One-Dimensional TiO2 Nanostructured Photoanodes: From Dye-Sensitised Solar Cells to Perovskite Solar Cells
Abstract
:1. Introduction
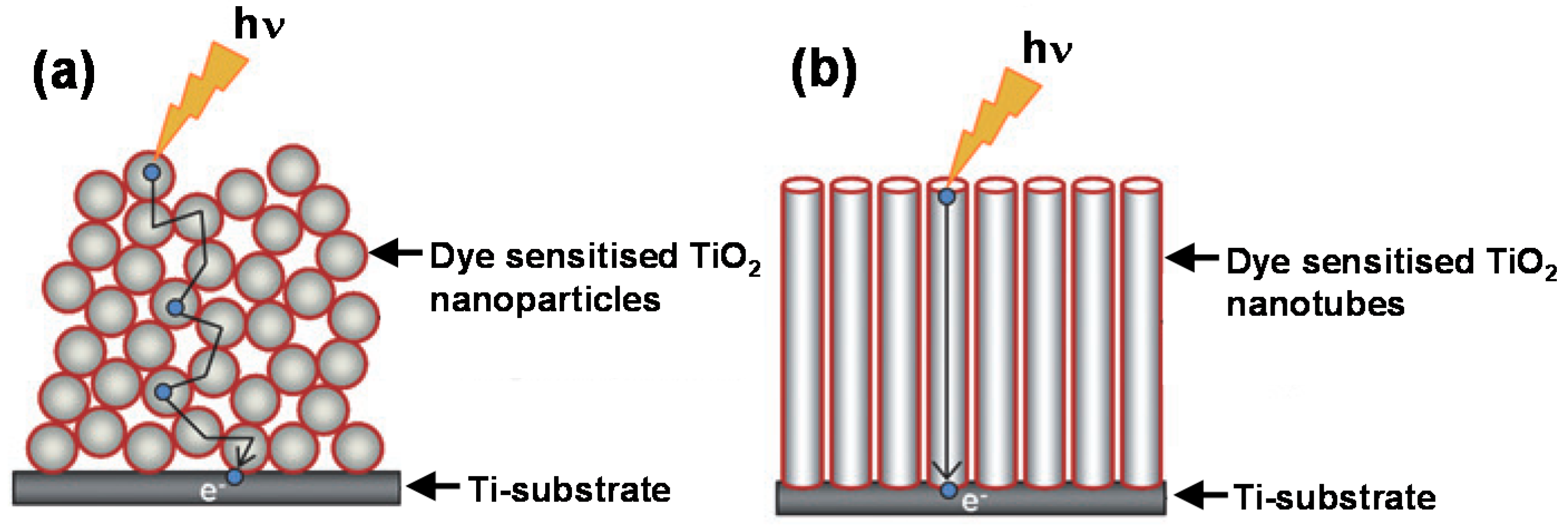
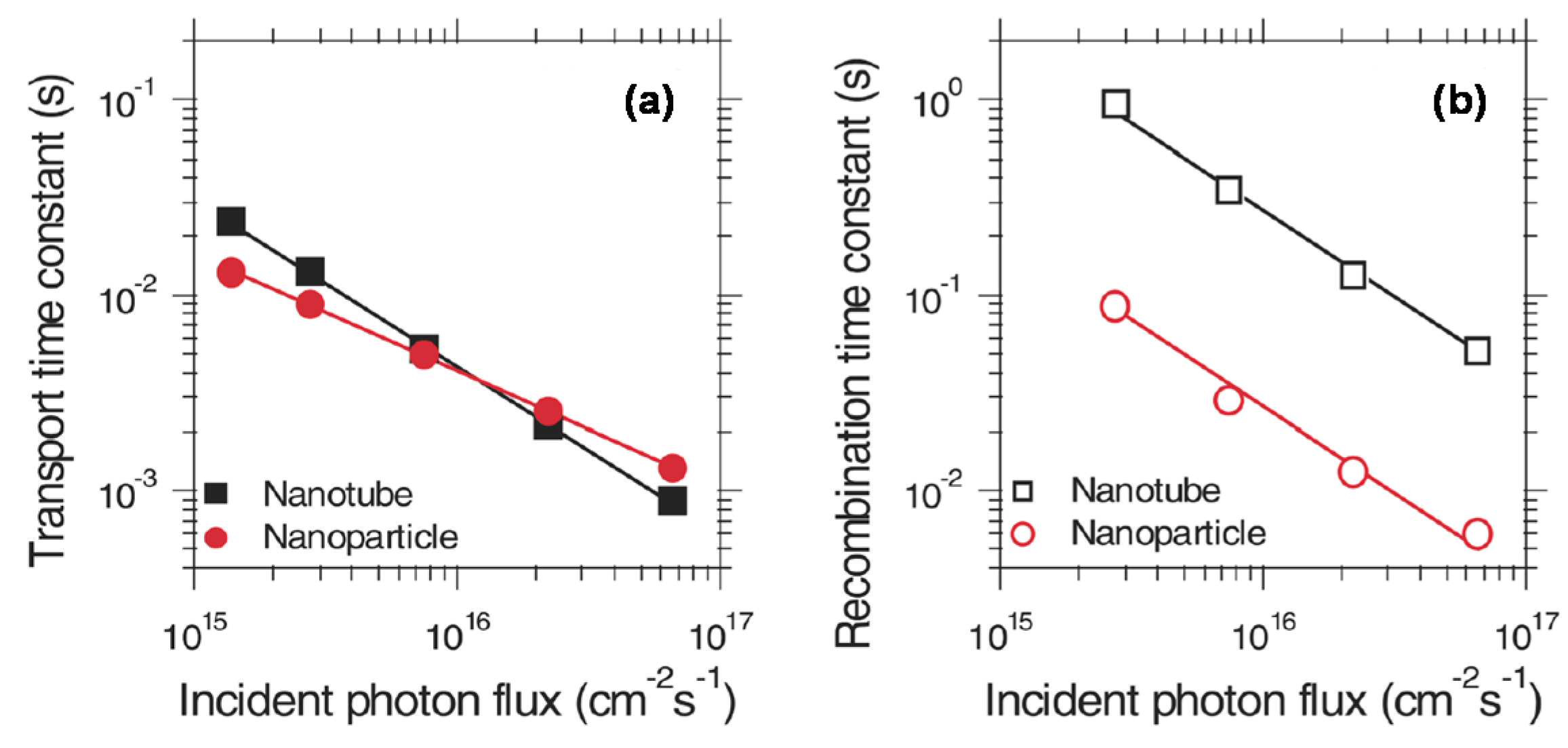
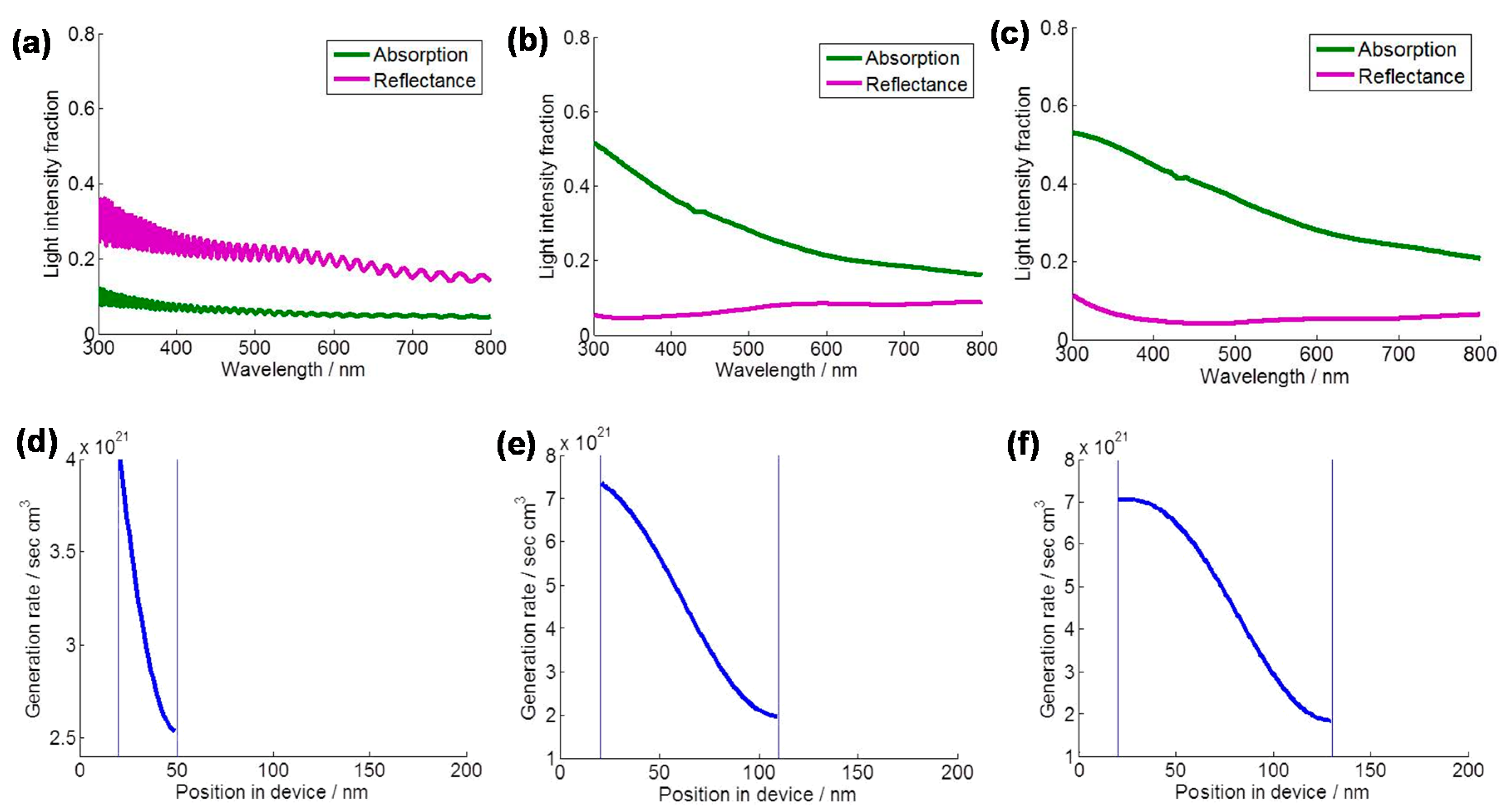
2. Novel Charge Transport and Optical Properties of 1D TiO2 Nanostructured Materials
3. Synthesis of 1D TiO2 Nanostructured Materials
3.1. Hydrothermal Method
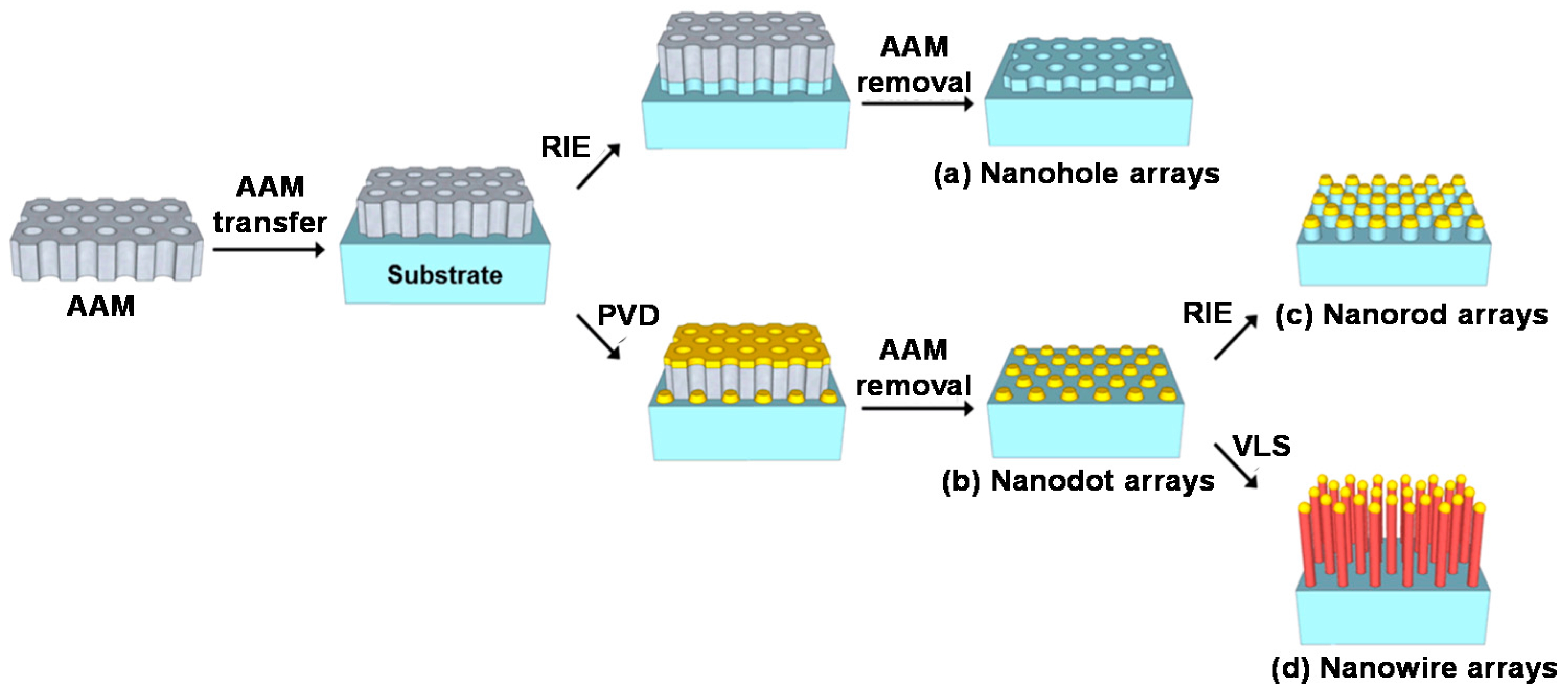
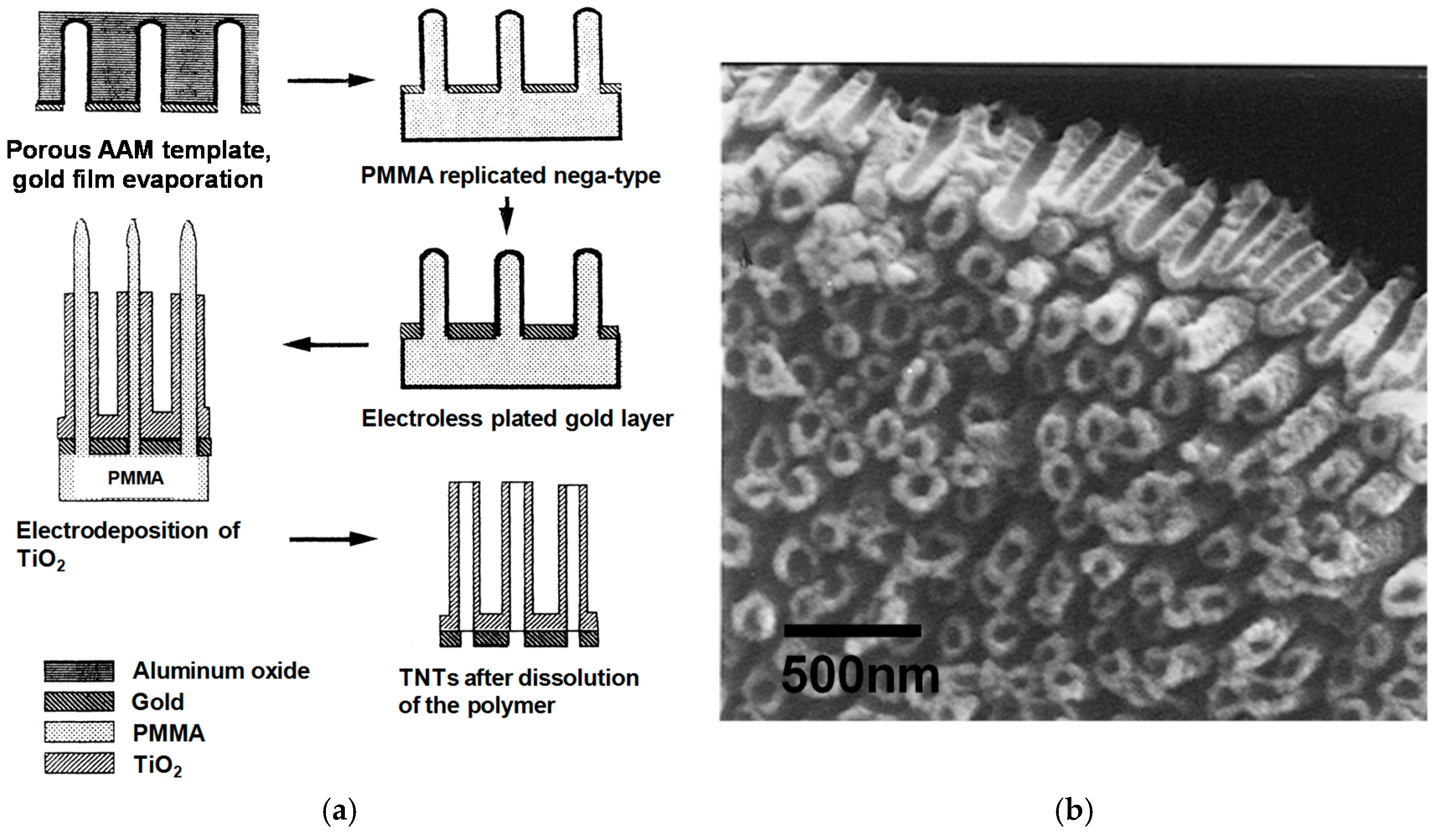
3.2. Template-Assisted Method
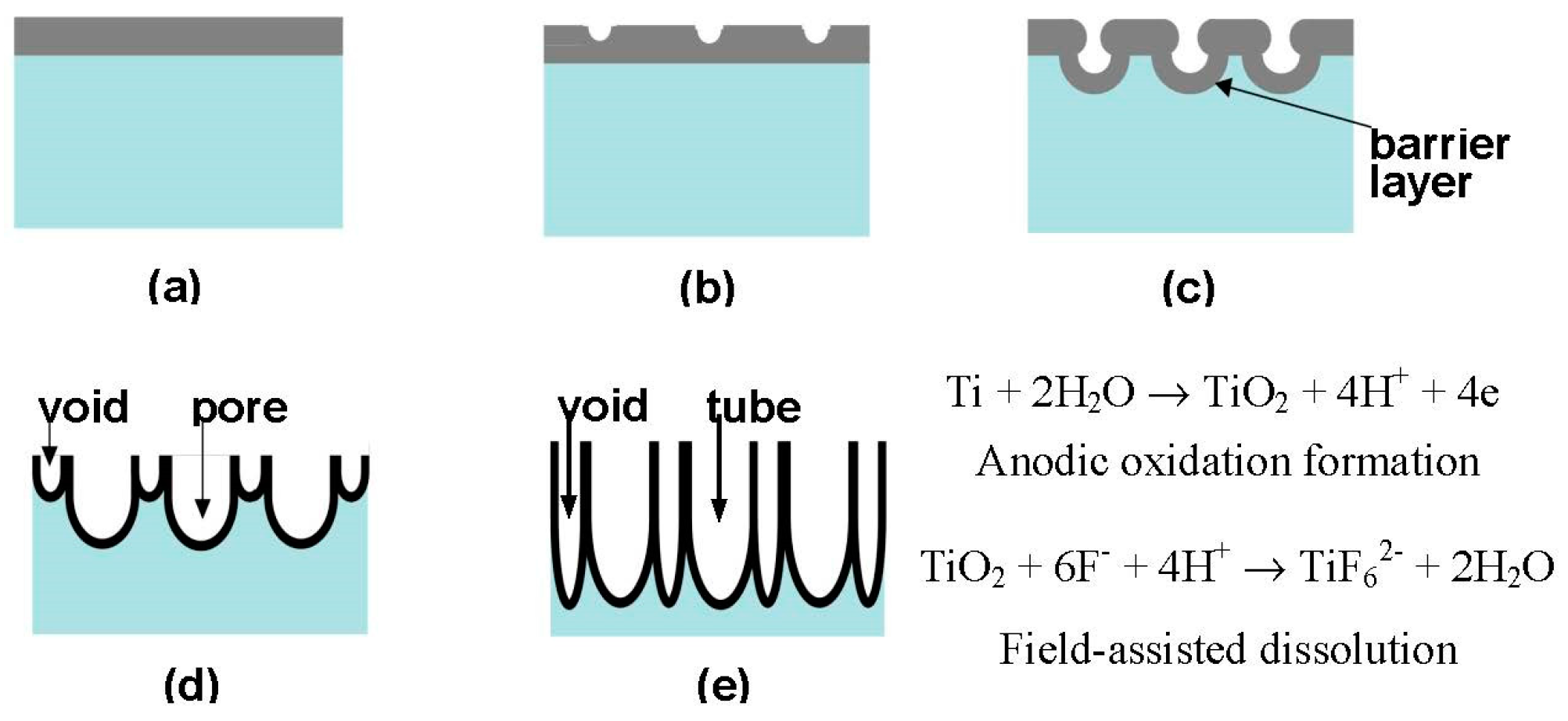
3.3. Electrochemical Anodisation Method
4. 1D TiO2 Nanostructure-Based Solar Cell Applications: DSCs and PSCs
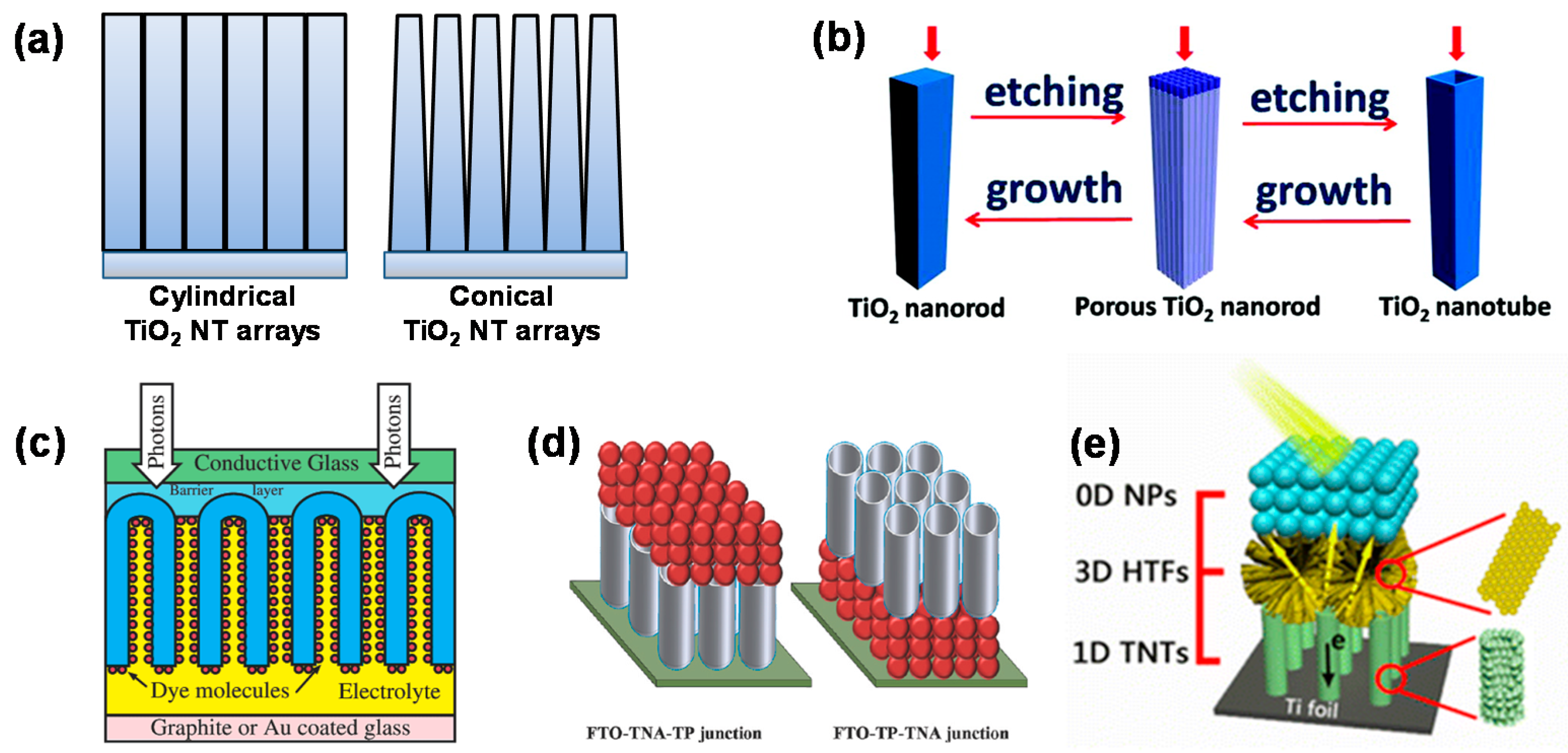
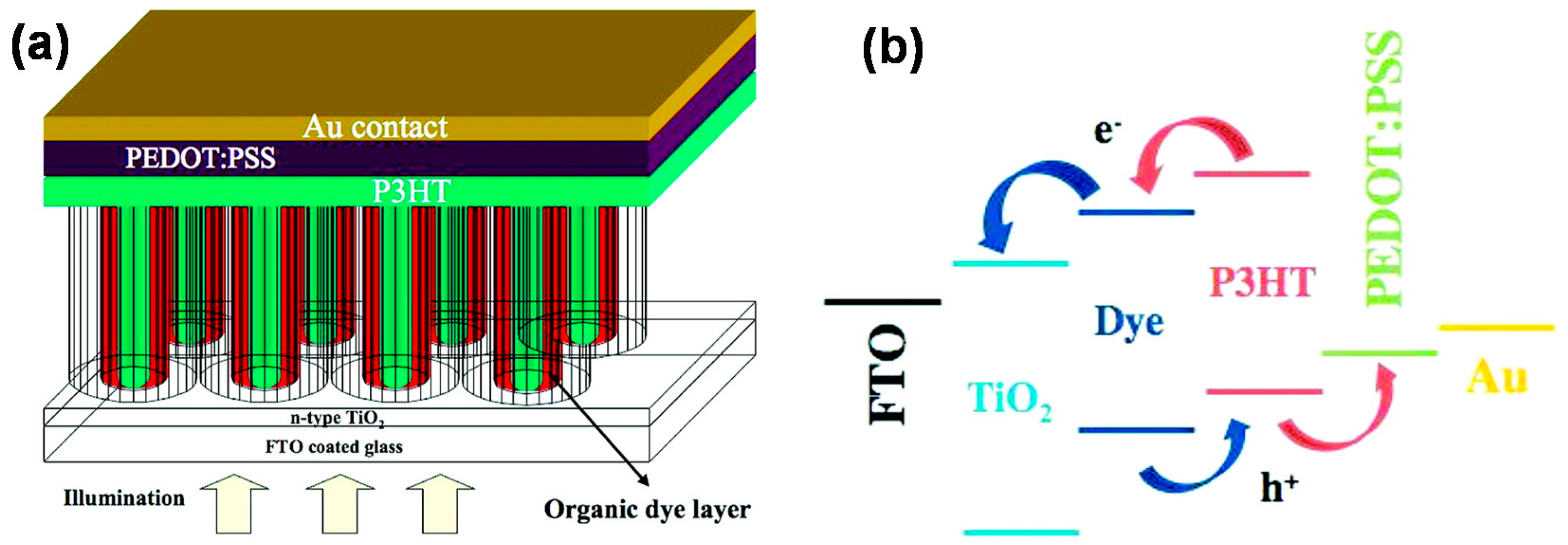
4.1. 1D TiO2 Nanostructure-Based DSCs
4.2. 1D TiO2 Nanostructure-Based PSCs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lewis, N.S.; Crabtree, G. Basic Research Needs for Solar Energy Utilization: Report of the Basic Energy Sciences Workshop on Solar Energy Utilization; US Department of Energy, Office of Basic Energy Science: Washington, DC, USA, 2005.
- Matsumura, M.; Matsudaira, S.; Tsubomura, H.; Takata, M.; Yanagida, H. Dye sensitization and surface structures of semiconductor electrodes. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 415–421. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- National Center for Photovoltaics (NCPV). Research Cell Efficiency Record Efficieny Chart. Available online: http://www.nrel.gov/pv/ (accessed on 20 October 2016).
- Jung, H.S.; Park, N.-G. Perovskite solar cells: From materials to devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.H.; Ikeda, S.; Harada, T.; Higashida, S.; Sakata, T.; Mori, H.; Matsumura, M. Fabrication of hollow carbon nanospheres encapsulating platinum nanoparticles using a photocatalytic reaction. Adv. Mater. 2007, 19, 597–601. [Google Scholar] [CrossRef]
- Ng, Y.H.; Ikeda, S.; Harada, T.; Park, S.; Sakata, T.; Mori, H.; Matsumura, M. Photocatalytic route for synthesis of hollow porous carbon/Pt nanocomposites with controllable density and porosity. Chem. Mater. 2008, 20, 1154–1160. [Google Scholar] [CrossRef]
- Ng, Y.H.; Lightcap, I.V.; Goodwin, K.; Matsumura, M.; Kamat, P.V. To what extent do graphene scaffolds improve the photovoltaic and photocatalytic response of TiO2 nanostructured films? J. Phys. Chem. Lett. 2010, 1, 2222–2227. [Google Scholar] [CrossRef]
- Bell, N.J.; Ng, Y.H.; Du, A.; Coster, H.; Smith, S.C.; Amal, R. Understanding the enhancement in photoelectrochemical properties of photocatalytically prepared TiO2-reduced graphene oxide composite. J. Phys. Chem. C 2011, 115, 6004–6009. [Google Scholar] [CrossRef]
- Jing, L.; Tan, H.L.; Amal, R.; Ng, Y.H.; Sun, K.-N. Polyurethane sponge facilitating highly dispersed TiO2 nanoparticles on reduced graphene oxide sheets for enhanced photoelectro-oxidation of ethanol. J. Mater. Chem. A 2015, 3, 15675–15682. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, K.; Frank, A.J.; Grimes, C.A.; Mallouk, T.E. Rapid charge transport in dye-sensitized solar cells made from vertically aligned single-crystal rutile TiO2 nanowires. Angew. Chem. 2012, 124, 2781–2784. [Google Scholar] [CrossRef]
- Yun, J.-H.; Ng, Y.H.; Ye, C.; Mozer, A.J.; Wallace, G.G.; Amal, R. Sodium fluoride-assisted modulation of anodized TiO2 nanotube for dye-sensitized solar cells application. ACS Appl. Mater. Interfaces 2011, 3, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.P.; Zhang, Q.; Cao, G. Effects of dye loading conditions on the energy conversion efficiency of zno and TiO2 dye-sensitized solar cells. J. Phys. Chem. C 2007, 111, 18804–18811. [Google Scholar] [CrossRef]
- Guo, M.Y.; Fung, M.K.; Fang, F.; Chen, X.Y.; Ng, A.M.C.; Djurišić, A.B.; Chan, W.K. ZnO and TiO2 1D nanostructures for photocatalytic applications. J. Alloy. Compd. 2011, 509, 1328–1332. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-dimensional titanium dioxide nanomaterials: Nanowires, nanorods, and nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S. An affordable green energy source—Evolving through current developments of organic, dye sensitized, and perovskite solar cells. Int. J. Green Energy 2016, 13, 859–906. [Google Scholar] [CrossRef]
- Roy, P.; Kim, D.; Lee, K.; Spiecker, E.; Schmuki, P. TiO2 nanotubes and their application in dye-sensitized solar cells. Nanoscale 2009, 2, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Kopidakis, N.; Schiff, E.A.; Park, N.G.; van de Lagemaat, J.; Frank, A.J. Ambipolar diffusion of photocarriers in electrolyte-filled, nanoporous TiO2. J. Phys. Chem. B 2000, 104, 3930–3936. [Google Scholar] [CrossRef]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Choi, S.H.; Kang, M.S.; Kim, J.Y.; Kim, H.S.; Hyeon, T.; Sung, Y.E. Nanorod-based dye-sensitized solar cells with improved charge collection efficiency. Adv. Mater. 2008, 20, 54–58. [Google Scholar] [CrossRef]
- Nakayama, K.; Kubo, T.; Nishikitani, Y. TiO2 nanotube layers on Ti substrates for high efficiency flexible dye-sensitized solar cells. Appl. Phys. Exp. 2008, 1. [Google Scholar] [CrossRef]
- Yun, J.-H.; Kim, I.K.; Ng, Y.H.; Wang, L.; Amal, R. Optical modeling-assisted characterization of dye-sensitized solar cells using TiO2 nanotube arrays as photoanodes. Beilstein J. Nanotechnol. 2014, 5, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Tétreault, N.; Horváth, E.; Moehl, T.; Brillet, J.; Smajda, R.; Bungener, S.; Cai, N.; Wang, P.; Zakeeruddin, S.M.; Forró, L.; et al. High-efficiency solid-state dye-sensitized solar cells: Fast charge extraction through self-assembled 3D fibrous network of crystalline TiO2 nanowires. ACS Nano 2010, 4, 7644–7650. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.-E.; Kang, S.H.; Kim, J.-Y. Nanotube-and nanorod-based dye-sensitized solar cells. In Energy Efficiency and Renewable Energy through Nanotechnology; Springer: Berlin, Germany, 2011; pp. 317–350. [Google Scholar]
- Matthews, A. The crystallization of anatase and rutile from amorphous titanium dioxide under hydrothermal conditions. Am. Mineral. 1976, 61, 419–424. [Google Scholar]
- Izumi, F. The polymorphic crystallization of titanium (IV) oxide under hydro-thermal conditions. II. The roles of inorganic anions in the nucleation of rutile and anatase from acid solutions. Bull. Chem. Soc. Jpn. 1978, 51, 1771–1776. [Google Scholar] [CrossRef]
- Lan, Y.; Gao, X.P.; Zhu, H.Y.; Zheng, Z.F.; Yan, T.Y.; Wu, F.; Ringer, S.P.; Song, D.Y. Titanate nanotubes and nanorods prepared from rutile powder. Adv. Funct. Mater. 2005, 15, 1310–1318. [Google Scholar] [CrossRef]
- Cho, I.S.; Chen, Z.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X. Branched TiO2 nanorods for photoelectrochemical hydrogen production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wen, Z.; Li, J. Solvent-controlled synthesis and electrochemical lithium storage of one-dimensional TiO2 nanostructures. Inorg. Chem. 2006, 45, 6944–6949. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Q.; Rao, H.-S.; Xu, Y.-F.; Wang, Y.-F.; Su, C.-Y.; Kuang, D.-B. Hierarchical oriented anatase TiO2 nanostructure arrays on flexible substrate for efficient dye-sensitized solar cells. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Park, S.-J. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, P. Formation of a titanium dioxide nanotube array. Langmuir 1996, 12, 1411–1413. [Google Scholar] [CrossRef]
- Yodyingyong, S.; Zhou, X.; Zhang, Q.; Triampo, D.; Xi, J.; Park, K.; Limketkai, B.; Cao, G. Enhanced photovoltaic performance of nanostructured hybrid solar cell using highly oriented TiO2 nanotubes. J. Phys. Chem. C 2010, 114, 21851–21855. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Huang, H.; Li, H.; Han, X.; Liu, Y.; Kang, Z. Carbon quantum dot sensitized TiO2 nanotube arrays for photoelectrochemical hydrogen generation under visible light. Nanoscale 2013, 5, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Yun, J.-H. Anodic Formation of Ordered TiO2 Nanotube Arrays for Solar Energy Conversion Applications; The University of New South Wales: Sydney, Australia, 2012. [Google Scholar]
- Wang, D.; Yu, B.; Wang, C.; Zhou, F.; Liu, W. A novel protocol toward perfect alignment of anodized TiO2 nanotubes. Adv. Mater. 2009, 21, 1964–1967. [Google Scholar] [CrossRef]
- Yun, J.-H.; Ng, Y.H.; Huang, S.; Conibeer, G.; Amal, R. Wrapping the walls of n-TiO2 nanotubes with p-CuInS2 nanoparticles using pulsed-electrodeposition for improved heterojunction photoelectrodes. Chem. Commun. 2011, 47, 11288–11290. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Wong, R.J.; Ng, Y.H.; Du, A.; Amal, R. Combined electrophoretic deposition–anodization method to fabricate reduced graphene oxide–TiO2 nanotube films. RSC Adv. 2012, 2, 8164–8171. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.-H.; Ng, Y.H.; Wong, R.J.; Amal, R. Reduced graphene oxide: Control of water miscibility, conductivity, and defects by photocatalysis. ChemCatChem 2013, 5, 3060–3067. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Lindström, H.; Södergren, S.; Lindquist, S.-E. Photoelectrochemical studies of colloidal TiO2 films: The effect of oxygen studied by photocurrent transients. J. Electroanal. Chem. 1995, 381, 39–46. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (x = Cl−, Br−, I−, CN−, and SCN−) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Masaki, N.; Kitamura, T.; Wada, Y.; Okamoto, T.; Sekino, T.; Niihara, K.; Yanagida, S. Dye-sensitized TiO2 nanotube solar cells: Fabrication and electronic characterization. Phys. Chem. Chem. Phys. 2005, 7, 4157–4163. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Gopal, K.M.; Haripriya, E.P.; Sorachon, Y.; Maggie, P.; Oomman, K.V.; Craig, A.G. Highly-ordered TiO2 nanotube arrays up to 220 μm in length: Use in water photoelectrolysis and dye-sensitized solar cells. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- So, S.; Kriesch, A.; Peschel, U.; Schmuki, P. Conical-shaped titania nanotubes for optimized light management in dsscs reach back-side illumination efficiencies >8%. J. Mater. Chem. A 2015, 3, 12603–12608. [Google Scholar] [CrossRef]
- Lv, M.; Zheng, D.; Ye, M.; Xiao, J.; Guo, W.; Lai, Y.; Sun, L.; Lin, C.; Zuo, J. Optimized porous rutile TiO2 nanorod arrays for enhancing the efficiency of dye-sensitized solar cells. Energy Environ. Sci. 2013, 6, 1615–1622. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Kang, H.; Yun, J.; Lee, J.; Park, J.H.; Baik, S. Hierarchical construction of self-standing anodized titania nanotube arrays and nanoparticles for efficient and cost-effective front-illuminated dye-sensitized solar cells. ACS Nano 2011, 5, 5088–5093. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Q.; Xu, Y.-F.; Rao, H.-S.; Su, C.-Y.; Kuang, D.-B. Trilayered photoanode of TiO2 nanoparticles on a 1d–3d nanostructured TiO2-grown flexible ti substrate for high-efficiency (9.1%) dye-sensitized solar cells with unprecedentedly high photocurrent density. J. Phys. Chem. C 2014, 118, 16426–16432. [Google Scholar] [CrossRef]
- Guo, W.; Xu, C.; Wang, X.; Wang, S.; Pan, C.; Lin, C.; Wang, Z.L. Rectangular bunched rutile TiO2 nanorod arrays grown on carbon fiber for dye-sensitized solar cells. J. Am. Chem. Soc. 2012, 134, 4437–4441. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Swain, B.S.; Amassian, A. Highly efficient hybrid photovoltaics based on hyperbranched three-dimensional TiO2 electron transporting materials. Adv. Mater. 2015, 27, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Bach, U.; Lupo, D.; Comte, P.; Moser, J.E.; Weissortel, F.; Salbeck, J.; Spreitzer, H.; Gratzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 1998, 395, 583–585. [Google Scholar]
- Snaith, H.J.; Moule, A.J.; Klein, C.; Meerholz, K.; Friend, R.H.; Grätzel, M. Efficiency enhancements in solid-state hybrid solar cells via reduced charge recombination and increased light capture. Nano Lett. 2007, 7, 3372–3376. [Google Scholar] [CrossRef] [PubMed]
- Ding, I.K.; Tétreault, N.; Brillet, J.; Hardin, B.E.; Smith, E.H.; Rosenthal, S.J.; Sauvage, F.; Grätzel, M.; McGehee, M.D. Pore-filling of Spiro-OMeTAD in solid-state dye sensitized solar cells: Quantification, mechanism, and consequences for device performance. Adv. Funct. Mater. 2009, 19, 2431–2436. [Google Scholar] [CrossRef]
- Mor, G.K.; Kim, S.; Paulose, M.; Varghese, O.K.; Shankar, K.; Basham, J.; Grimes, C.A. Visible to near-infrared light harvesting in TiO2 nanotube array–P3HT based heterojunction solar cells. Nano Lett. 2009, 9, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Roh, D.K.; Chi, W.S.; Jeon, H.; Kim, S.J.; Kim, J.H. High efficiency solid-state dye-sensitized solar cells assembled with hierarchical anatase pine tree-like TiO2 nanotubes. Adv. Funct. Mater. 2014, 24, 379–386. [Google Scholar] [CrossRef]
- Yang, K.H.; Chen, C.C. Alumina template assistance in titania nanotubes dye-sensitized solar cell (TiO2 NT-DSSC) device fabrication. ISRN Nanotechnol. 2012, 2012, 132797. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Xu, Y.-F.; Su, C.-Y.; Kuang, D.-B. Ultra-long anatase TiO2 nanowire arrays with multi-layered configuration on fto glass for high-efficiency dye-sensitized solar cells. Energy Environ. Sci. 2014, 7, 644–649. [Google Scholar] [CrossRef]
- Maggie, P.; Karthik, S.; Oomman, K.V.; Gopal, K.M.; Brian, H.; Craig, A.G. Backside illuminated dye-sensitized solar cells based on titania nanotube array electrodes. Nanotechnology 2006, 17. [Google Scholar] [CrossRef]
- Lin, C.-J.; Yu, W.-Y.; Chien, S.-H. Transparent electrodes of ordered opened-end TiO2-nanotube arrays for highly efficient dye-sensitized solar cells. J. Mater. Chem. 2010, 20, 1073–1077. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, H.-S.; Shin, H.; Jung, H.S.; Yoo, P.J.; Park, J.H.; Jung, D.-Y.; Park, N.-G. A sharp focus on perovskite solar cells at Sungkyun International Solar Forum (SISF). ACS Energy Lett. 2016, 1, 500–502. [Google Scholar] [CrossRef]
- Gonzalez-Pedro, V.; Juarez-Perez, E.J.; Arsyad, W.-S.; Barea, E.M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J. General working principles of CH3NH3PbX3 perovskite solar cells. Nano Lett. 2014, 14, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Ponseca, C.S.; Savenije, T.J.; Abdellah, M.; Zheng, K.; Yartsev, A.; Pascher, T.; Harlang, T.; Chabera, P.; Pullerits, T.; Stepanov, A.; et al. Organometal halide perovskite solar cell materials rationalized: Ultrafast charge generation, high and microsecond-long balanced mobilities, and slow recombination. J. Am. Chem. Soc. 2014, 136, 5189–5192. [Google Scholar] [CrossRef] [PubMed]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, J.-W.; Yantara, N.; Boix, P.P.; Kulkarni, S.A.; Mhaisalkar, S.; Grätzel, M.; Park, N.-G. High efficiency solid-state sensitized solar cell-based on submicrometer rutile TiO2 nanorod and CH3NH3PbI3 perovskite sensitizer. Nano Lett. 2013, 13, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Q.; Huang, F.; Chen, D.; Cheng, Y.-B.; Caruso, R.A. Solvent-mediated dimension tuning of semiconducting oxide nanostructures as efficient charge extraction thin films for perovskite solar cells with efficiency exceeding 16%. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
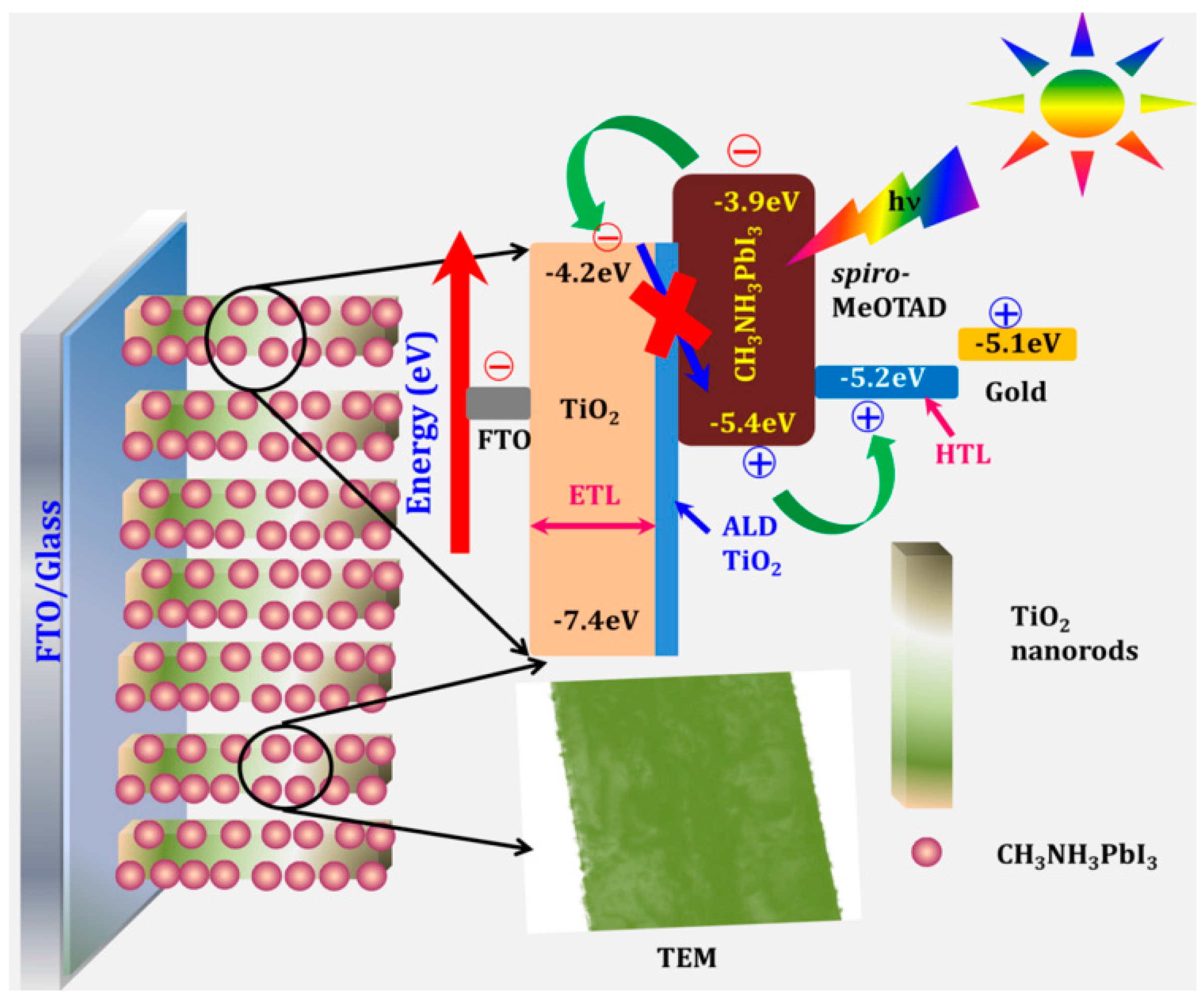
- Mali, S.S.; Shim, C.S.; Park, H.K.; Heo, J.; Patil, P.S.; Hong, C.K. Ultrathin atomic layer deposited TiO2 for surface passivation of hydrothermally grown 1D TiO2 nanorod arrays for efficient solid-state perovskite solar cells. Chem. Mater. 2015, 27, 1541–1551. [Google Scholar] [CrossRef]
- Li, X.; Dai, S.-M.; Zhu, P.; Deng, L.-L.; Xie, S.-Y.; Cui, Q.; Chen, H.; Wang, N.; Lin, H. Efficient perovskite solar cells depending on TiO2 nanorod arrays. ACS Appl. Mater. Interfaces 2016, 8, 21358–21365. [Google Scholar] [CrossRef] [PubMed]






| TiO2 Type; Fabrication Method 1 | Architecture 2 | η | Jsc | Voc | FF | Dye Type; Dye Concentration | Reference |
|---|---|---|---|---|---|---|---|
| % | mA·cm−2 | V | % | nmol·cm−2 | |||
| DSCs with 1D TiO2 Nanostructured Photoanodes | |||||||
| Layered NTs; HT | FI; 10 μm thick NT particulate layer on FTO | 7.10 | 13.3 | 0.79 | 0.68 | N719; 150 | [46] |
| Layered and Hyper-branched NRs; HT/ES | FI; 29 μm thick 4 layered Hyperbranched NRs on FTO | 11.22 | 18.60 | 0.84 | 0.69 | N719; 240 | [54] |
| Vertical NT arrays; SG + AAO Template | FI; 25 μm thick NT-AAO arrays on ITO | 2.70 | 5.5 | 0.72 | 0.69 | N3 | [60] |
| Vertical NW arrays; HT | FI; 47 μm thick NW arrays on FTO | 9.40 | 18.25 | 0.79 | 0.65 | N719; 160 | [61] |
| Vertical NT arrays; Anodisation | BI; 6 μm thick NT arrays on Ti foil | 4.24 | 8.79 | 0.84 | 0.57 | N719 | [62] |
| Vertical and Conical shaped NT arrays; Anodisation | BI; 13 μm thick NT arrays on Ti foil | 8.09 | 15.63 | 0.79 | 0.66 | N719; 144 | [48] |
| Vertical NT arrays; Anodisation | FI; 63 μm thick NT arrays on FTO | 9.10 | 18.50 | 0.77 | 0.64 | N719; 152 | [63] |
| Vertical and Porous rutile NR arrays; HT | FI; 30 μm thick NR arrays on FTO | 7.91 | 20.49 | 0.71 | 0.54 | N719; 133 | [49] |
| Vertical and Hierarchical NT arrays; Anodisation/SG | FI; 17 μm thick; FTO/double 0D NP/1D NT | 8.80 | 18.89 | 0.75 | 0.62 | N719 | [51] |
| FTO/ 1D NT/double 0D NP | 6.03 | 13.07 | 0.75 | 0.62 | |||
| Vertical and Hierarchical NT arrays; HT/SG | BI; 28 μm thick; 0D NP/3D Microsphere/1D NT/Ti foil | 9.10 | 17.90 | 0.74 | 0.69 | N719; 148 | [52] |
| SSDSCs with 1D TiO2 Nanostructured Photoanodes | |||||||
| Vertical NT arrays; Anodisation | FI; FTO/600–700 nm thick NT-dyes/P3HT/PEDOT:PSS/Au | 3.2 | 10.75 | 0.55 | 0.55 | SQ-1 | [58] |
| Vertical and Hierarchical NT arrays; HT | FI; FTO/19 μm thick Hierarchical NT arrays-dyes/PEBII/Pt | 8.00 | 17.70 | 0.74 | 0.62 | N719; 106 | [59] |
| TiO2 Type/Fabrication Method 1 | Architecture 2 | η (%) | Jsc (mA·cm−2) | Voc (V) | FF (%) | References |
|---|---|---|---|---|---|---|
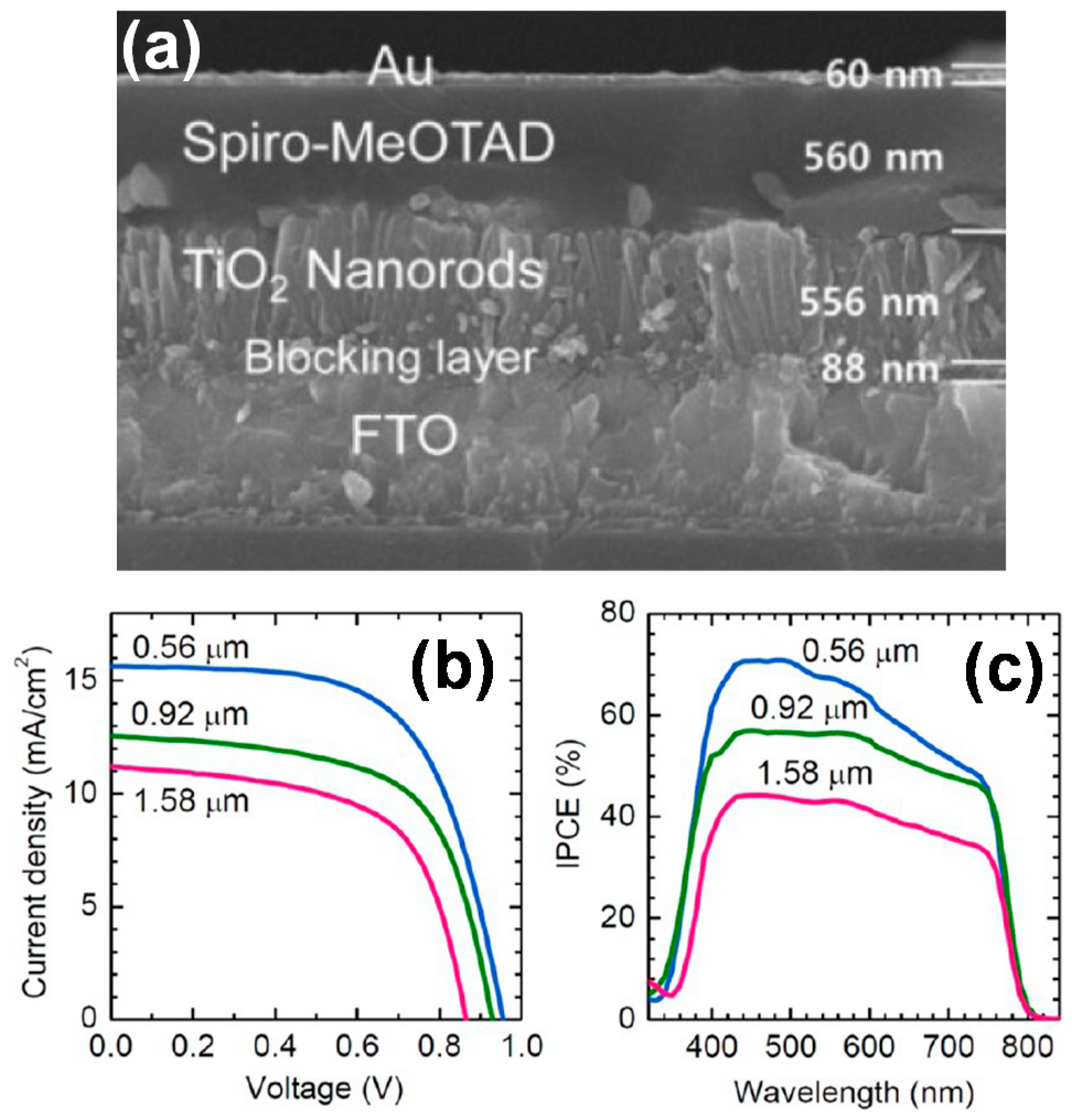
| Vertical and Rutile NR arrays; HT | FTO/NR-MAPbI3/Spiro-MeOTAD/Au | [69] | ||||
| 0.56 μm thick NR | 9.4 | 15.6 | 0.96 | 0.63 | ||
| 0.92 μm thick NR | 7.3 | 12.6 | 0.93 | 0.62 | ||
| 1.58 μm thick NR | 5.9 | 11.2 | 0.87 | 0.61 | ||
| Vertical NW arrays; ST | FTO/0.15–0.20 μm thick NW-MAPbI3/Spiro-MeOTAD/Au | 16.04 | 22.60 | 1.01 | 0.71 | [70] |
| 0.03–0.05 μm thick NP | 11.61 | 21.83 | 0.90 | 0.59 | ||
| 0.07–0.10 μm thick NS | 13.09 | 21.26 | 0.95 | 0.65 | ||
| Layered Hyper-branched NR arrays; HT/ES | FTO/1 layered Hyperbranched NR-MAPbI3/Spiro-MeOTAD/Au | [54] | ||||
| 0.60 μm thick NR | 15.50 | 20.5 | 1.00 | 0.75 | ||
| 0.85 μm thick NR | 6.85 | 11.0 | 0.77 | 0.75 | ||
| 1.23 μm thick NR | 5.88 | 9.5 | 0.75 | 0.74 | ||
| Vertical and Anatase NR arrays; HT/ALD | FTO/1.8 μm thick NR-MAPbI3/Spiro-MeOTAD/Au | [71] | ||||
| TiCl4-treated NR arrays | 7.98 | 16.16 | 0.85 | 0.58 | ||
| ALD-treated NR arrays (4.8 nm thick) | 13.45 | 19.78 | 0.94 | 0.72 | ||
| Vertical NR arrays; ST/UV-O3 | FTO/0.18 μm thick NR-MAPbI3/Spiro-MeOTAD/Au | [72] | ||||
| UV-O3 treatment | 18.22 | 22.09 | 1.04 | 0.76 | ||
| No treatment | 13.80 | 20.10 | 1.02 | 0.67 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.-H.; Wang, L.; Amal, R.; Ng, Y.H. One-Dimensional TiO2 Nanostructured Photoanodes: From Dye-Sensitised Solar Cells to Perovskite Solar Cells. Energies 2016, 9, 1030. https://doi.org/10.3390/en9121030
Yun J-H, Wang L, Amal R, Ng YH. One-Dimensional TiO2 Nanostructured Photoanodes: From Dye-Sensitised Solar Cells to Perovskite Solar Cells. Energies. 2016; 9(12):1030. https://doi.org/10.3390/en9121030
Chicago/Turabian StyleYun, Jung-Ho, Lianzhou Wang, Rose Amal, and Yun Hau Ng. 2016. "One-Dimensional TiO2 Nanostructured Photoanodes: From Dye-Sensitised Solar Cells to Perovskite Solar Cells" Energies 9, no. 12: 1030. https://doi.org/10.3390/en9121030





