Steering the Properties of MoOx Hole Transporting Layers in OPVs and OLEDs: Interface Morphology vs. Electronic Structure
Abstract
:1. Introduction
2. Results and Discussion
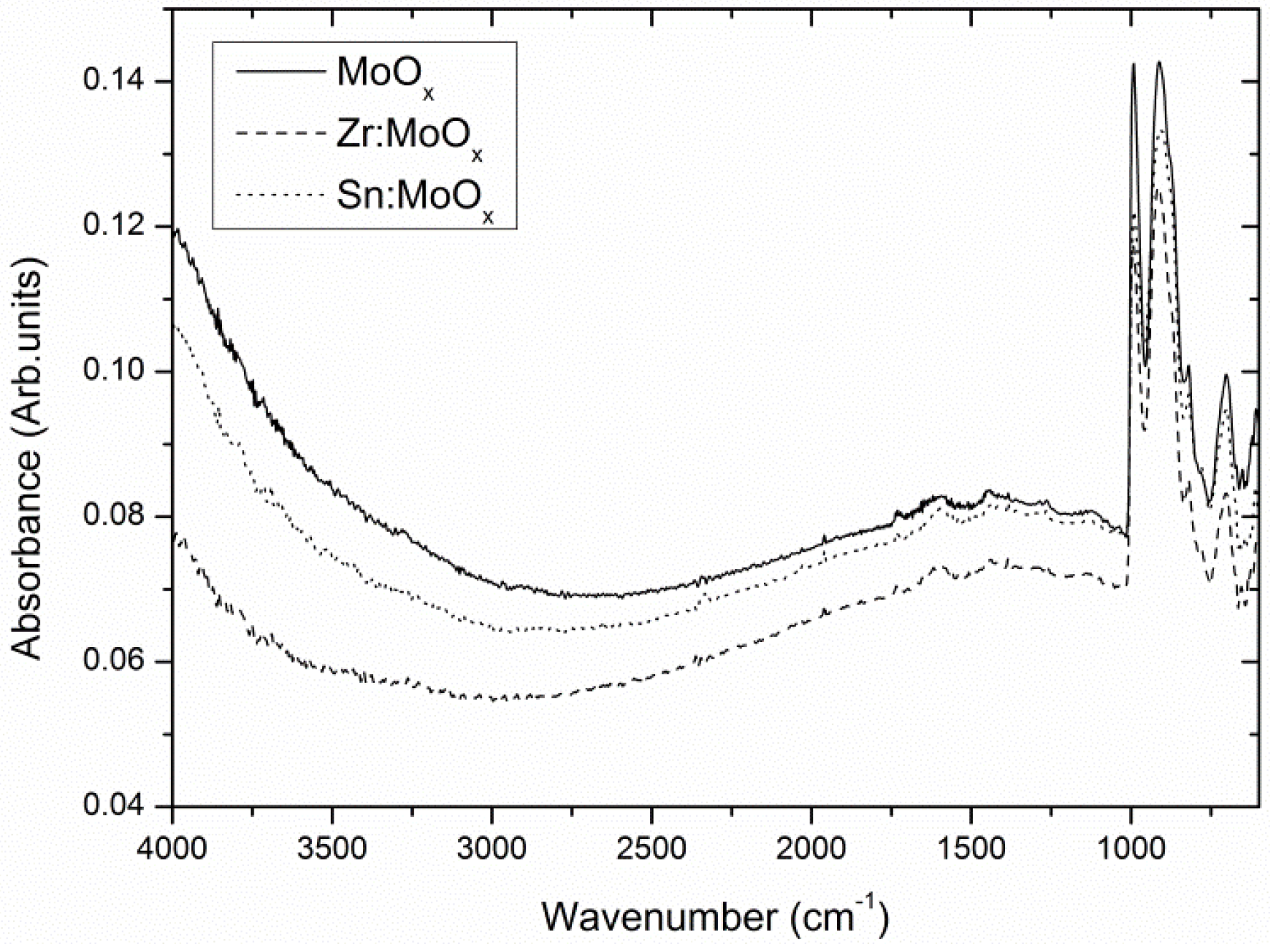
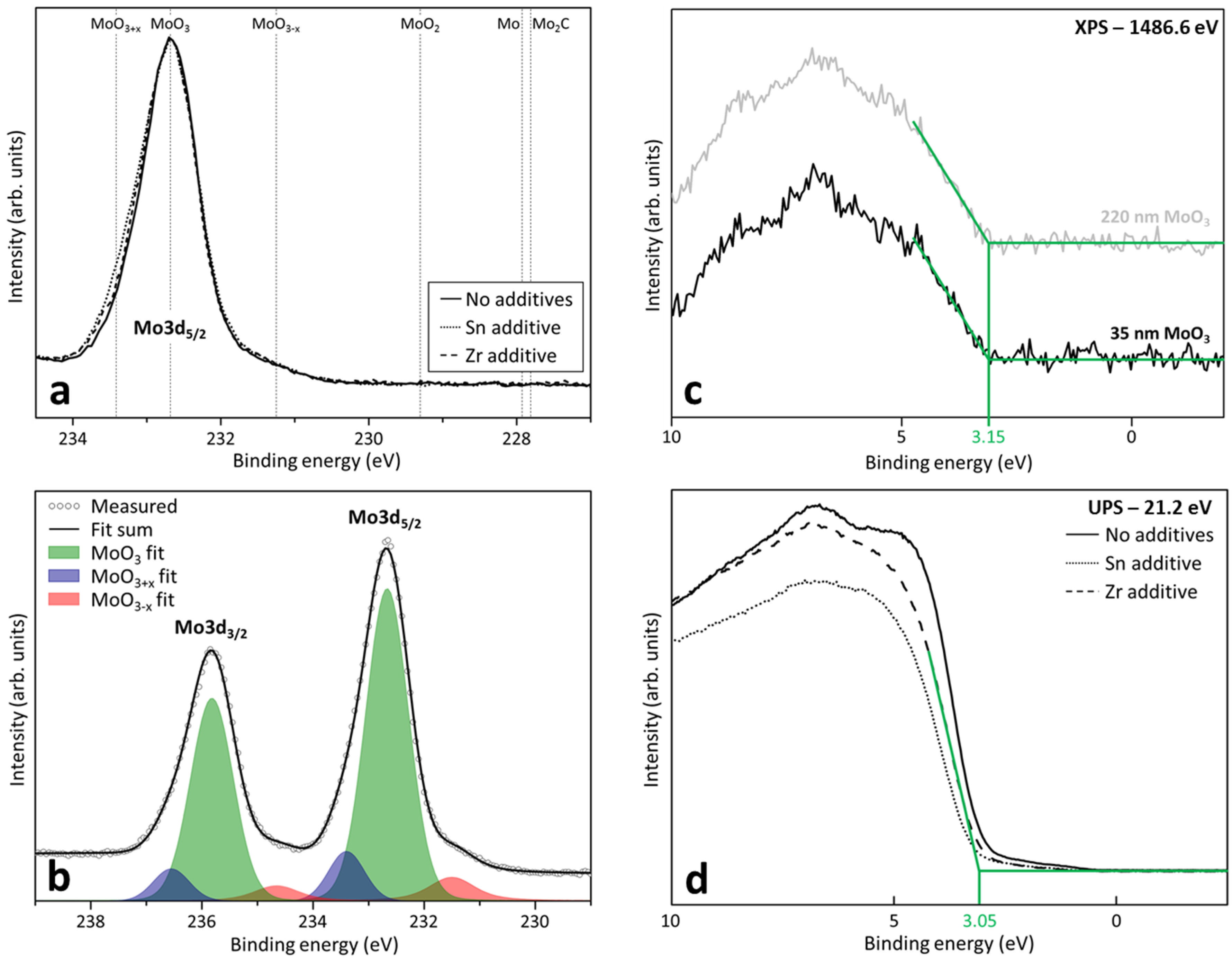
2.1. Composition and Electronic Structure
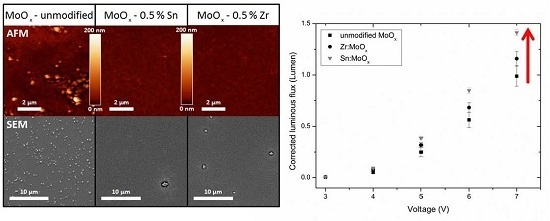
2.2. Morphology and Crystallinity
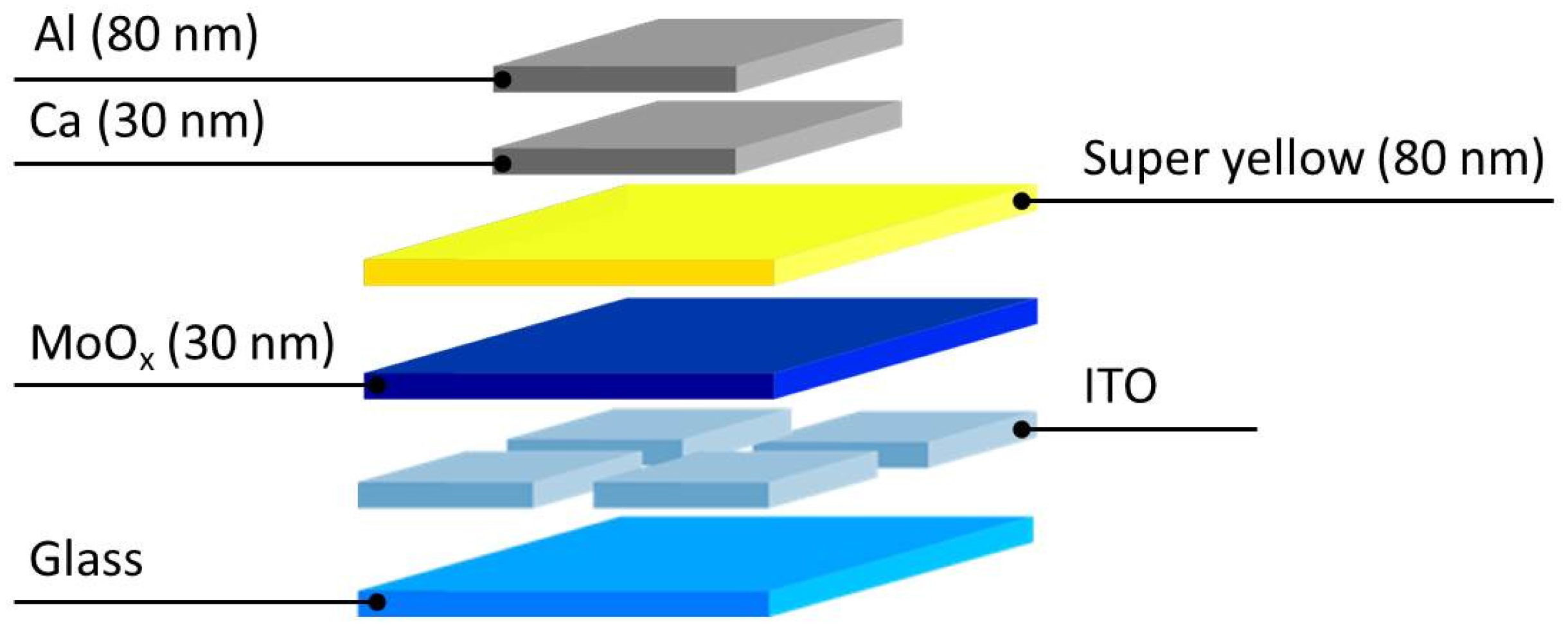
2.3. Device Output Characteristics
3. Materials and Methods
3.1. Precursor Synthesis
3.2. Film Deposition
3.3. Film Characterization
3.4. Device Processing
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Youn, H.; Park, H.J.; Guo, L.J. Organic photovoltaic cells: From performance improvement to manufacturing processes. Small 2015, 11, 2228–2246. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S. Wide-bandgap semiconductor materials: For their full bloom. Jpn. J. Appl. Phys. 2015, 54, 030101. [Google Scholar] [CrossRef]
- Alivov, Y.I.; Kalinina, E.V.; Cherenkov, A.E.; Look, D.C.; Ataev, B.M.; Omaev, A.K.; Chukichev, M.V.; Bagnall, D.M. Fabrication and characterization of n-ZnO/p-AlGaN heterojunction light-emitting diodes on 6h-sic substrates. Appl. Phys. Lett. 2003, 83, 4719. [Google Scholar] [CrossRef]
- Thejokalyani, N.; Dhoble, S.J. Novel approaches for energy efficient solid state lighting by RGB organic light emitting diodes—A review. Renew. Sustain. Energy Rev. 2014, 32, 448–467. [Google Scholar] [CrossRef]
- Zhan, X.; Zhu, D. Conjugated polymers for high-efficiency organic photovoltaics. Polym. Chem. 2010, 1, 409–419. [Google Scholar] [CrossRef]
- Suzuki, Y.; Zhang, Q.; Adachi, C. A solution-processable host material of 1,3-bis{3-[3-(9-carbazolyl)phenyl]-9-carbazolyl}benzene and its application in organic light-emitting diodes employing thermally activated delayed fluorescence. J. Mater. Chem. C 2015, 3, 1700–1706. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, Y.; Huang, J. Towards high performance organic photovoltaic cells: A review of recent development in organic photovoltaics. Polymers 2014, 6, 2473–2509. [Google Scholar] [CrossRef]
- Ray, B.; Alam, M.A. Random vs regularized opv: Limits of performance gain of organic bulk heterojunction solar cells by morphology engineering. Sol. Energy Mater. Sol. Cells 2012, 99, 204–212. [Google Scholar] [CrossRef]
- Gilissen, K.; Stryckers, J.; Verstappen, P.; Drijkoningen, J.; Heintges, G.H.L.; Lutsen, L.; Manca, J.; Maes, W.; Deferme, W. Ultrasonic spray coating as deposition technique for the light-emitting layer in polymer leds. Org. Electron. 2015, 20, 31–35. [Google Scholar] [CrossRef]
- Eggenhuisen, T.M.; Galagan, Y.; Coenen, E.W.C.; Voorthuijzen, W.P.; Slaats, M.W.L.; Kommeren, S.A.; Shanmuganam, S.; Coenen, M.J.J.; Andriessen, R.; Groen, W.A. Digital fabrication of organic solar cells by inkjet printing using non-halogenated solvents. Sol. Energy Mater. Sol. Cells 2015, 134, 364–372. [Google Scholar] [CrossRef]
- Meyer, J.; Hamwi, S.; Kroger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition metal oxides for organic electronics: Energetics, device physics and applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, Q.; Zeng, W.; Ma, D. High-efficiency fluorescent organic light-emitting diodes with MoO3 and PEDOT: PSS composition film as a hole injection layer. J. Mater. Chem. C 2014, 2, 9620–9624. [Google Scholar] [CrossRef]
- Salim, T.; Yin, Z.; Sun, S.; Huang, X.; Zhang, H.; Lam, Y.M. Solution-processed nanocrystalline TiO2 buffer layer used for improving the performance of organic photovoltaics. ACS Appl. Mater. Interfaces 2011, 3, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Tokmoldin, N.; Griffiths, N.; Bradley, D.D.C.; Haque, S.A. A hybrid inorganic-organic semiconductor light-emitting diode using ZrO2 as an electron-injection layer. Adv. Mater. 2009, 21, 3475–3478. [Google Scholar] [CrossRef]
- Wang, Z.; Nayak, P.K.; Caraveo-Frescas, J.A.; Alshareef, H.N. Recent developments in p-type oxide semiconductor materials and devices. Adv. Mater. 2016, 28, 3831–3892. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.; Yang, Y. Solution processed MoO3 interfacial layer for organic photovoltaics prepared by a facile synthesis method. Adv. Mater. 2012, 24, 2459–2462. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zakutayev, A.; Stowers, J.; Anderson, M.D.; Tate, J.; McIntyre, D.H.; Johnson, D.C.; Keszler, D.A. Low-temperature, solution processing of TiO2 thin films and fabrication of multilayer dielectric optical elements. Solid State Sci. 2009, 11, 1692–1699. [Google Scholar] [CrossRef]
- Hammond, S.R.; Meyer, J.; Widjonarko, N.E.; Ndione, P.F.; Sigdel, A.K.; Garcia, A.; Miedaner, A.; Lloyd, M.T.; Kahn, A.; Ginley, D.S.; et al. Low-temperature, solution-processed molybdenum oxide hole-collection layer for organic photovoltaics. J. Mater. Chem. 2012, 22, 3249–3254. [Google Scholar] [CrossRef]
- Jasieniak, J.J.; Seifter, J.; Jo, J.; Mates, T.; Heeger, A.J. A solution-processed MoOx anode interlayer for use within organic photovoltaic devices. Adv. Funct. Mater. 2012, 22, 2594–2605. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Chen, S.; Shi, X.; Wang, J.; Huang, S.; Guo, X.; He, G. Low-temperature MoO3 film from a facile synthetic route for an efficient anode interfacial layer in organic optoelectronic devices. J. Mater. Chem. C 2014, 2, 158–163. [Google Scholar] [CrossRef]
- Zilberberg, K.; Trost, S.; Meyer, J.; Kahn, A.; Behrendt, A.; Lützenkirchen-Hecht, D.; Frahm, R.; Riedl, T. Inverted organic solar cells with sol-gel processed high work-function vanadium oxide hole-extraction layers. Adv. Funct. Mater. 2011, 21, 4776–4783. [Google Scholar] [CrossRef]
- Choi, H.; Kim, B.; Ko, M.J.; Lee, D.K.; Kim, H.S.H.; Kim, K. Solution processed WO3 layer for the replacement of PEDOT:PSS layer in organic photovoltaic cells. Org. Electron. 2012, 13, 959–968. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Soultati, A.; Georgiadou, D.G.; Stergiopoulos, T.; Palilis, L.C.; Kennou, S.; Stathopoulos, N.A.; Davazoglou, D.; Argitis, P. Hydrogenated under-stoichiometric tungsten oxide anode interlayers for efficient and stable organic photovoltaics. J. Mater. Chem. A 2014, 2, 1738–1749. [Google Scholar] [CrossRef]
- Girotto, C.; Voroshazi, E.; Cheyns, D.; Heremans, P.; Rand, B.P. Solution-processed MoO3 thin films as a hole-injection layer for organic solar cells. ACS Appl. Mater. Interfaces 2011, 3, 3244–3247. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.W.; Liang, Z.; Jin, C.; Jiang, X.Y.; Zhang, Z.L. Enhanced power efficiency for white oled with MoO3 as hole injection layer and optimized charge balance. Solid State Commun. 2009, 149, 214–217. [Google Scholar] [CrossRef]
- Griffin, J.; Watters, D.C.; Yi, H.; Iraqi, A.; Lidzey, D.; Buckley, A.R. The influence of mooxanode stoicheometry on the performance of bulk heterojunction polymer solar cells. Adv. Energy Mater. 2013, 3, 903–908. [Google Scholar] [CrossRef]
- Choy, W.C.; Zhang, D. Solution-processed metal oxides as efficient carrier transport layers for organic photovoltaics. Small 2016, 12, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shao, S.; Guo, X.; Zhao, Y.; Xie, Z. Efficient polymer photovoltaic cells using solution-processed MoO3 as anode buffer layer. Sol. Energy Mater. Sol. Cells 2010, 94, 842–845. [Google Scholar] [CrossRef]
- Xie, F.; Choy, W.C.; Wang, C.; Li, X.; Zhang, S.; Hou, J. Low-temperature solution-processed hydrogen molybdenum and vanadium bronzes for an efficient hole-transport layer in organic electronics. Adv. Mater. 2013, 25, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.a.; Qian, D.; Zhang, W.; Li, L.; Ding, Y.; Xu, Q.; Wang, F.; Li, Y. Efficient and stable polymer solar cells with solution-processed molybdenum oxide interfacial layer. J. Mater. Chem. A 2013, 1, 657–664. [Google Scholar] [CrossRef]
- Marchal, W.; De Dobbelaere, C.; Kesters, J.; Bonneux, G.; Vandenbergh, J.; Damm, H.; Junkers, T.; Maes, W.; D’Haen, J.; Van Bael, M.K.; et al. Combustion deposition of MoO3 films: From fundamentals to opv applications. RSC Adv. 2015, 5, 91349–91362. [Google Scholar] [CrossRef]
- Li, X.; Choy, W.C.H.; Xie, F.; Zhang, S.; Hou, J. Room-temperature solution-processed molybdenum oxide as a hole transport layer with ag nanoparticles for highly efficient inverted organic solar cells. J. Mater. Chem. A 2013, 1, 6614–6621. [Google Scholar] [CrossRef] [Green Version]
- Gershon, T. Metal oxide applications in organic-based photovoltaics. Mater. Sci. Technol. 2011, 27, 1357–1371. [Google Scholar]
- Boukhachem, A.; Kamoun, O.; Mrabet, C.; Mannai, C.; Zouaghi, N.; Yumak, A.; Boubaker, K.; Amlouk, M. Structural, optical, vibrational and photoluminescence studies of Sn-doped MoO3 sprayed thin films. Mater. Res. Bull. 2015, 72, 252–263. [Google Scholar] [CrossRef]
- Kim, P.L.; Yusoff, A.R.M.; Kim, H.M.; Lee, H.J.; Seo, G.J.; Jang, J. Inverted organic photovoltaic device with a new electron transport layer. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Su, H.-C.; Chen, C.-H.; Liu, K.-L.; Tsai, C.-M.; Yen, S.-J.; Yew, T.-R. Indium-doped molybdenum oxide as a new p-type transparent conductive oxide. J. Mater. Chem. 2011, 21, 5745–5752. [Google Scholar] [CrossRef]
- Li, X.; Xie, F.; Zhang, S.; Hou, J.; Choy, W.C.H. Over 1.1 ev workfunction tuning of cesium intercalated metal oxides for functioning as both electron and hole transport layers in organic optoelectronic devices. Adv. Funct. Mater. 2014, 24, 7348–7356. [Google Scholar] [CrossRef]
- Li, X.; Xie, F.; Zhang, S.; Hou, J.; Choy, W.C.H. MoOx and V2Ox as hole and electron transport layers through functionalized intercalation in normal and inverted organic optoelectronic devices. Light Sci. Appl. 2015, 4, e273. [Google Scholar] [CrossRef] [Green Version]
- Moon, B.S.; Lee, S.-H.; Huh, Y.H.; Park, B. Hole-selective and impedance characteristics of an aqueous solution-processable MoO3 layer for solution-processable organic semiconducting devices. J. Korean. Phys. Soc. 2015, 66, 635–645. [Google Scholar] [CrossRef]
- Seguin, L.; Figlarz, M.; Cavagnat, R.; Lassègues, J.-C. Infrared and raman spectra of MoO3 molybdenum trioxides and MoO3.XH2O molybdenum trioxide hydrates. Spectrochim. Acta A 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Eda, K. Longitudinal-transverse splitting effects in ir absorption spectra of MoO3. J. Solid State Chem. 1991, 95, 64–73. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Wang, Q.; Zhang, D.-A.; Xing, L.; Xue, X. Electrochemical performance of α-MoO3–In2O3 core–shell nanorods as anode materials for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 5083–5091. [Google Scholar] [CrossRef]
- Chang, J.H.; Liu, S.Y.; Wu, I.W.; Chen, T.C.; Liu, C.W.; Wu, C.I. Stability improvement of organic light emitting diodes by the insertion of hole injection materials on the indium tin oxide substrate. J. Appl. Phys. 2014, 115, 1–5. [Google Scholar] [CrossRef]
- Dasgupta, B.; Goh, W.P.; Ooi, Z.E.; Wong, L.M.; Jiang, C.Y.; Ren, Y.; Tok, E.S.; Pan, J.; Zhang, J.; Chiam, S.Y. Enhanced extraction rates through gap states of molybdenum oxide anode buffer. J. Phys. Chem. C 2013, 117, 9206–9211. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides; Noyes Publications: Albuquerque, NM, USA, 1996. [Google Scholar]
- Bernède, J.C.; Houari, S.; Nguyen, D.; Jouan, P.Y.; Khelil, A.; Mokrani, A.; Cattin, L.; Predeep, P. Xps study of the band alignment at ito/oxide (n-type MoO3 or p-type NiO) interface. Phys. Status Solidi A 2012, 209, 1291–1297. [Google Scholar] [CrossRef] [Green Version]
- Bottois, C.; Tarasov, K.; Poncelet, O.; Pecquet, R.; Lemaitre, N. Tungstite nanoparticles prepared by microwave-assisted synthesis and their application as a hole transporting layer in organic solar cells. RSC Adv. 2015, 5, 23271–23277. [Google Scholar] [CrossRef]
- Garin, J.L.; Blanc, J.M. Synthesis and chrystal chemistry of NH3(MoO3)3. J. Solid State Chem. 1985, 58, 98–102. [Google Scholar] [CrossRef]
- Robertson, J.; Xiong, K.; Clark, S.J. Band gaps and defect levels in functional oxides. Thin Solid Films 2006, 496, 1–7. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, F.; Xu, K.W.; Liang, H.F. Optical properties and structural characterization of bias sputtered ZrO2 films. J. Alloys Compd. 2008, 453, 453–457. [Google Scholar] [CrossRef]
- Novikov, S.V. Rough electrode surface: Effect on charge carrier injection and transport in organic devices. Macromol. Symp. 2004, 212, 191–200. [Google Scholar] [CrossRef]
- Qin, W.; Ding, G.; Xu, X.; Yang, L.; Yin, S. Annealing effect of ZnO on the performance of inverted organic photovoltaic devices. J. Mater. Sci. Technol. 2014, 30, 197–202. [Google Scholar] [CrossRef]
- Wang, G.; Jiu, T.; Li, P.; Li, J.; Sun, C.; Lu, F.; Fang, J. Preparation and characterization of MoO3 hole-injection layer for organic solar cell fabrication and optimization. Sol. Energy Mater. Sol. Cells 2014, 120, 603–609. [Google Scholar] [CrossRef]
- Hu, X.; Chen, L.; Chen, Y. Universal and versatile MoO3-based hole transport layers for efficient and stable polymer solar cells. J. Phys. Chem. C 2014, 118, 9930–9938. [Google Scholar] [CrossRef]
- Shao, S.; Liu, J.; Bergqvist, J.; Shi, S.; Veit, C.; Würfel, U.; Xie, Z.; Zhang, F. In situ formation of MoO3 in PEDOT:PSS matrix: A facile way to produce a smooth and less hygroscopic hole transport layer for highly stable polymer bulk heterojunction solar cells. Adv. Energy Mater. 2013, 3, 349–355. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. Wsxm: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
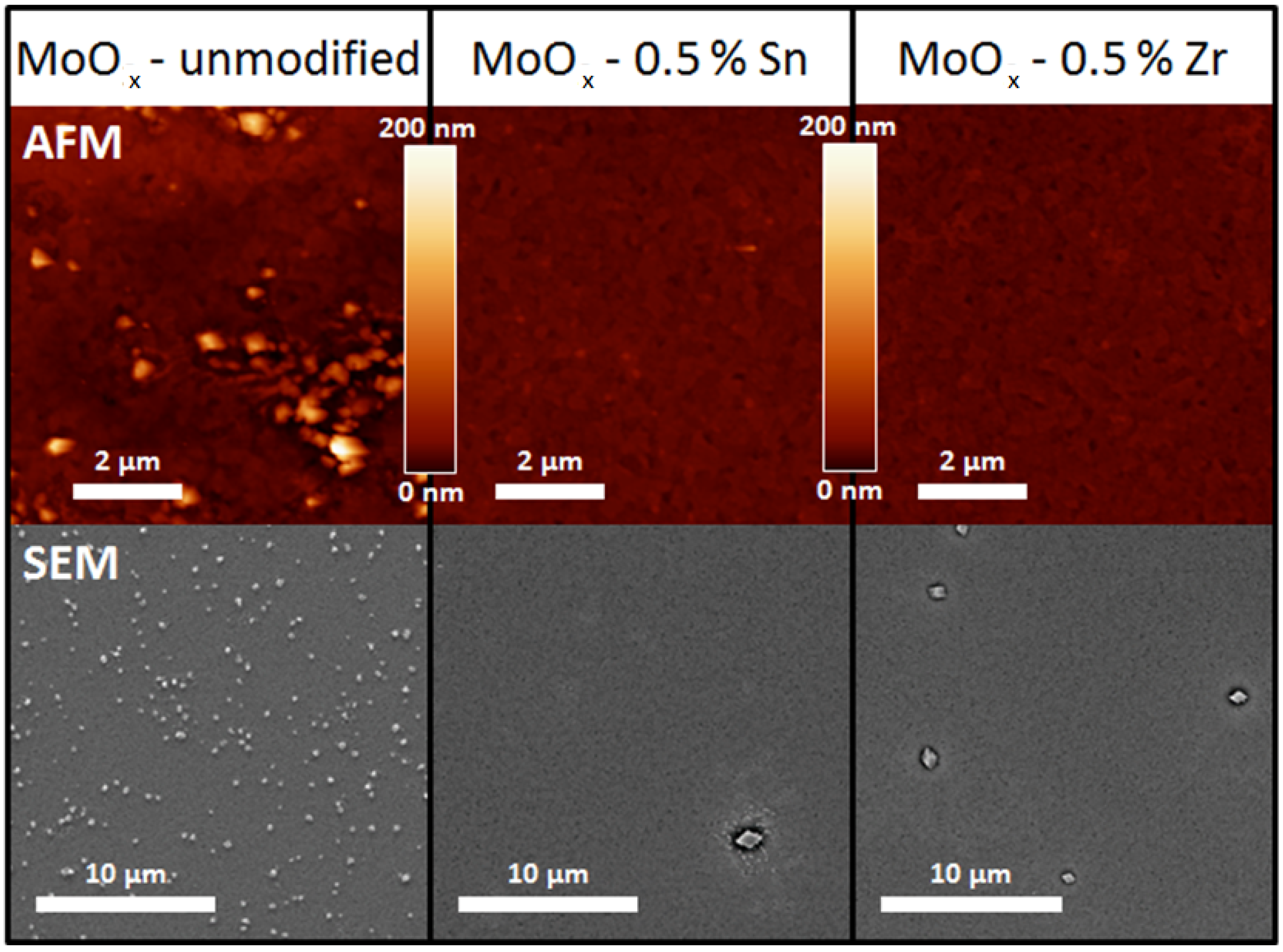
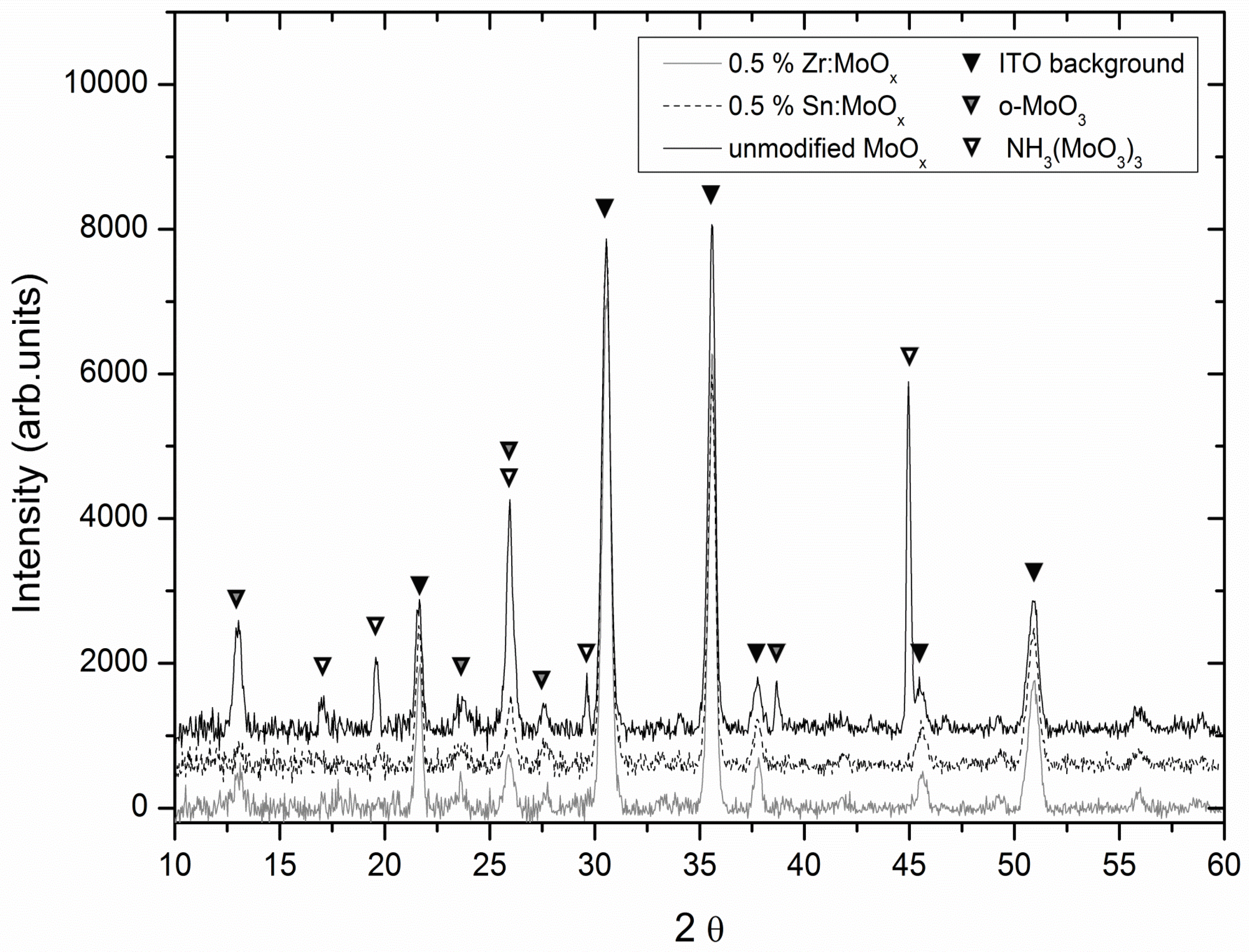
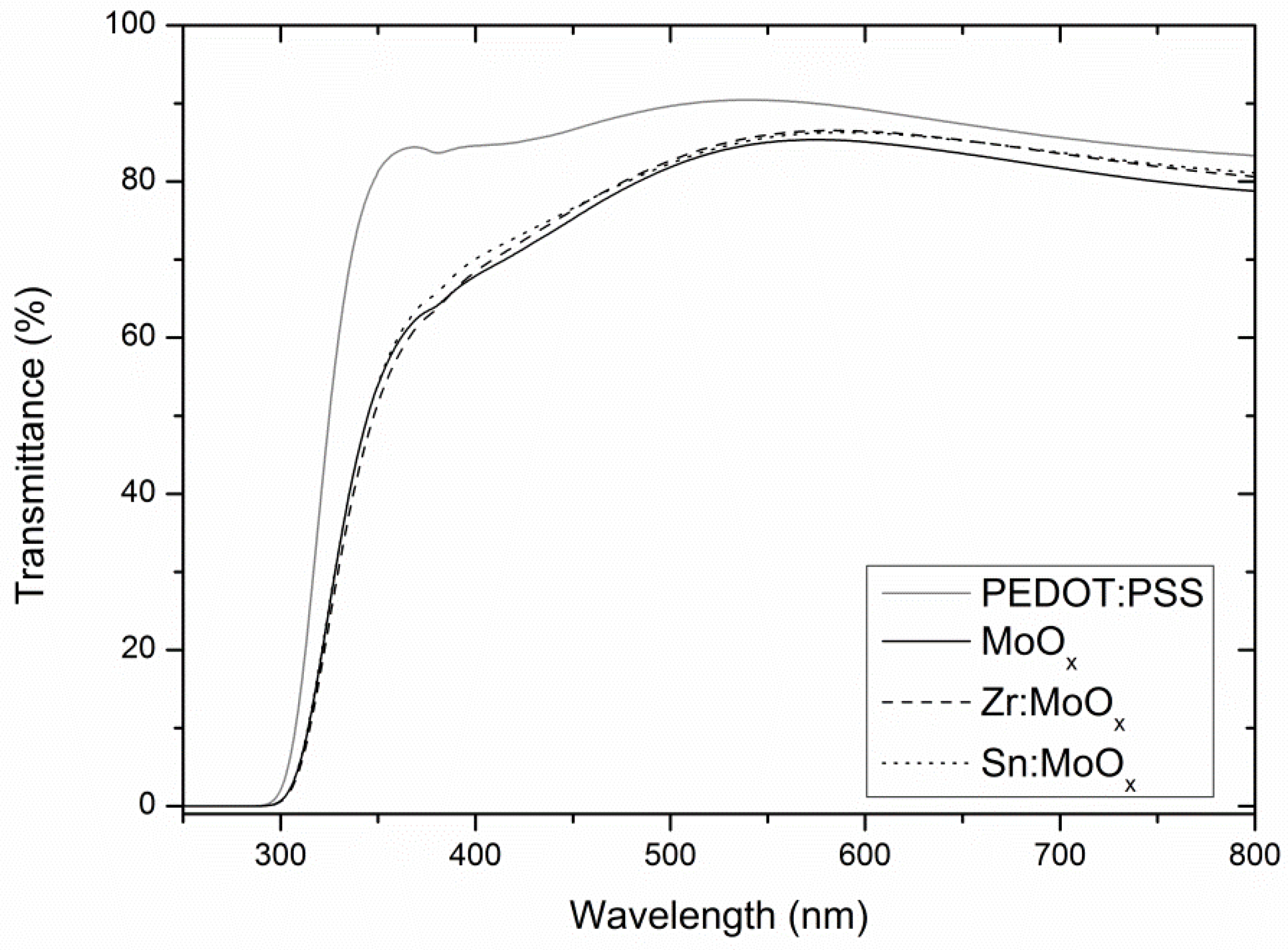
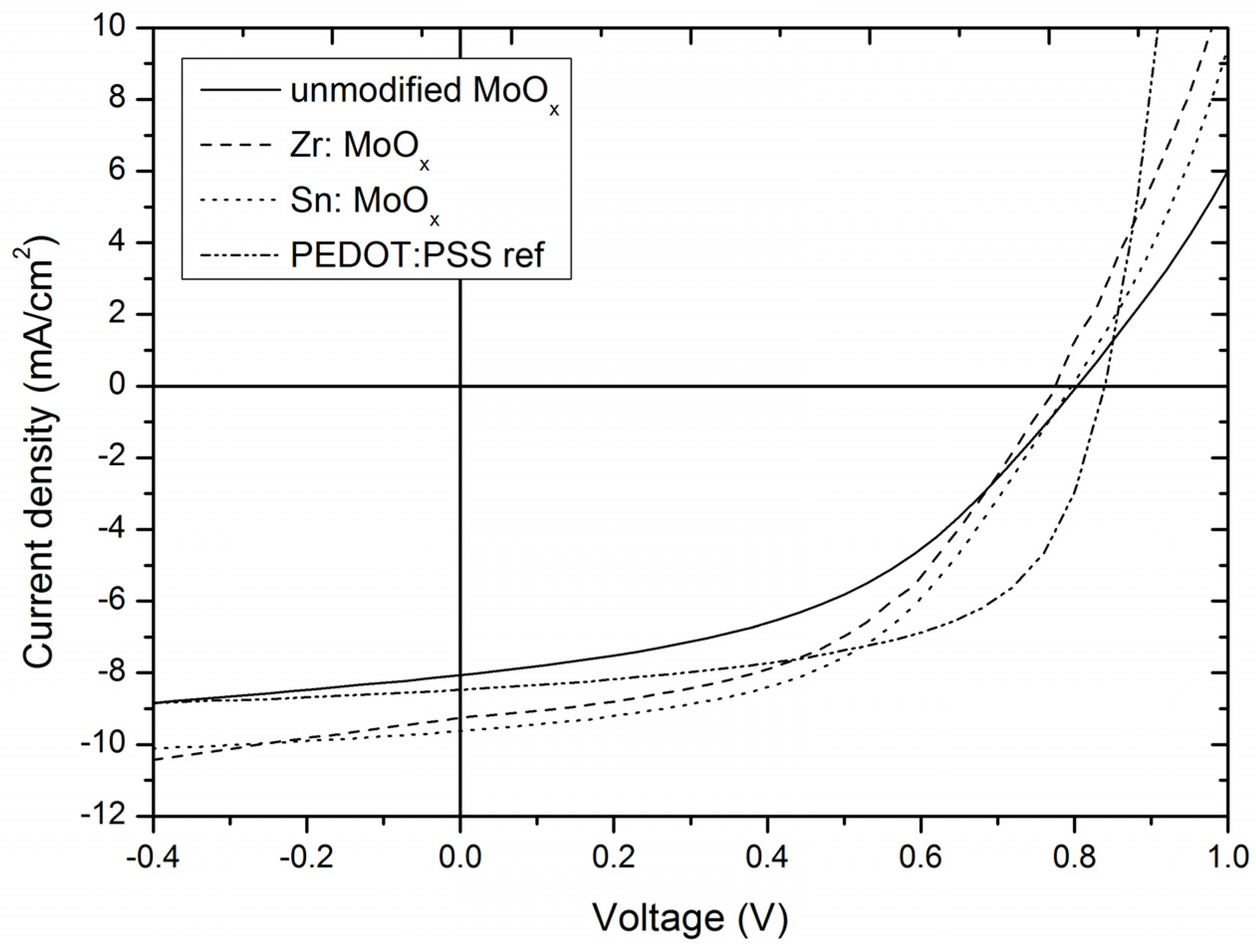

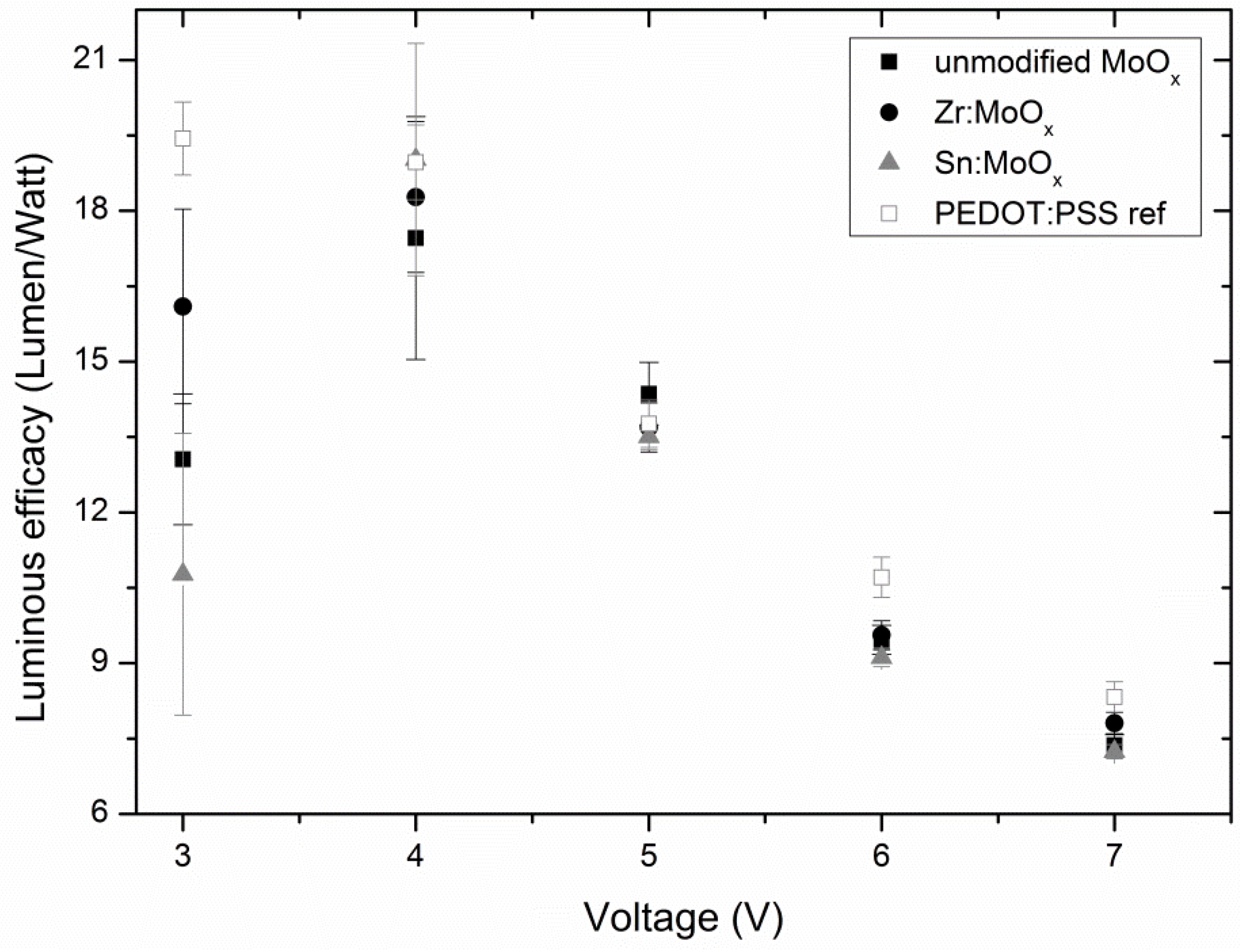
| RMS Roughness | Mean (nm) | S (nm) | nsamples |
|---|---|---|---|
| MoOx | 18.8 | 5.6 | 6 |
| Zr:MoOx | 2.2 | 0.3 | 3 |
| Sn:MoOx | 2.7 | 0.4 | 3 |
| PEDOT:PSS ref. | 1.4 | 0.1 | 1 |
| HTL | Voc (V) | Jsc (mA/cm2) | FF | Average PCE (%) |
|---|---|---|---|---|
| MoOx layer | 0.82 ± 0.04 | 8.13 ± 0.51 | 0.47 ± 0.03 | 3.10 ± 0.28 |
| Zr additive | 0.77 ± 0.02 | 9.03 ± 0.28 | 0.47 ± 0.01 | 3.30 ± 0.21 |
| Sn additive | 0.79 ± 0.02 | 9.32 ± 0.56 | 0.49 ± 0.01 | 3.62 ± 0.20 |
| PEDOT:PSS | 0.84 ± 0.01 | 8.71 ± 0.10 | 0.56 ± 0.01 | 4.11 ± 0.05 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchal, W.; Verboven, I.; Kesters, J.; Moeremans, B.; De Dobbelaere, C.; Bonneux, G.; Elen, K.; Conings, B.; Maes, W.; Boyen, H.G.; et al. Steering the Properties of MoOx Hole Transporting Layers in OPVs and OLEDs: Interface Morphology vs. Electronic Structure. Materials 2017, 10, 123. https://doi.org/10.3390/ma10020123
Marchal W, Verboven I, Kesters J, Moeremans B, De Dobbelaere C, Bonneux G, Elen K, Conings B, Maes W, Boyen HG, et al. Steering the Properties of MoOx Hole Transporting Layers in OPVs and OLEDs: Interface Morphology vs. Electronic Structure. Materials. 2017; 10(2):123. https://doi.org/10.3390/ma10020123
Chicago/Turabian StyleMarchal, Wouter, Inge Verboven, Jurgen Kesters, Boaz Moeremans, Christopher De Dobbelaere, Gilles Bonneux, Ken Elen, Bert Conings, Wouter Maes, Hans Gerd Boyen, and et al. 2017. "Steering the Properties of MoOx Hole Transporting Layers in OPVs and OLEDs: Interface Morphology vs. Electronic Structure" Materials 10, no. 2: 123. https://doi.org/10.3390/ma10020123
APA StyleMarchal, W., Verboven, I., Kesters, J., Moeremans, B., De Dobbelaere, C., Bonneux, G., Elen, K., Conings, B., Maes, W., Boyen, H. G., Deferme, W., Van Bael, M., & Hardy, A. (2017). Steering the Properties of MoOx Hole Transporting Layers in OPVs and OLEDs: Interface Morphology vs. Electronic Structure. Materials, 10(2), 123. https://doi.org/10.3390/ma10020123