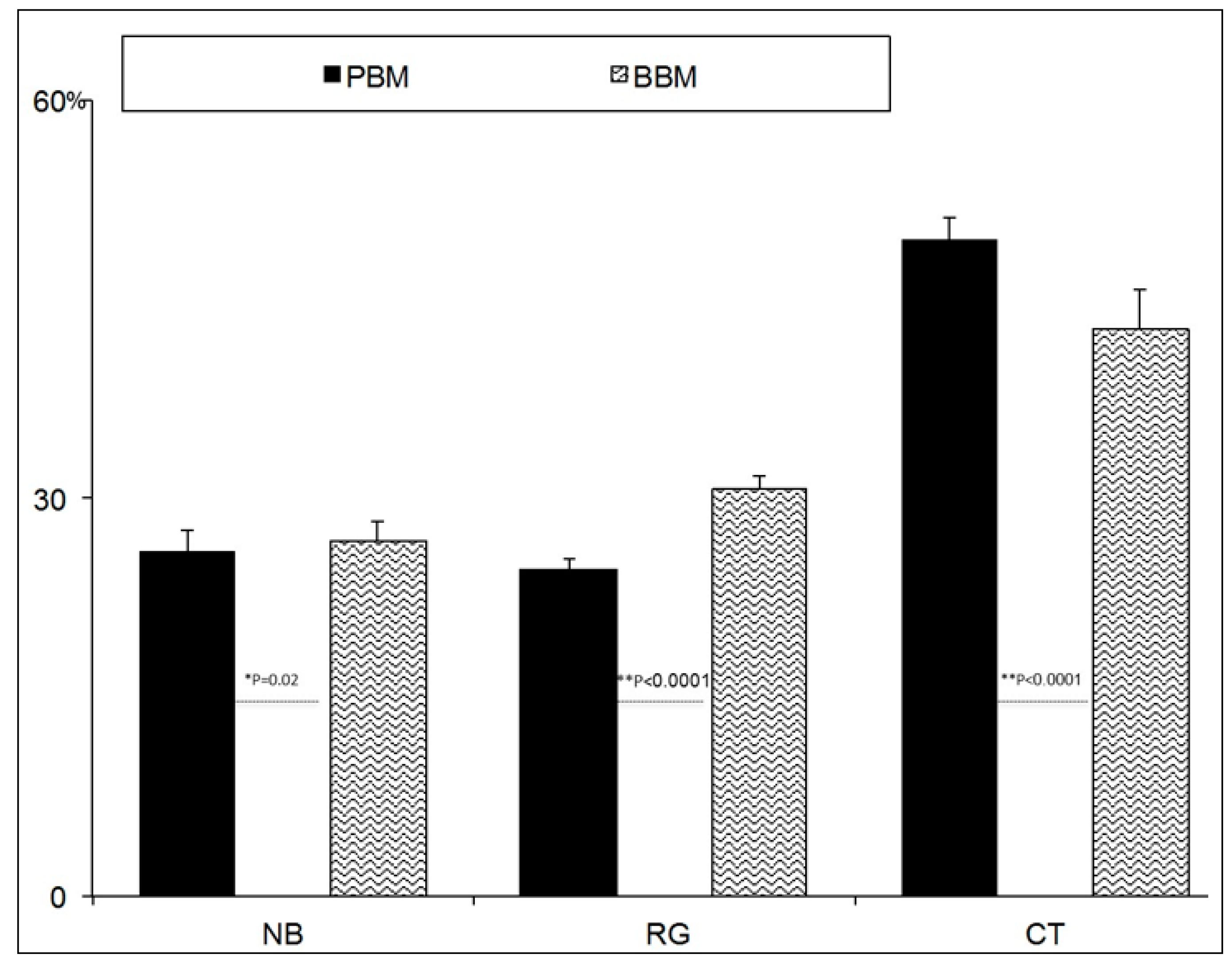
2.3. Histomorphometric Results
SEM-BSE Analysis
3. Discussion
4. Materials and Methods
4.1. Grafts
- (PBM, OsteoBiol® mp3 deproteinized porcine bone substitute material) was made up of granulated small bone particles, 600–1000 μm in size, and is a ceramic that derives from cancellous-cortical porcine bone. The material is obtained at a low processing temperature (130 °C). According to its commercial specifications, this material is claimed to preserve the structure and composition of natural bone components.
- (BBM, Endobon® deproteinized bovine bone substitute material) was made up of granulated small bone particles, 500–1000 μm in size, and is a ceramic that derives from cancellous bovine bone, which is fully deproteinated by a high temperature manufacturing process for safety from bacteria, viruses and prions. This process consists of two steps: firstly, pyrolysis at a temperature above 900 °C to eliminate the organic element; secondly, a ceramization process at temperatures above 1200 °C to creates a crystalline structure.
4.2. Graft Characterization
4.2.1. SEM-EDX
4.2.2. X-ray Diffraction (XRD)
4.2.3. Mercury Intrusion Porosimetry (MIP)
4.2.4. Statistical Analysis
4.3. Implant Procedure
4.3.1. Patient Selection and Protocol
4.3.2. Inclusion and Exclusion Criteria
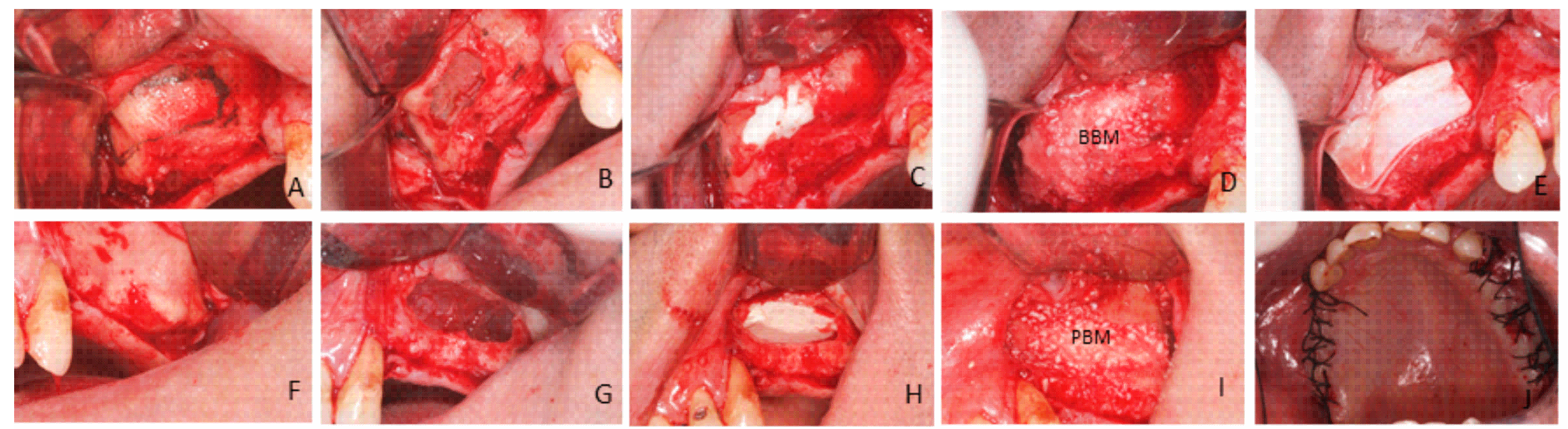
4.3.3. Surgical Procedure: First Phase
4.3.4. Surgical Procedure: Second Phase (Bone Biopsy Harvesting)
4.4. Sample Processing and Analysis
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beretta, M.; Poli, P.P.; Grossi, G.B.; Pieroni, S.; Maiorana, C. Long-term survival rate of implants placed in conjunction with 246 sinus floor elevation procedures: Results of a 15-year retrospective study. J. Dent. 2015, 43, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sani, A.S.; Loomer, P.M.; Wallace, S.S. A comprehensive clinical review of maxillary sinus floor elevation: Anatomy, techniques, biomaterials and complications. Br. J. Oral Maxillofac. Surg. 2016, 54, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. 1965. Clin. Orthop. Relat. Res. 2002, 395, 4–10. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sani, S.A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: A systematic review and meta-analysis. J. Periodontal Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Frenken, J.W.; Bouwman, W.F.; Bravenboer, N.; Zijderveld, S.A.; Schulten, E.A.; ten Bruggenkate, C.M. The use of Straumanns Bone Ceramic in a maxillary sinus floor elevation procedure: A clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin. Oral Implant Res. 2010, 21, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Simunek, A.; Kopecka, D.; Somanathan, R.V.; Pilathadka, S.; Brazda, T. Deproteinized bovine bone versus beta-tricalcium phosphate in sinus augmentation surgery: A comparative histologic and histomorphometric study. Int. J. Oral Maxillofac. Implant 2008, 23, 935–942. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Rees, J.; Karasoulos, D.; Felice, P.; Alissa, R.; Worthington, H.; Coulthard, P. Effectiveness of sinus lift procedures for dental implant rehabilitation: A Cochrane systematic review. Eur. J. Oral Implantol. 2010, 23, 7–26. [Google Scholar]
- Tadic, D.; Epple, M. A thorough physicochemical characterization of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft: A systematic review. Clin. Oral Implant Res. 2012, 23, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Froum, S.J.; Tarnow, D.P.; Wallace, S.S.; Rohrer, M.D.; Cho, S.C. Sinus floor elevation using anorganic bovine bone matrix (OsteoGraf/N) with and without autogenous bone: A clinical, histologic, radiographic, and histomorphometric analysis—Part 2 of an ongoing prospective study. Int. J. Periodontics Restor. Dent. 1998, 18, 528–543. [Google Scholar]
- Carvalho, A.L.; Faria, P.E.; Grisi, M.F.; Souza, S.L.; Taba, M.J.; Palioto, D.B.; Novaes, A.B.; Fraga, A.F.; Ozyegin, L.S.; Oktar, F.N.; et al. Effects of granule size on the osteoconductivity of bovine and synthetic hydroxyapatite: A histologic and histometric study in dogs. J. Oral Implantol. 2007, 33, 267–276. [Google Scholar] [CrossRef]
- Orsini, G.; Traini, T.; Scarano, A.; Degidi, M.; Perrotti, V.; Piccirilli, M.; Piattelli, A. Maxillary sinus augmentation with Bio-Oss particles: A light, scanning, and transmission electron microscopy study in man. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, L.; Bosshardt, D.D.; Palatella, P.; Rao, W.; Serino, G.; Chiapasco, M. Maxillary sinus grafting with Bio-Oss® or Straumann® Bone Ceramic: Histomorphometric result from a randomized controlled multicenter clinical trial. Clin. Oral Implant Res. 2008, 19, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Valentini, P.; Lezzi, G.; Piatelli, A. A histologic and histomorphometric evaluation of anorganic bovine bone retrieved 9 years after a sinus augmentation procedure. J. Periodontol. 2007, 78, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Degidi, M.; Sammons, R.; Stanley, P.; Piattelli, A. Histologic and elemental microanalytical study of anorganic bovine bone substitution following sinus floor augmentation in humans. J. Periodontol. 2008, 79, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Camelo, M.; de Angelis, N.; Hanratty, J.J.; Khang, W.G.; Kwon, J.J.; Rasperini, G.; Rocchietta, I.; Schupbach, P.; Kim, D.M. The clinical and histologic efficacy of xenograft granules for maxillary sinus floor augmentation. Int. J. Periodontics Restor. Dent. 2011, 31, 227–235. [Google Scholar] [CrossRef]
- Wenz, B.; Oesch, B.; Horst, M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials 2001, 22, 1599–1606. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, H.K.; Yun, J.H.; Cho, K.S. Randomized Clinical Trial of Maxillary Sinus Grafting using Deproteinized Porcine and Bovine Bone Mineral. Clin. Implant Dent. Relat. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Scarano, A.; Piattelli, M.; Piccirilli, M.; Caputi, S.; Piattelli, A. Histologic and ultrastructural analysis of regenerated bone in maxillary sinus augmentation using a porcine bone-derived biomaterial. J. Periodontol. 2006, 77, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piatelli, A.; Perrotti, V.; Manzon, L.; Lezzi, G. Maxillary sinus augementation in human using cortical porcine bone: A histological and histomorphometrical evaluation after 4 and 6 months. Clin. Implant Dent. Relat. Res. 2011, 13, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Ricci, M.; Covani, U.; Nannmark, U.; Azarmehr, I.; Calvo-Guirado, J.L. Maxillary sinus augmentation using prehydrated corticocancellous porcine bone: Hystomorphometric evaluation after 6 months. Clin. Implant Dent. Relat. Res 2012, 14, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ceseña, A.B.; Sánchez-Saavedra, M.P.; Novitskaya, E.E.; Chen, P.Y.; Hirata, G.A.; McKittrick, J. Kinetic characterization of the deproteinization of trabecular and cortical bovine femur bones. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4958–4964. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ye, X.; Wang, H.; Zhu, M.; Wang, B.; Yan, H. The influence of pH and temperature on the morphology of hydroxyapatite synthesized by hydrothermal method. Ceram. Int. 2003, 29, 629–633. [Google Scholar] [CrossRef]
- Le Geros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Blokhuis, T.J.; Termaat, M.F.; den Boer, F.C.; Patka, P.; Bakker, F.C.; Haarman, H.J. Properties of calcium phosphate ceramics in relation to their in vivo behavior. J. Trauma 2000, 48, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Danoux, C.; Pereira, D.; Döbelin, N.; Stähli, C.; Barralet, J.; van Blitterswijk, C.; Habibovic, P. The Effects of Crystal Phase and Particle Morphology of Calcium Phosphates on Proliferation and Differentiation of Human Mesenchymal Stromal Cells. Adv. Healthc. Mater. 2016, 5, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dennison, D.; Ong, J.L. Protein Adsorption and Osteoblast Precursor Cell Attachment to Hydroxyapatite of Different Crystallinities. Int. J. Oral Maxillofac. Implant 2005, 20, 187–192. [Google Scholar]
- Rosa, A.L.; Beloti, M.M.; Oliveira, P.T.; van Noort, R. Osseointegration and osseoconductivity of hydroxyapatite of different microporosities. J. Mater. Sci. Mater. Med. 2002, 13, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Von Doernberg, M.C.; von Rechenberg, B.; Bohner, M.; Grünenfelder, S.; van Lenthe, G.H.; Müller, R.; Gasser, B.; Mathys, R.; Baroud, G.; Auer, J. In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials 2006, 27, 5186–5198. [Google Scholar] [CrossRef] [PubMed]
- Ducheyne, P.E.; Qiu, Q. Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999, 20, 2287–2303. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Detsch, R.; Deisinger, U.; Hilbig, U.; Rausch, V.; Sader, R.; Unger, R.E.; Ziegler, G.; Kirkpatrick, C.J. The chemical composition of synthetic bone substitutes influences tissue reactions in vivo: Histological and histomorphometrical analysis of the cellular inflammatory response to hydroxyapatite, beta-tricalcium phosphate and biphasic calcium phosphate ceramics. Biomed. Mater. 2012, 7, 015005. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Kruyt, M.C.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; van Blitterswijk, C.A. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J. Orthop. Res. 2008, 26, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Favero, G.; Labanca, M. Biomaterials in maxillofacial surgery: Membranes and grafts. Int. J. Biomed. Sci. 2011, 7, 81–88. [Google Scholar] [PubMed]
- Barakata, N.A.M.; Khalil, K.A.; Sheikh, F.A.; Omran, A.M.; Gaihre, B.; Khild, S.M.; Kim, H.Y. Physiochemical characterizations of hydroxyapatite extracted from bovine bones by three different methods: Extraction of biologically desirable Hap. Mater. Sci. Eng. C Mater. Biol. Appl. 2008, 28, 1381–1387. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, Y.J.; Lee, H.W.; Lee, W.K.; Ko, J.S.; Kim, H.M. Osteoblastic cell response to thin film of poorly crystalline calcium phosphate apatite formed at low temperatures. Biomaterials 2003, 24, 2977–2984. [Google Scholar] [CrossRef]
- Herliansyah, M.K.; Hamdia, M.; de-Ektessabic, A.I.; Wildanb, M.W.; Toque, J.A. The influence of sintering temperature on the properties of compacted bovine. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 1674–1680. [Google Scholar] [CrossRef]
- Muralithran, G.; Ramesh, S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram. Int. 2000, 26, 221–230. [Google Scholar] [CrossRef]
- Annaz, B.; Hing, K.A.; Kayser, M.; Buckland, T.; di Silvio, L. An ultrastructural study of cellular response to variation in porosity in phase-pure hydroxyapatite. J. Microsc. 2004, 216, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Cyster, L.A.; Grant, D.M.; Howdle, S.M.; Rose, F.R.; Irvine, D.J.; Freeman, D.; Scotchford, C.A.; Shakesheff, K.M. The influence of dispersant concentration on the pore morphology of hydroxyapatite ceramics for bone tissue engineering. Biomaterials 2005, 26, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Coathup, M.J.; Hing, K.A.; Samizadeh, S.; Chan, O.; Fang, Y.S.; Campion, C.; Buckland, T.; Blunn, G.W. Effect of increased strut porosity of calcium phosphate bone graft substitute biomaterials on osteoinduction. J. Biomed. Mater. Res. A 2012, 100, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.; Coimbra, P.; Cabrita, A.; Guerra, F.; Figueiredo, M. Comparison of a xenogeneic and an alloplastic material used in dental implants in terms of physico-chemical characteristics and in vivo inflammatory response. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 3, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, D.; Ozkan Karaca, E.; Dirikan Ipci, S.; Cakar, G.; Olgac, V.; Yilmaz, S. Comparison of two different xenografts in bilateral sinus augmentation: Radiographic and histologic findings. Quintessence Int. 2015, 46, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Riachi, F.; Naaman, N.; Tabarani, C.; AboeIsaad, N.; Berberi, A.; Salameh, Z. Influence of material properties on rate of resorption of two bone graft materials. Int. J. Dent. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Fienitz, T.; Moses, O.; Klemm, C.; Happe, A.; Ferrari, D.; Kreppel, M.; Ormianer, Z.; Gal, M.; Rothamel, D. Histological and radiological evaluation of sintered and non-sintered deproteinized bovine bone substitute materials in sinus augmentation procedures. A prospective, randomized-controlled, clinical multicenter study. Clin. Oral Investig. 2016. [Google Scholar] [CrossRef] [PubMed]
- Conz, M.B.; Granjeiro, J.M.; Soares, G.A. Physicochemical characterization of six commercial hydroxyapatites for medical-dental applicatons as bone graft. J. Appl. Oral Sci. 2005, 13, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.M.; da Rocha, N.C.C.; Rossi, A.M.; de Almeida Soares, G. Dissolution properties of calcium phosphate granules with different compositions in simulated body fluid. J. Biomed. Mater. Res. A 2003, 65, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Joschek, S.; Nies, B.; Krotz, R.; Göferich, A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000, 21, 1645–1658. [Google Scholar] [CrossRef]
- Rodrigues, C.V.M.; Serricella, P.; Linhares, A.B.; Guerdes, R.M.; Borojevic, R.; Rossi, A.M. Characterization of a bovine collagen-hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials 2003, 24, 4987–4997. [Google Scholar] [CrossRef]
- Alayan, J.; Vaquette, C.; Farah, C.; Ivanovski, S. A histomorphometric assessment of collagen-stabilized anorganic bovine bone mineral in maxillary sinus augmentation—A prospective clinical trial. Clin. Oral Implants Res. 2016, 27, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Taschieri, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric outcomes after lateral sinus floor elevation procedure: A systematic review of the literature and meta-analysis. Clin. Oral Implants Res. 2016, 27, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
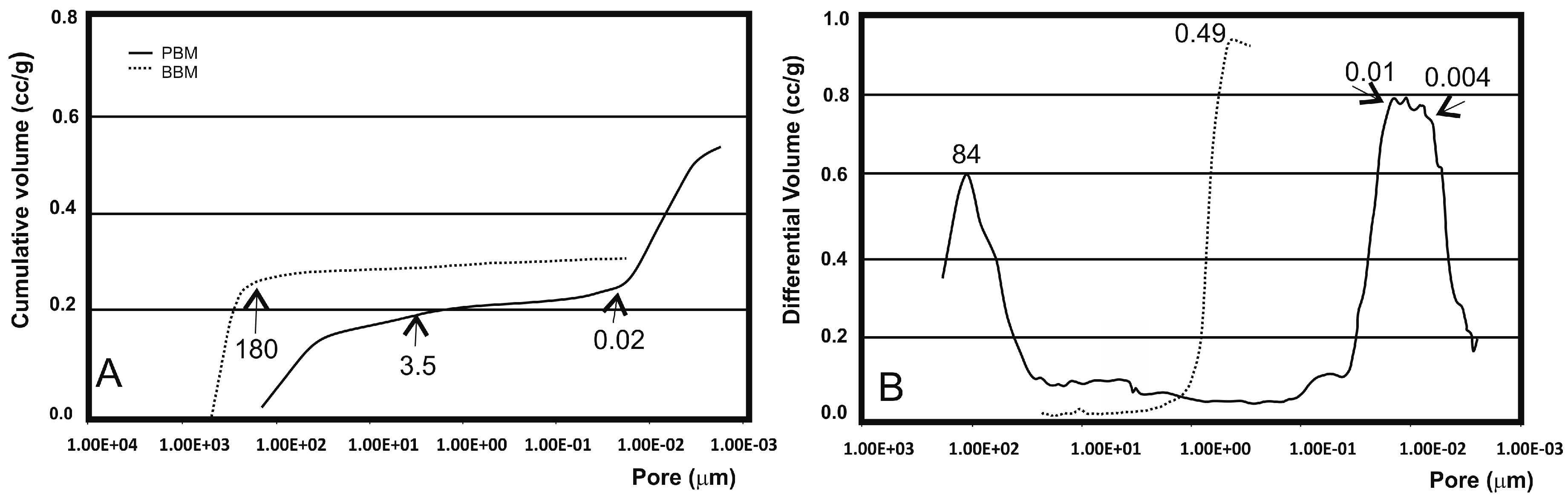


| Materials | Intruded Volume (cc/g) | Mode of Intraparticle Pores (μm) | Total Porosity (%) a | Intraparticle Porosity (%) b |
|---|---|---|---|---|
| PBM | 0.524 | 0.01–0.004 | 59.90 | 38.11 |
| BBM | 0.323 | 0.49 | 49.13 | 3.66 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez Fernández, M.P.; Mazón, P.; Gehrke, S.A.; Calvo-Guirado, J.L.; De Aza, P.N. Comparison of Two Xenograft Materials Used in Sinus Lift Procedures: Material Characterization and In Vivo Behavior. Materials 2017, 10, 623. https://doi.org/10.3390/ma10060623
Ramírez Fernández MP, Mazón P, Gehrke SA, Calvo-Guirado JL, De Aza PN. Comparison of Two Xenograft Materials Used in Sinus Lift Procedures: Material Characterization and In Vivo Behavior. Materials. 2017; 10(6):623. https://doi.org/10.3390/ma10060623
Chicago/Turabian StyleRamírez Fernández, María Piedad, Patricia Mazón, Sergio A. Gehrke, Jose Luis Calvo-Guirado, and Piedad N. De Aza. 2017. "Comparison of Two Xenograft Materials Used in Sinus Lift Procedures: Material Characterization and In Vivo Behavior" Materials 10, no. 6: 623. https://doi.org/10.3390/ma10060623










