Low-Temperature, Chemically Grown Titanium Oxide Thin Films with a High Hole Tunneling Rate for Si Solar Cells
Abstract
:1. Introduction
2. Experimental
- (1)
- an environment that was temperature controlled at an accuracy of ±0.1 °C;
- (2)
- a Teflon vessel containing the deposition solution.
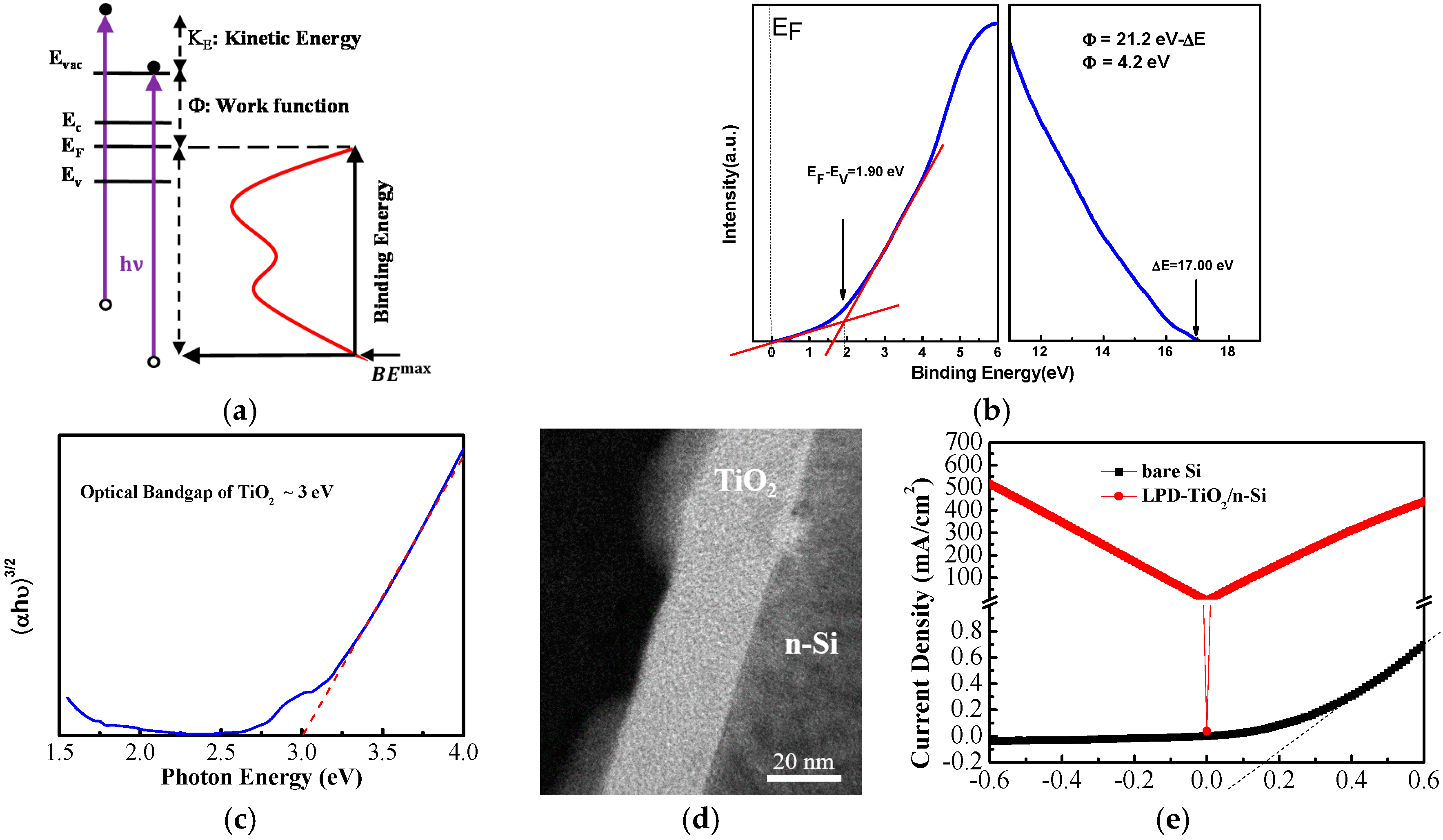
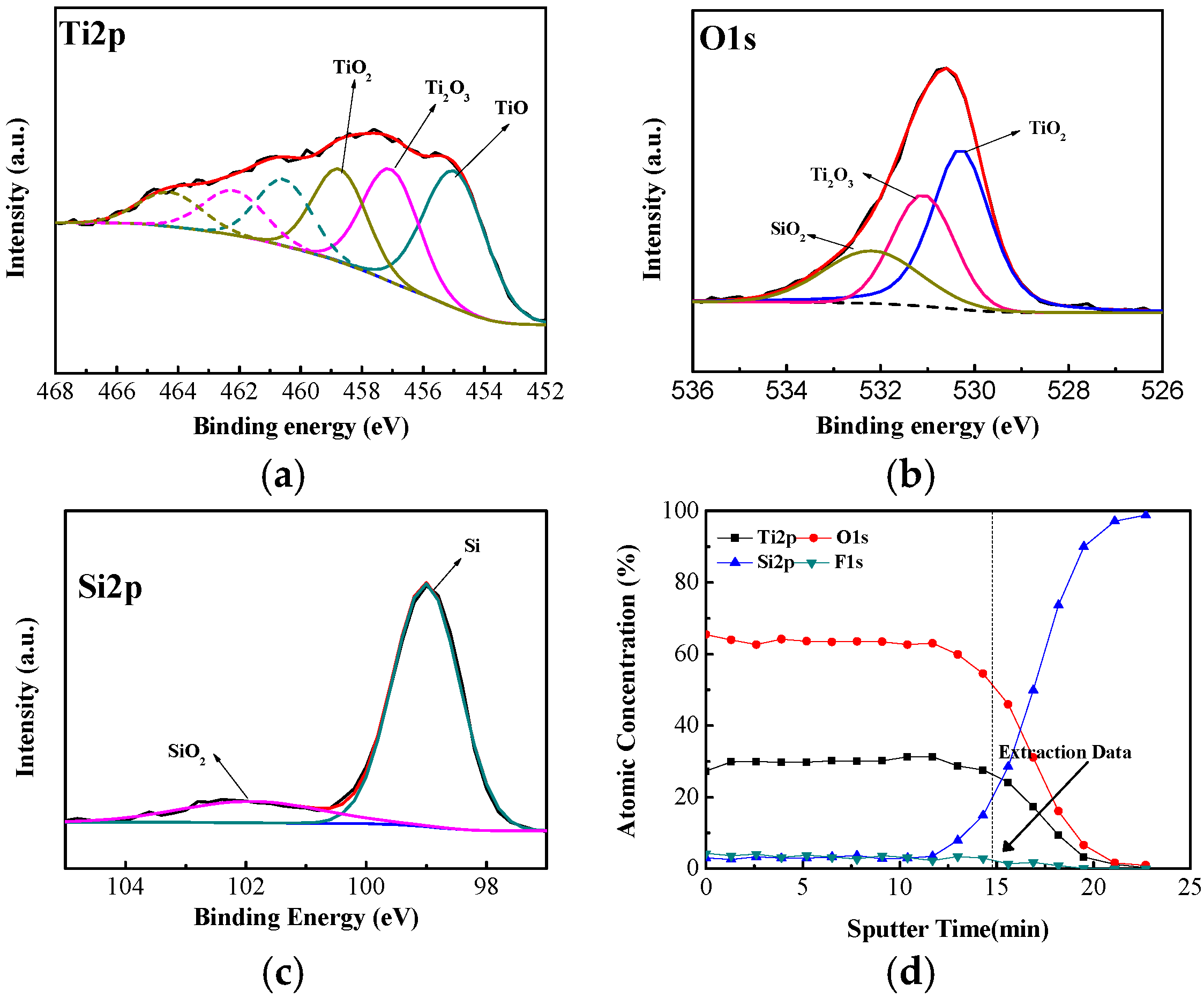
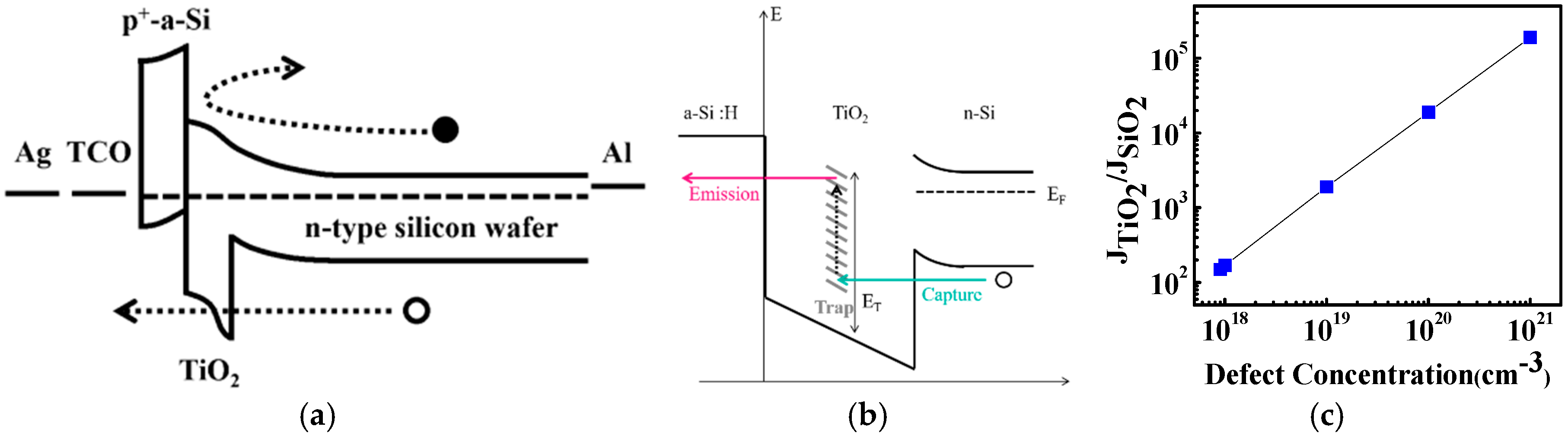
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kang, H.; Kim, G.; Hwang, I.W.; Kim, Y.; Lee, K.C.; Park, S.H.; Lee, K. High-performance polymer tandem devices combining solar cell and light-emitting diode. Sol. Energy Mater. Sol. Cells 2012, 107, 148–153. [Google Scholar] [CrossRef]
- Morteani, A.C.; Dhoot, A.S.; Kim, J.S.; Silva, C.; Greenham, N.C.; Murphy, C.; Moons, E.; Cina, S.; Burroughes, J.H.; Friend, R.H. Barrier-free electron-hole capture in polymer blend heterojunction light-emitting diodes. Adv. Mater. 2003, 15, 1708–1712. [Google Scholar] [CrossRef]
- Chen, C.P.; Ke, M.Y.; Liu, C.C.; Chang, Y.J. Observation of 394 nm electroluminescence from low-temperature sputtered n-ZnO/SiO2 thin films on top of the p-GaN heterostructure. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef]
- You, J.B.; Zhang, X.W.; Zhang, S.G.; Wang, J.X.; Yin, Z.G.; Tan, H.R.; Zhang, W.J.; Chu, P.K.; Cui, B.; Wowchak, A.M.; et al. Improved electroluminescence from n-ZnO/AlN/p-GaN heterojunction light-emitting diodes. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Liang, H.K.; Yu, S.F.; Yang, H.Y. Directional and controllable edge-emitting ZnO ultraviolet random laser diodes. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Zhu, H.; Shan, C.X.; Yao, B.; Li, B.H.; Zhang, J.Y.; Zhang, Z.Z.; Zhao, D.X.; Shen, D.Z.; Fan, X.W.; Lu, Y.M.; et al. Ultralow-threshold laser realized in zinc oxide. Adv. Mater. 2009, 21, 1613–1617. [Google Scholar] [CrossRef]
- Wang, D.Y.; Jiang, Y.T.; Lin, C.C.; Li, S.S.; Wang, Y.T.; Chen, C.C.; Chen, C.W. Solution-processable pyrite fes2 nanocrystals for the fabrication of heterojunction photodiodes with visible to NIR photodetection. Adv. Mater. 2012, 24, 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Yu, M.; Lian, J.; Sawyer, S. Heterojunction photodiode fabricated from hydrogen treated ZnO nanowires grown on p-silicon substrate. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Yu, M.; Lian, J.; Sawyer, S. Heterojunction photodiode fabricated from multiwalled carbon nanotube/ZnO nanowire/p-silicon composite structure. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Wolf, S.D.; Descoeudres, A.; Holman, Z.C.; Ballif, C. High-efficiency silicon heterojunction solar cells: A review. Green 2012, 2, 7–24. [Google Scholar] [CrossRef]
- Pudasaini, P.R.; Zepeda, F.R.; Sharma, M.; Elam, D.; Ponce, A.; Ayon, A.A. High Efficiency Hybrid Silicon Nanopillar-Polymer Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 9620–9627. [Google Scholar] [CrossRef] [PubMed]
- Pudasaini, P.R.; Elam, D.; Ayon, A.A. Aluminum oxide passivated radial junction sub-micrometre pillar array textured silicon solar cells. J. Phys. D Appl. Phys. 2013, 46. [Google Scholar] [CrossRef]
- Pudasaini, P.R.; Sharma, M.; Zepeda, F.R.; Ayon, A.A. Efficiency improvement of a nanostructured polymer solar cell employing atomic layer deposited Al2O3 as a passivation layer. Microelectron. Eng. 2014, 119, 6–10. [Google Scholar] [CrossRef]
- Sheng, J.; Fan, K.; Wang, D.; Han, C.; Fang, J.; Gao, P.; Ye, J. Improvement of the SiOx passivation layer for high-efficiency Si/PEDOT:PSS heterojunction solar cells. Appl. Mater. Interfaces 2014, 6, 16027–16034. [Google Scholar] [CrossRef] [PubMed]
- Neamen, D.A. Semiconductor Physics and Devices, 4th ed.; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Avasthi, S.; McClain, W.E.; Man, G.; Kahn, A.; Schwartz, J.; Sturm, J.C. Hole-blocking titanium-oxide/silicon heterojunction and its application to photovoltaics. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Salim, T.; Yin, Z.; Sun, S.; Huang, X.; Zhang, H.; Lam, Y.M. Solution-processed nanocrystalline TiO2 buffer layer used for improving the performance of organic photovoltaics. ACS Appl. Mater. Interfaces 2011, 3, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Richards, B.S.; Cotter, J.E.; Honsberg, C.B. Enhancing the surface passivation of TiO2 coated silicon wafers. Appl. Phys. Lett. 2002, 80. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, H.C.; Hsu, C.M. High dielectric constant TiO2 film grown on polysilicon by liquid phase deposition. Mater. Sci. Semicond. Process. 2007, 10, 61–67. [Google Scholar] [CrossRef]
- Li, C.Y.; Wen, T.C.; Lee, T.H.; Guo, T.F.; Huang, J.C.A.; Lind, Y.C.; Hsu, Y.J. An inverted polymer photovoltaic cell with increased air stability obtained by employing novel hole/electron collecting layers. J. Mater. Chem. 2009, 19, 1643–1647. [Google Scholar] [CrossRef]
- Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Energy level alignment and interfacial electronic structures at organic/metal and organic/organic interfaces. Adv. Mater. 1999, 11, 605–625. [Google Scholar] [CrossRef]
- Fang, Q.; Meier, M.; Yu, J.J.; Wang, Z.M.; Zhang, J.Y.; Wu, J.X.; Kenyon, A.; Hoffmann, P.; Boyd, I.W. FTIR and XPS investigation of Er-doped SiO2–TiO2 films. Mater. Sci. Eng. B 2003, 105, 209–213. [Google Scholar] [CrossRef]
- Anandan, C.; Bera, P. XPS studies on the interaction of CeO2 with silicon in magnetron sputtered CeO2 thin films on Si and Si3N4 substrates. Appl. Surf. Sci. 2013, 283, 297–303. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Liu, Y.; Chen, T. Evolution of Si suboxides into Si nanocrystals during rapid thermal annealing as revealed by XPS and Raman studies. J. Cryst. Growth 2009, 311, 1296–1301. [Google Scholar] [CrossRef]
- Lee, W.C.; Hu, C. Modeling CMOS tunneling currents through ultrathin gate oxide due to conduction- and valence-band electron and hole tunneling. IEEE Trans. Electron Devices 2001, 48, 1366–1373. [Google Scholar]
- Yu, S.; Guan, X.; Wong, H.-S.P. Conduction mechanism of TiN/HfOx/Pt resistive switching memory: A trap-assisted-tunneling model. Appl. Phys. Lett. 2011, 99. [Google Scholar] [CrossRef]
- Entner, R.; Gehring, A.; Kosina, H.; Grasser, T.; Selberherr, S. Impact of multi-trap assisted tunneling on gate leakage of CMOS memory devices. NSTI Nanotech 2005, 3, 45–48. [Google Scholar]
- Vandelli, L.; Padovani, A.; Larcher, L.; Southwick, R.G.; Knowlton, W.B.; Bersuker, G. A Physical model of the temperature dependence of the current through SiO2/HfO2 stacks. IEEE Trans. Electron Devices 2011, 58, 2878–2887. [Google Scholar] [CrossRef]
- Gehring, A.; Selberherr, S. Modeling of Tunneling Current and Gate Dielectric Reliability for Nonvolatile Memory Devices. IEEE Trans. Electron Devices 2004, 4, 306–319. [Google Scholar] [CrossRef]
- Coenen, T.; Van de Groep, J.; Polman, A. Resonant modes of single silicon nanocavities excited by electron irradiation. ACS Nano 2013, 7, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, P.; Macco, B.; Verschuuren, M.A.; Kessels, W.M.M.; Polman, A. Al2O3/TiO2 nano-pattern antireflection coating with ultralow surface recombination. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Cui, Y.; Fan, S. Light management for photovoltaics using high-index nanostructures. Nat. Mater. 2014, 13, 451–460. [Google Scholar] [CrossRef] [PubMed]
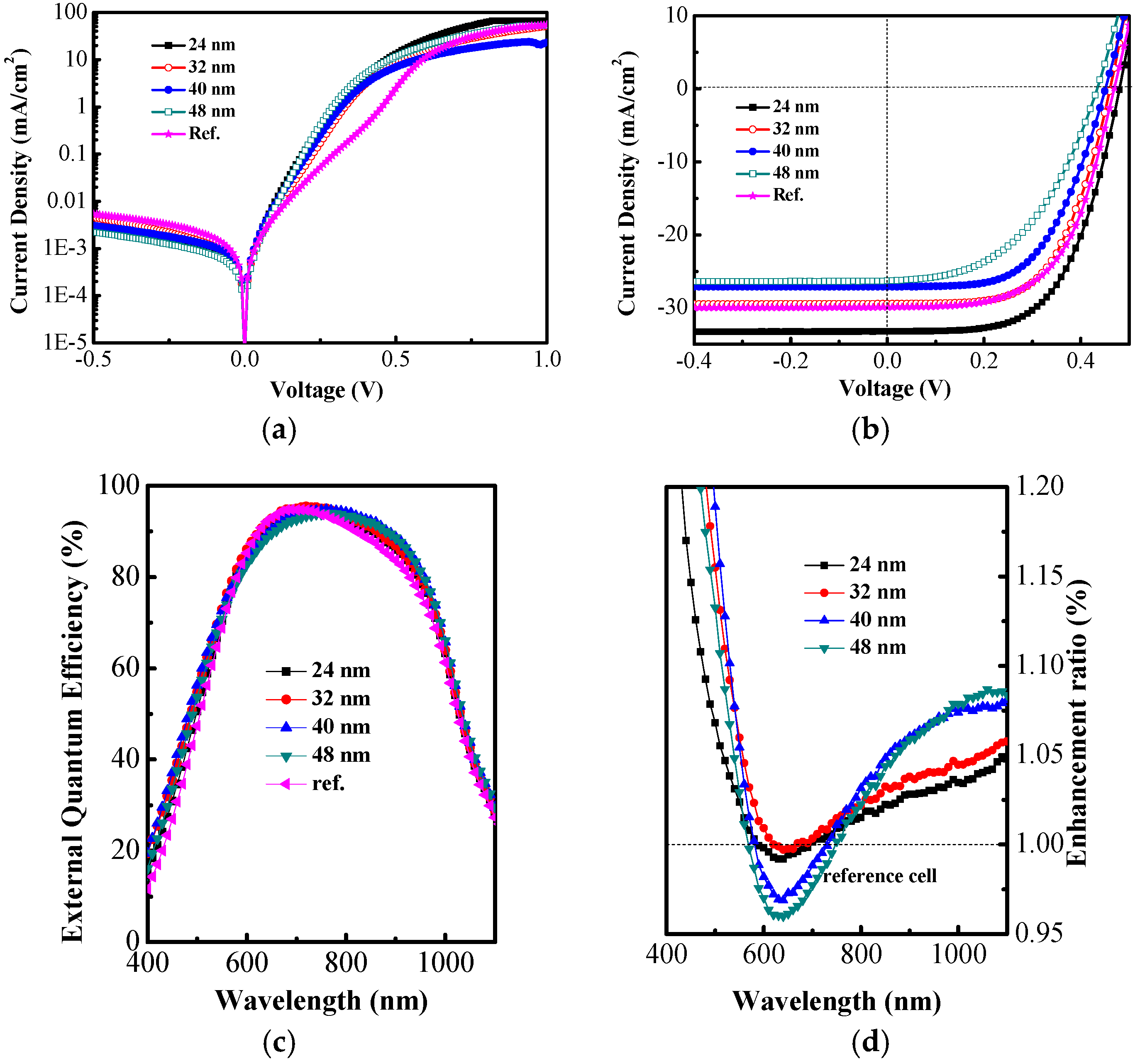
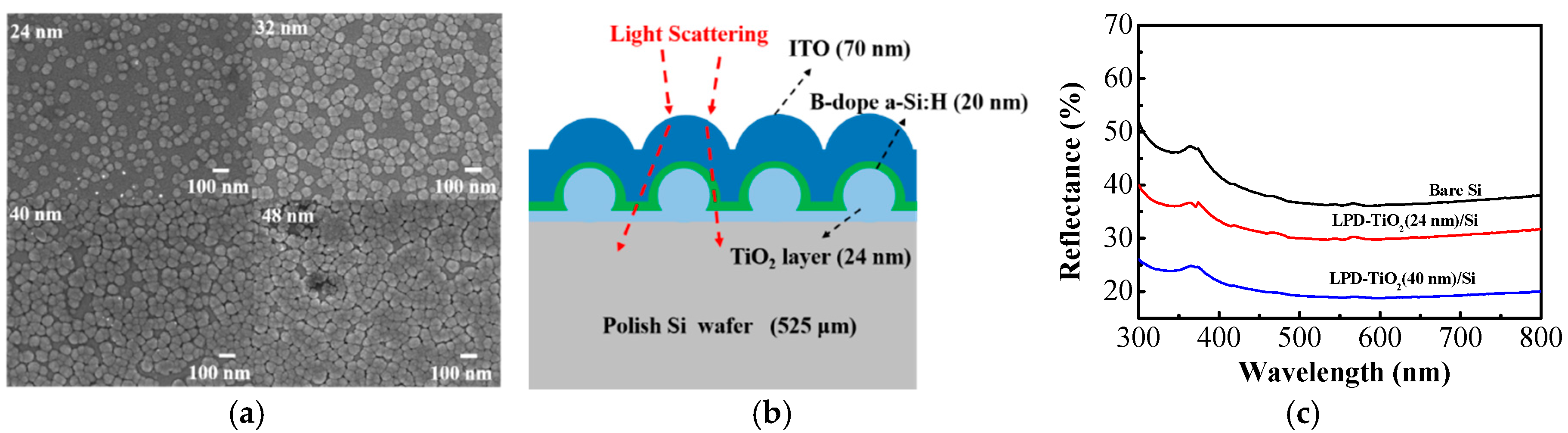
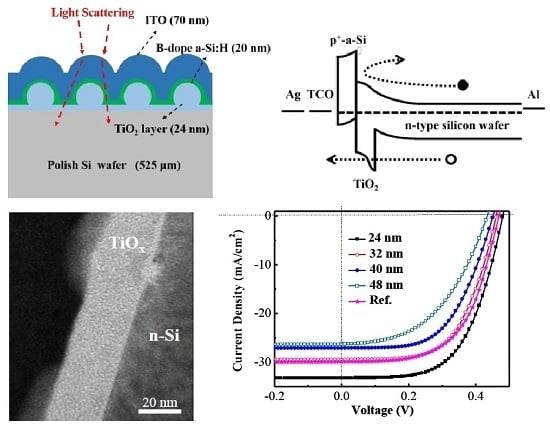
| Parameter | Ref. a | 24 nm | 32 nm | 40 nm | 48 nm |
|---|---|---|---|---|---|
| Voc (V) | 0.47 | 0.49 | 0.47 | 0.46 | 0.44 |
| Jsc (mA/cm2) | 29.9 | 33.2 | 29.5 | 27.1 | 26.3 |
| Jsc (EQE) (mA/cm2) | 30.5 | 31.77 | 31.88 | 31.42 | 30.59 |
| Fill Factor | 0.586 | 0.579 | 0.583 | 0.556 | 0.477 |
| Efficiency (%) | 8.23 | 9.42 | 8.09 | 6.93 | 5.52 |
| Efficiency EQE (%) | 8.40 | 9.01 | 8.73 | 7.98 | 6.42 |
| Rs (Ω-cm2) | 3.8 | 3 | 3.5 | 4.44 | 5.35 |
| Rsh (Ω-cm2) | 1855 | 1875 | 1727 | 11,875 | 5369 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-T.; Lin, F.-R.; Lin, T.-C.; Chen, C.-H.; Pei, Z. Low-Temperature, Chemically Grown Titanium Oxide Thin Films with a High Hole Tunneling Rate for Si Solar Cells. Energies 2016, 9, 402. https://doi.org/10.3390/en9060402
Lee Y-T, Lin F-R, Lin T-C, Chen C-H, Pei Z. Low-Temperature, Chemically Grown Titanium Oxide Thin Films with a High Hole Tunneling Rate for Si Solar Cells. Energies. 2016; 9(6):402. https://doi.org/10.3390/en9060402
Chicago/Turabian StyleLee, Yu-Tsu, Fang-Ru Lin, Ting-Chun Lin, Chien-Hsun Chen, and Zingway Pei. 2016. "Low-Temperature, Chemically Grown Titanium Oxide Thin Films with a High Hole Tunneling Rate for Si Solar Cells" Energies 9, no. 6: 402. https://doi.org/10.3390/en9060402