In Vitro Evaluation of PCL and P(3HB) as Coating Materials for Selective Laser Melted Porous Titanium Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Osteoblast Isolation and Cultivation
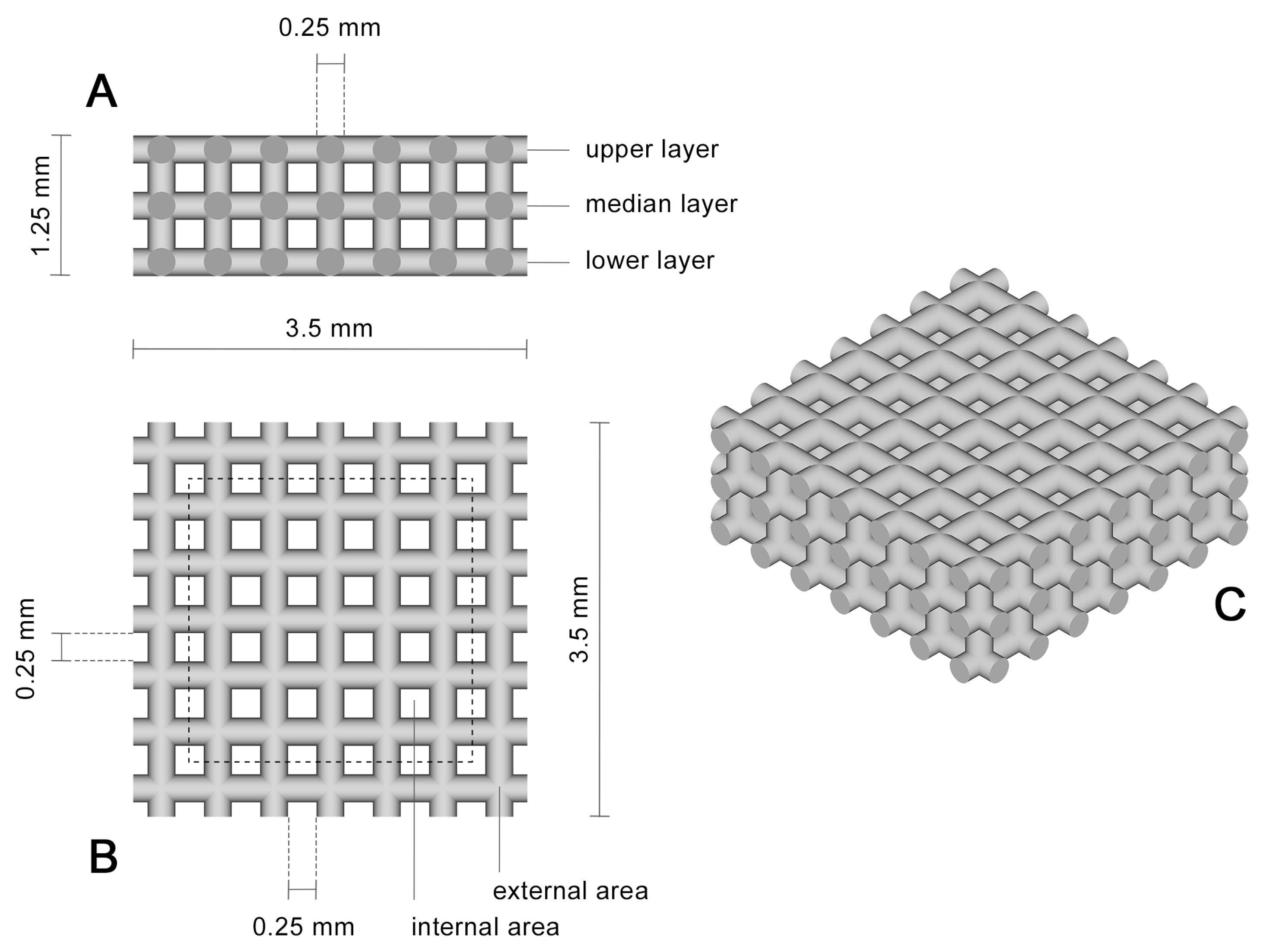
2.2. Selective Laser Melting of Titanium Scaffolds
2.3. Viscosimetry of PCL and P(3HB) Coating Solutions
2.4. PCL and P(3HB) Coating of Titanium Scaffolds
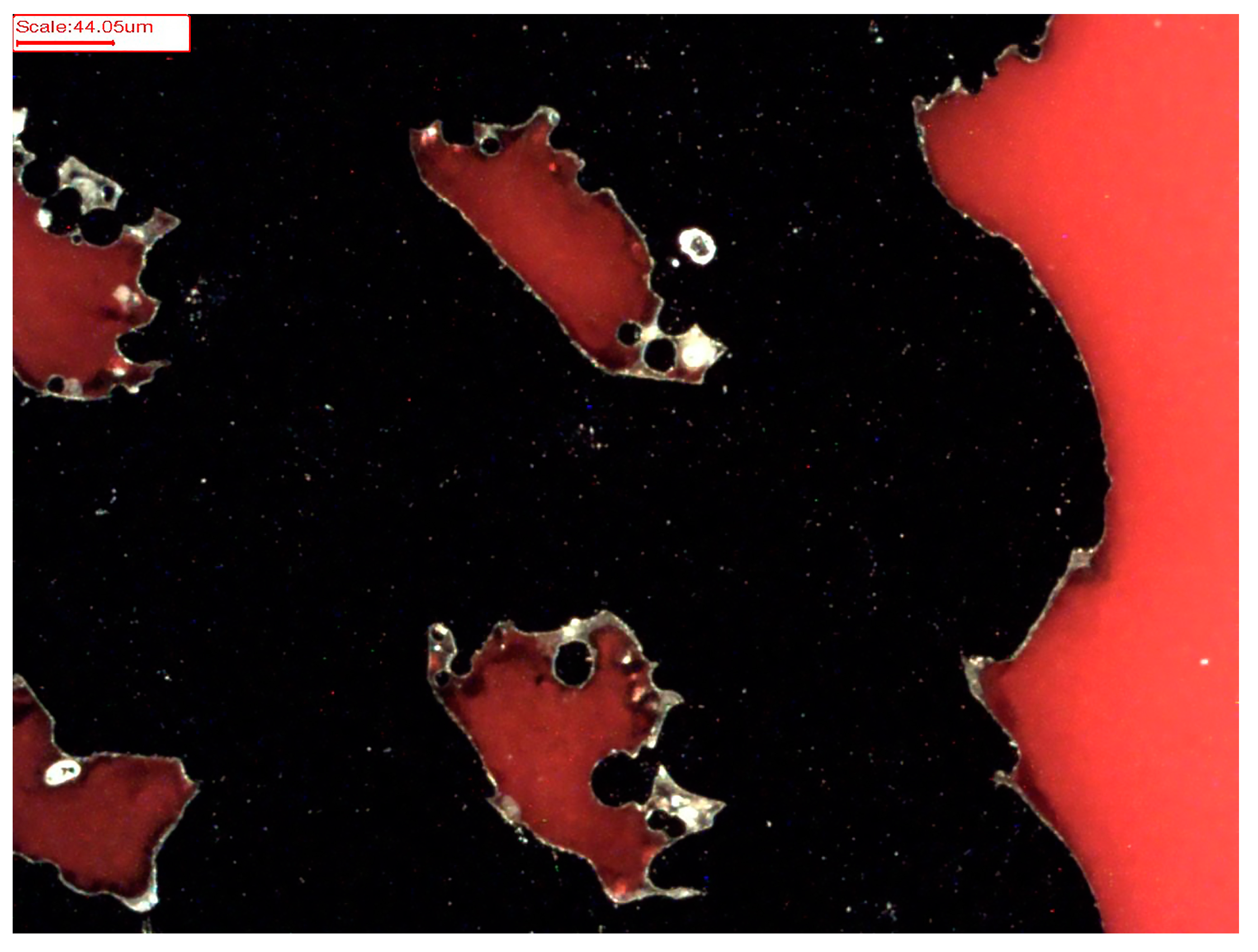
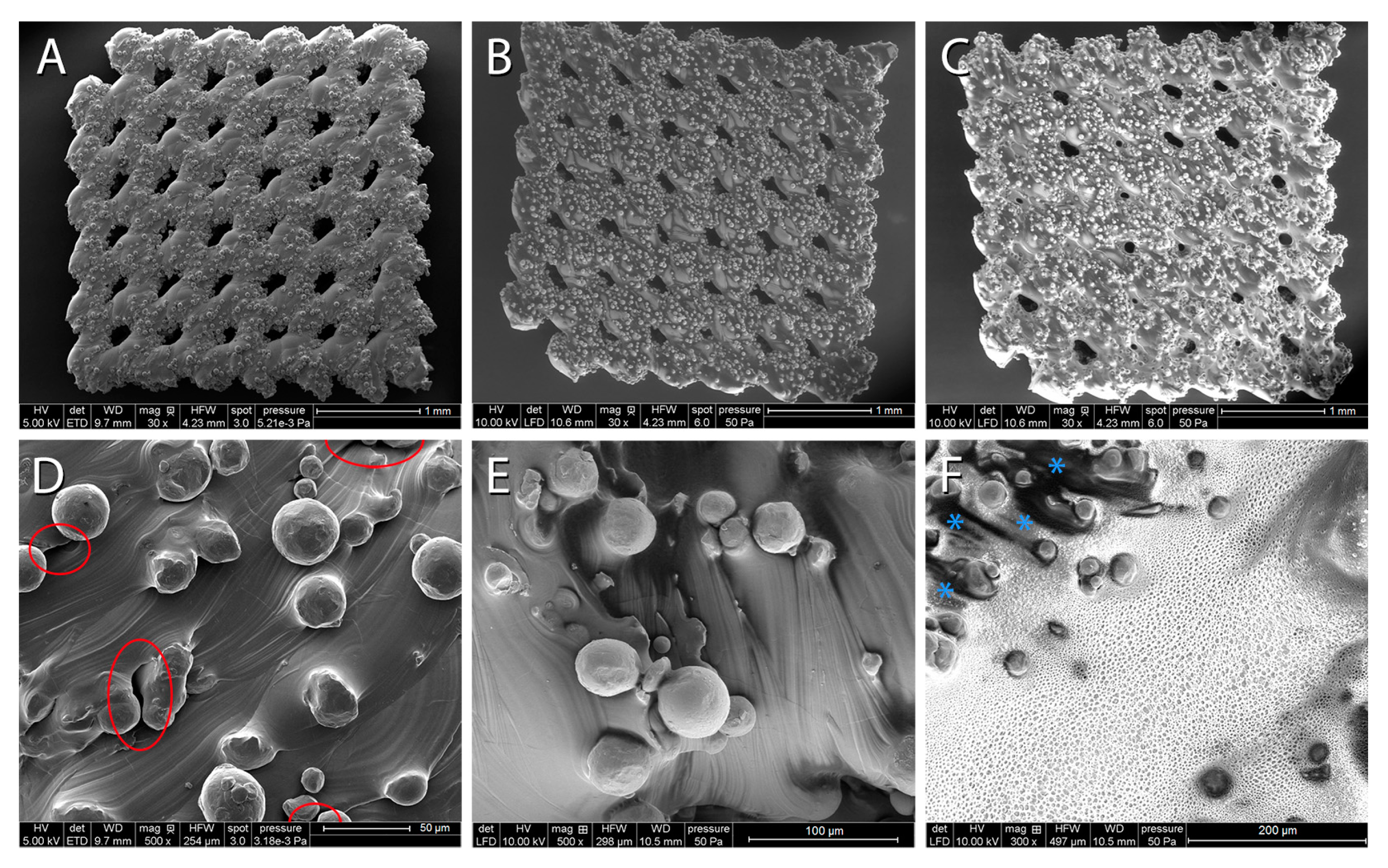
2.5. Characterisation of PCL and P(3HB) Coatings via ESEM and EDX Analysis
2.6. Cross-Sections of PCL-Coated Titanium Scaffolds
2.7. Preparation and Characterisation of PCL and P(3HB) Foils
2.8. Vitality and Proliferation Assays
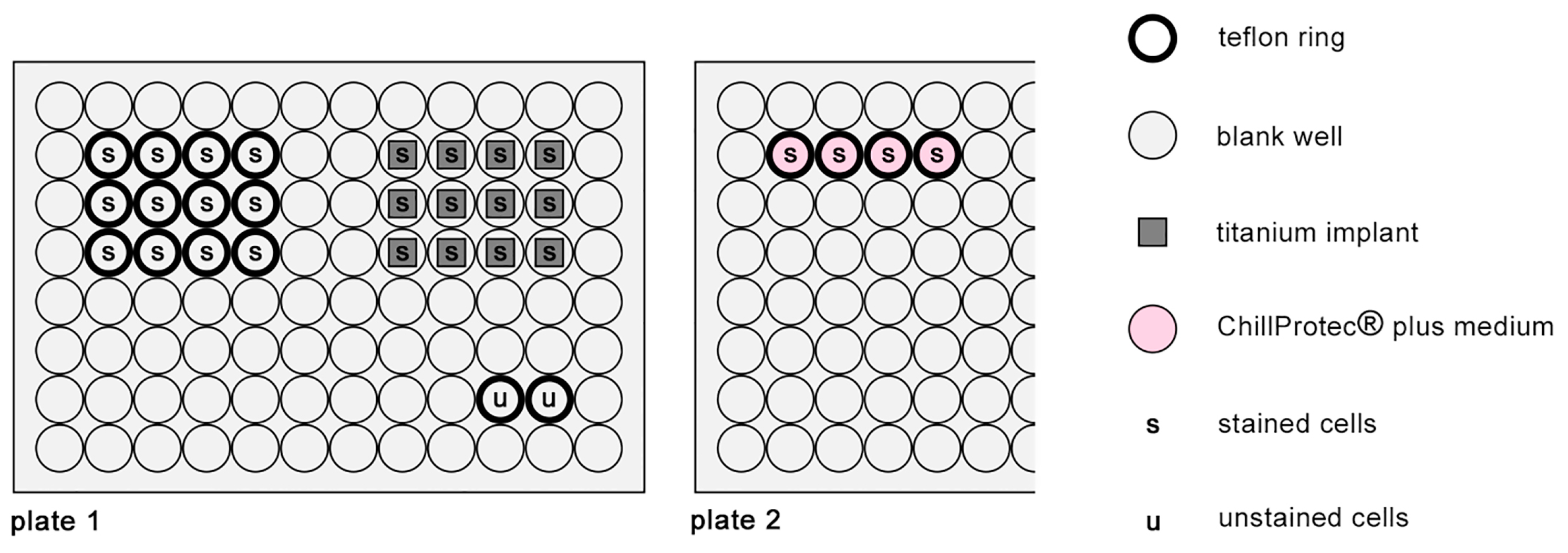
2.8.1. CFSE Staining of Osteoblasts
2.8.2. Vitality and Proliferation Assay on Polymer Foils
2.8.3. Vitality and Proliferation Assay on Titanium Scaffolds
2.8.4. Data Analysis
2.9. Live Cell Imaging
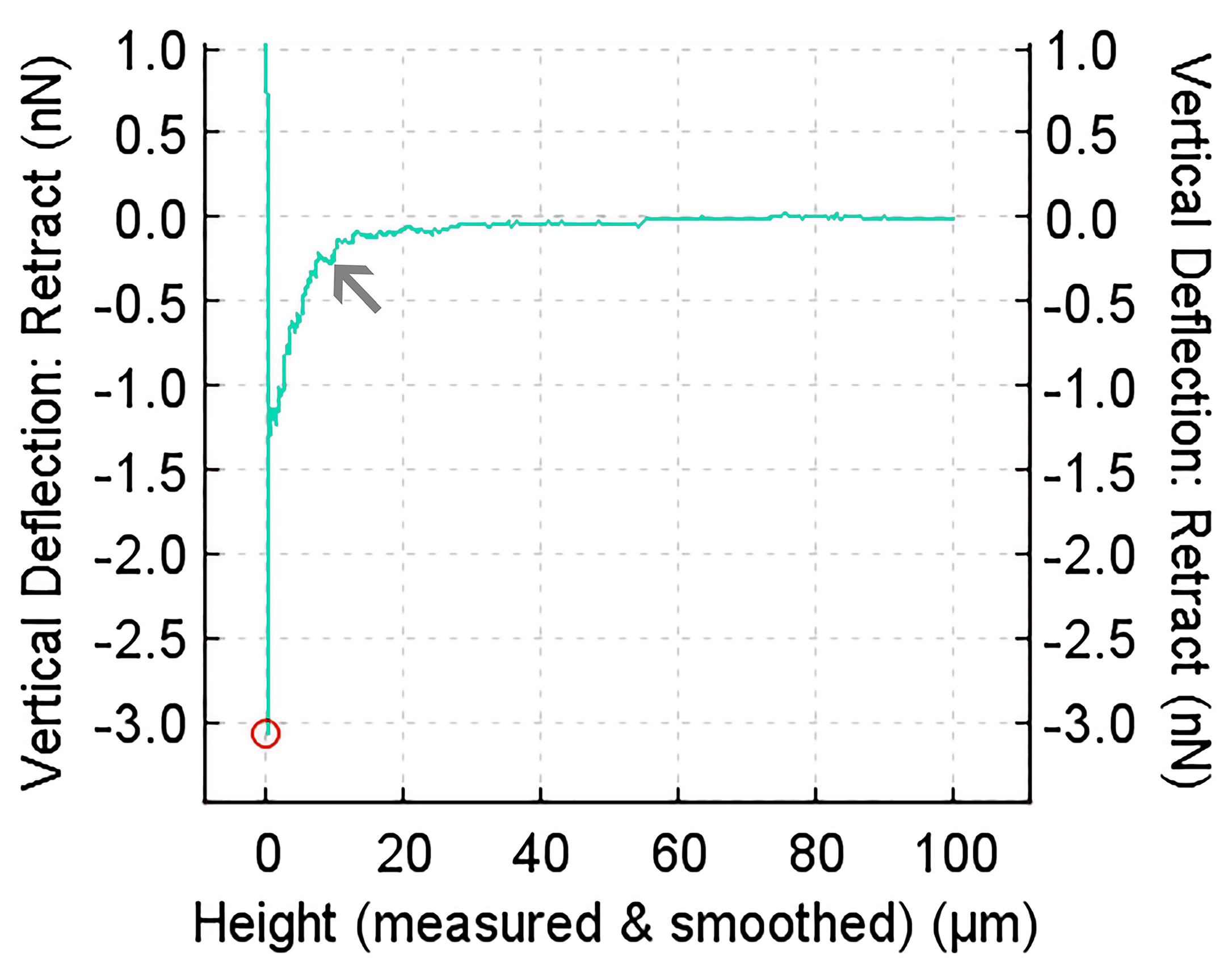
2.10. Single Cell Force Spectroscopy
2.11. Statistical Analysis
3. Results
3.1. Viscosimetry of PCL and P(3HB) Coating Solutions
3.2. ESEM and EDX Analysis of PCL and P(3HB) Coatings
3.3. Cross-Sections of PCL-Coated Titanium Scaffolds
3.4. ESEM Analysis of PCL and P(3HB) Foils
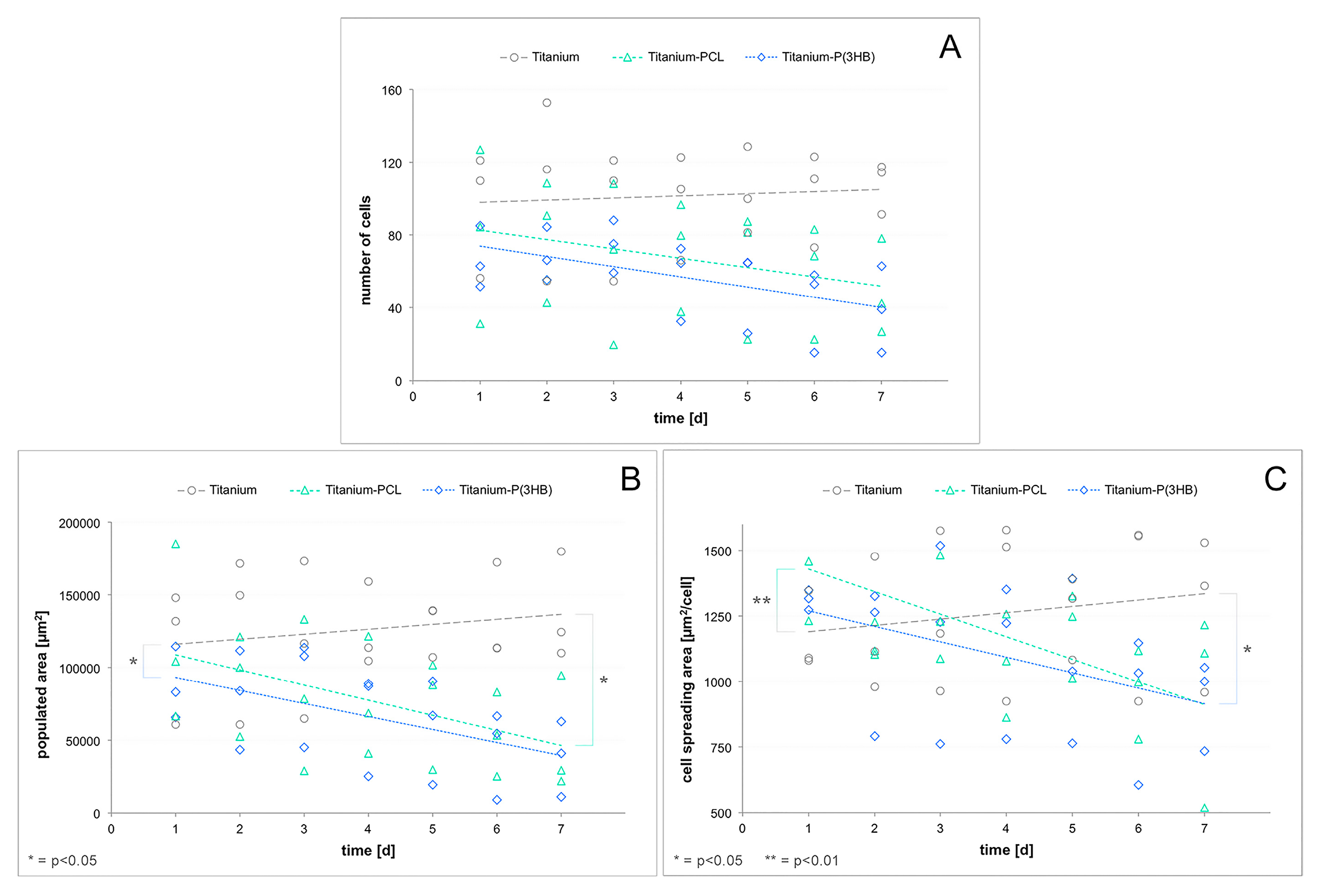
3.5. Vitality and Proliferation Assays
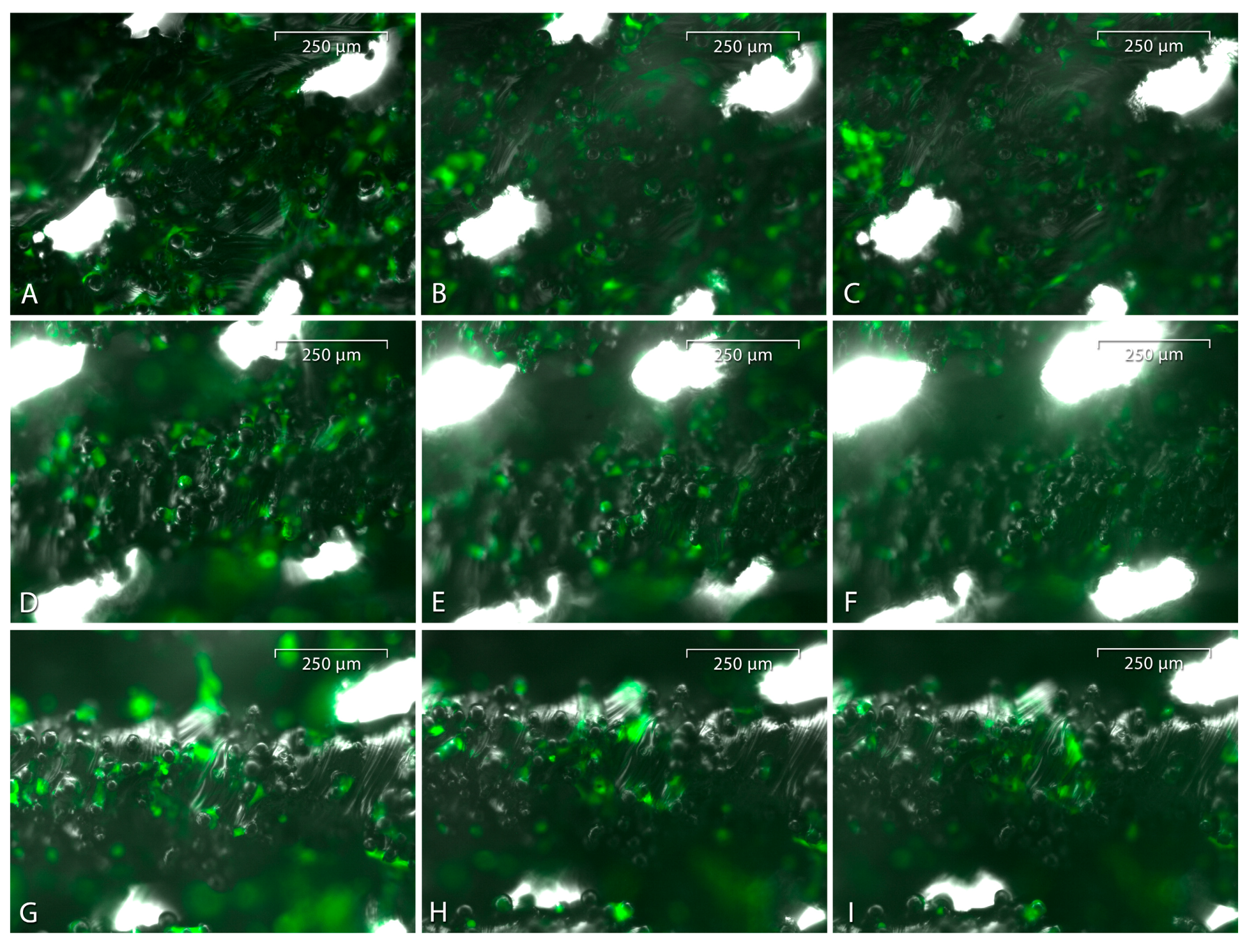
3.6. Live Cell Imaging
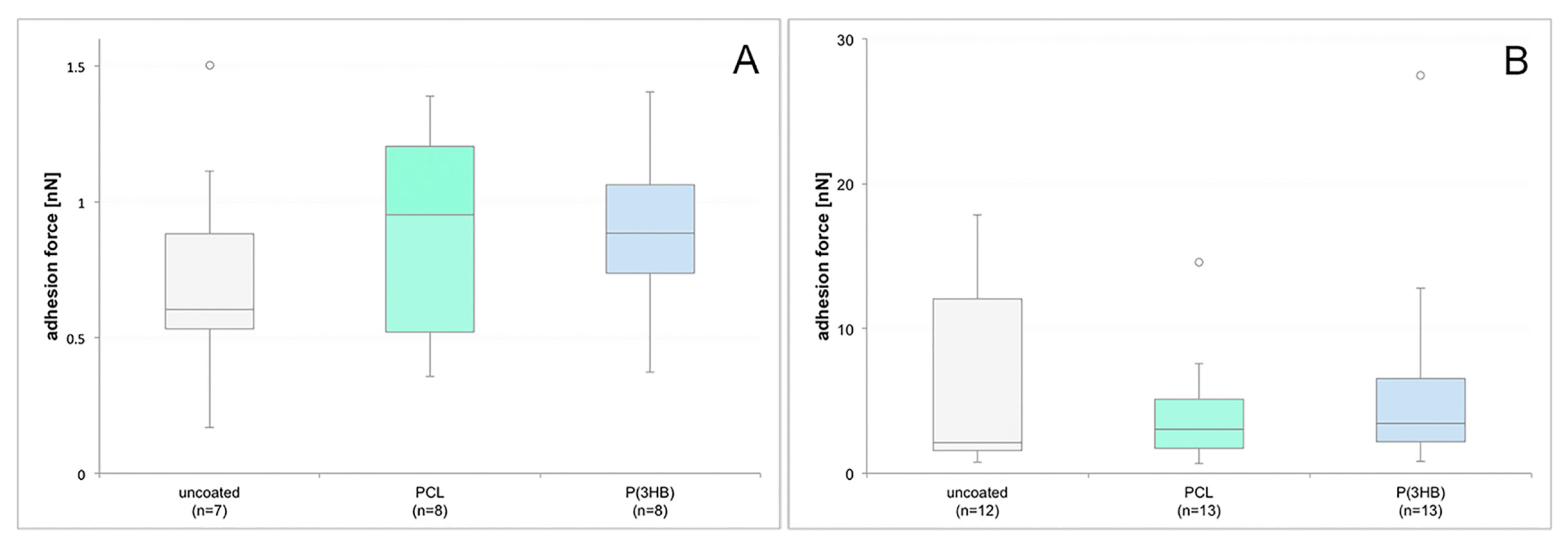
3.7. Single Cell Force Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, K.; Li, C.; Zhu, Z.; Liu, C.S. Measurement of the dynamic Young’s modulus of porous titanium and Ti6Al4V. J. Mater. Sci. 2007, 42, 7348–7353. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 124–134. [Google Scholar] [CrossRef]
- Engh, C.A.; Bobyn, J.D.; Glassman, A.H. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J. Bone Jt. Surg. Br. 1987, 69, 45–55. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Wang, Z.; Yang, J.; Liu, N.; Huang, W. Development of highly porous titanium scaffolds by selective laser melting. Mater. Lett. 2010, 64, 674–676. [Google Scholar] [CrossRef]
- Gebhardt, A. Understanding Additive Manufacturing: Rapid Prototyping-Rapid Tooling-Rapid Manufacturing; Carl Hanser Verlag GmbH & Co., KG: Munich, Germany, 2012; pp. 40–44. ISBN 9783446431621. [Google Scholar]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Van der Stok, J.; Van der Jagt, O.P.; Amin Yavari, S.; De Haas, M.F.; Waarsing, J.H.; Jahr, H.; Van Lieshout, E.M.; Patka, P.; Verhaar, J.A.; Zadpoor, A.A.; et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J. Orthop. Res. 2013, 31, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Artel, A.; Mehdizadeh, H.; Chiu, Y.C.; Brey, E.M.; Cinar, A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. Part A 2011, 17, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Guillot, R.; Pignot-Paintrand, I.; Lavaud, J.; Decambron, A.; Burgeois, E.; Josserand, V.; Logeart-Avramoglou, D.; Viguier, E.; Picart, C. Assessment of a polyelectrolyte multilayer film coating loaded with BMP-2 on titanium and PEEK implants in the rabbit femoral condyle. Acta Biomater. 2016, 36, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Backhaus, S.; Grau, M.; Matena, J.; Teske, M.; Beyerbach, M.; Murua Escobar, H.; Haferkamp, H.; Gellrich, N.-C.; Nolte, I. Evaluation of functionalized porous titanium scaffolds for enhancing angiogenesis in vitro. Materials 2016, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Müller, U. In vitro biocompatibility testing of biomaterials and medical devices. Med. Device Technol. 2008, 19, 32–34. [Google Scholar]
- Cipitria, A.; Reichert, J.C.; Epari, D.R.; Saifzadeh, S.; Berner, A.; Schell, H.; Mehta, M.; Schuetz, M.A.; Duda, G.N.; Hutmacher, D.W. Polycaprolactone scaffold and reduced rhBMP-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 2013, 34, 9960–9968. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.T.; Teh, L.Y.; Tan, D.B.; Zhang, Z.; Teoh, S.H. Novel 3D polycaprolactone scaffold for ridge preservation—A pilot randomised controlled clinical trial. Clin. Oral Implant. Res. 2015, 26, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Shishatskaya, E.I.; Kamendov, I.V.; Starosvetsky, S.I.; Vinnik, Y.S.; Markelova, N.N.; Shageev, A.A.; Khorzhevsky, V.A.; Peryanova, O.V.; Shumilova, A.A. An in vivo study of osteoplastic properties of resorbable poly-3-hydroxybutyrate in models of segmental osteotomy and chronic osteomyelitis. Artif. Cells Nanomed. Biotechnol. 2014, 42, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Centeno-Leija, S.; Huerta-Beristain, G.; Giles-Gomez, M.; Bolivar, F.; Gosset, G.; Martinez, A. Improving poly-3-hydroxybutyrate production in Escherichia coli by combining the increase in the NADPH pool and acetyl-CoA availability. Antonie Leeuwenhoek 2014, 105, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Willbold, E.; Kalla, K.; Bartsch, I.; Bobe, K.; Brauneis, M.; Remennik, S.; Schechtman, D.; Nellesen, J.; Tillmann, W.; Vogt, C.; et al. Biocompatibility of rapidly solidified magnesium alloy RS66 as a temporary biodegradable metal. Acta Biomater. 2013, 9, 8509–8517. [Google Scholar] [CrossRef] [PubMed]
- Matena, J.; Petersen, S.; Gieseke, M.; Teske, M.; Beyerbach, M.; Kampmann, A.; Murua Escobar, H.; Gellrich, N.-C.; Haferkamp, H.; Nolte, I. Comparison of Selective Laser Melted Titanium and Magnesium Scaffolds Coated with PCL. Int. J. Mol. Sci. 2015, 16, 13287–13301. [Google Scholar] [CrossRef] [PubMed]
- Matena, J.; Petersen, S.; Gieseke, M.; Kampmann, A.; Teske, M.; Beyerbach, M.; Murua Escobar, H.; Haferkamp, H.; Gellrich, N.-C.; Nolte, I. SLM Produced Porous Titanium Scaffold Improvements for Enhanced Vascularization and Osteoblast Seeding. Int. J. Mol. Sci. 2015, 16, 7478–7492. [Google Scholar] [CrossRef] [PubMed]
- Vandrovcova, M.; Bacakova, L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone scaffolds. Physiol. Res. 2011, 60, 403–417. [Google Scholar] [PubMed]
- Aliuos, P.; Sen, A.; Reich, U.; Dempwolf, W.; Warnecke, A.; Hadler, C.; Lenarz, T.; Menzel, H.; Reuter, G. Inhibition of fibroblast adhesion by covalently immobilized protein repellent polymer coatings studied by single cell force spectroscopy. J. Biomed. Mater. Res. A 2014, 102, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wulf, K.; Teske, M.; Lobler, M.; Luderer, F.; Schmitz, K.P.; Sternberg, K. Surface functionalization of poly(ε-caprolactone) improves its biocompatibility as scaffold material for bioartificial vessel prostheses. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sancaktar, E. Polymer adhesion by ultrasonic welding. J. Adhes. Sci. Technol. 1999, 13, 179–201. [Google Scholar] [CrossRef]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Additively Manufactured 3D Porous Ti-6Al-4V Constructs Mimic Trabecular Bone Structure and Regulate Osteoblast Proliferation, Differentiation and Local Factor Production in a Porosity and Surface Roughness Dependent Manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef] [PubMed]
- Mour, M.; Das, D.; Winkler, T.; Hoenig, E.; Mielke, G.; Morlock, M.M.; Schilling, A.F. Advances in Porous Biomaterials for Dental and Orthopaedic Applications. Materials 2010, 3, 2947–2974. [Google Scholar] [CrossRef]
- Yulianto, W.; Andarwulan, N.; Giriwono, P.E.; Pamungkas, J. HPLC-based metabolomics to identify cytotoxic compounds from Plectranthus amboinicus (Lour.) Spreng against human breast cancer MCF-7 Cells. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1039, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ullh, F.; Zeb, A.; Ayaz, M.; Ullah, F.; Sadiq, A. Evaluation of Rumex hastatus D. Don for cytotoxic potential against HeLa and NIH/3T3 cell lines: Chemical characterization of chloroform fraction and identification of bioactive compounds. BMC Complement. Altern. Med. 2016, 16, 308. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Manakhov, A.; Zajickova, L.; Thomas, S. Structural and Surface Compatibility Study of Modified Electrospun Poly(ε-caprolactone) (PCL) Composites for Skin Tissue Engineering. AAPS PharmSciTech 2017, 18, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.H.; Moffatt, S.; Horton, M. Cell adhesion molecules in human osteoblasts: Structure and function. Histol. Histopathol. 2001, 16, 603–611. [Google Scholar] [PubMed]
- Brynda, E.; Pachernik, J.; Houska, M.; Pientka, Z.; Dvorak, P. Surface immobilized protein multilayers for cell seeding. Langmuir 2005, 21, 7877–7883. [Google Scholar] [CrossRef] [PubMed]
- Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO 10993–5:2009–10; International Organization of Standardization: Genève, Switzerland, 2009.
- Impurities: Guideline for Residual Solvents Q3C(R6); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Genève, Switzerland, 2016.



| Surface Type | Attachment Time | |
|---|---|---|
| 180 s | 5 s | |
| uncoated | 12 | 7 |
| PCL-coated | 13 | 8 |
| P(3HB)-coated | 13 | 8 |
| Uncoated Porous Titanium Scaffolds | PCL Coated Porous Titanium Scaffolds | P(3HB) Coated Porous Titanium Scaffolds |
|---|---|---|
| 230.8 ± 42.7 µm | 217.7 ± 46.4 µm | 88.7 ± 84.6 µm |
| Scaffold Modification | At-% Ti | At-% C |
|---|---|---|
| Uncoated | 93.74 | 6.26 |
| PCL coated | 3.90 | 96.10 |
| P(3HB) coated | 21.34 | 78.66 |
| Position | External Area | Internal Area |
|---|---|---|
| Upper layer | 4.6 ± 2.0 µm | 1.5 ± 0.9 µm |
| Median layer | 4.1 ± 1.8 µm | 4.8 ± 2.8 µm |
| Lower layer | 4.4 ± 1.6 µm | 4.5 ± 3.0 µm |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grau, M.; Matena, J.; Teske, M.; Petersen, S.; Aliuos, P.; Roland, L.; Grabow, N.; Murua Escobar, H.; Gellrich, N.-C.; Haferkamp, H.; et al. In Vitro Evaluation of PCL and P(3HB) as Coating Materials for Selective Laser Melted Porous Titanium Implants. Materials 2017, 10, 1344. https://doi.org/10.3390/ma10121344
Grau M, Matena J, Teske M, Petersen S, Aliuos P, Roland L, Grabow N, Murua Escobar H, Gellrich N-C, Haferkamp H, et al. In Vitro Evaluation of PCL and P(3HB) as Coating Materials for Selective Laser Melted Porous Titanium Implants. Materials. 2017; 10(12):1344. https://doi.org/10.3390/ma10121344
Chicago/Turabian StyleGrau, Michael, Julia Matena, Michael Teske, Svea Petersen, Pooyan Aliuos, Laura Roland, Niels Grabow, Hugo Murua Escobar, Nils-Claudius Gellrich, Heinz Haferkamp, and et al. 2017. "In Vitro Evaluation of PCL and P(3HB) as Coating Materials for Selective Laser Melted Porous Titanium Implants" Materials 10, no. 12: 1344. https://doi.org/10.3390/ma10121344




