Abstract
Hydrothermal liquefaction (HTL) presents a viable route for converting a vast range of materials into liquid fuel, without the need for pre-drying. Currently, HTL studies produce bio-crude with properties that fall short of diesel or biodiesel standards. Upgrading bio-crude improves the physical and chemical properties to produce a fuel corresponding to diesel or biodiesel. Properties such as viscosity, density, heating value, oxygen, nitrogen and sulphur content, and chemical composition can be modified towards meeting fuel standards using strategies such as solvent extraction, distillation, hydrodeoxygenation and catalytic cracking. This article presents a review of the upgrading technologies available, and how they might be used to make HTL bio-crude into a transportation fuel that meets current fuel property standards.
1. Introduction
In the first quarter of 2015, global atmospheric carbon dioxide levels have reached a record high of 400 parts per million (ppm) [1]. In order to restrict global temperature rise to 2 °C, greenhouse gas (GHG) emissions should be maintained in the range of 445–490 ppm CO2-equivalent [2]. This target requires a reduction in GHG emissions from energy production by shifting from a reliance on fossil fuels to renewable energy sources such as biomass and biofuels. With a climate change mitigation strategy that maintains CO2 levels at 450 ppm, it is projected the share of biofuels in the energy mix will rise to up to 11% by 2030 [3].
Biomass is used as a sustainable solid fuel largely for cooking and heating [3]. In recent years, however, energy from biomass has taken a different form. From 1990 to 2008, the use of liquid fuels from biomass increased at an average of 12.1% annually, taking biofuels’ share in the global transport fuel mix to 2% in 2008 [3]. Furthermore, the United Nations Sustainable Energy for All Strategy aims to “double the share of renewable energy in the global energy mix” by 2030 [4]. This demonstrates a need to develop highly-productive and cost-effective biofuel technologies not only to meet the growing energy demand, but also to support climate mitigation strategies. These imperatives provide motivation to make fuels from biomass viable for widespread use.
Converting biomass from its natural solid form to liquid fuels is not a spontaneous process. The liquid fuels that humans have harnessed on a large scale as fossil fuels took thousands of years of geochemical processing to convert biomass to crude oil and gas. Unprocessed biomass, however, has lower energy density, higher moisture content, and its physical form is not homogeneous and free-flowing [5] making it a problem as a feedstock for reciprocating engines. These issues have been partially addressed by a number of processing technologies. For example, the controlled burning of wood in the absence of air to produce charcoal results in a solid fuel with lower moisture content and a higher energy density than wood [5]. However, since charcoal is still a solid, it cannot be used in modern transportation applications.
In the 1940s, Berl [6] noted that the high conversion and thermal efficiencies for converting carbohydrate-containing materials into liquid fuel justified further research with a view to overcoming declining oil reserves.
In addition to addressing climate change and energy security, it can be expected that wide use of biofuels may bring about benefits towards improving overall health. Sulphur dioxide, among other pollutants, are significantly lower when biodiesel is used instead of conventional diesel [4]. Moreover, reduction of air pollution from fossil fuels is projected to cause a decline in mortalities and health care costs quantified in the range of US$ 1.9–4.6 per gigajoule [7].
As of 2010, world biofuel production has been largely focused on first-generation fuels producing ethanol and biodiesel from starch, sugars, and vegetable oils. Advanced biofuels or biofuels produced from lignocellulosic materials such as wood waste and straw made up only 0.2% of total biofuel production [8]. In recent years, research on using biomass for liquid fuels has been robust, ranging from studies of pyrolysis and hydrothermal liquefaction of lignocellulosic materials, gasification and biomass-to-liquid technologies, to upgrading processes.
Several technologies have been employed to harness the energy content of biomass and make it more available for a variety of uses [9]. Of these, thermochemical processes are of significant importance due to their ability to transform biomass into fluids, increase heating value, and enable easier handling, distribution and storage. Pyrolysis, initially developed to produce chemicals such as methanol, acetic acid and acetone from wood [5] has been widely researched and developed to an industrial-scale process to produce oils from biomass. Among different pyrolysis processes, fast pyrolysis has been determined to maximise liquid products [10]. However, fast pyrolysis is limited by its requirements for low moisture content feedstocks, rapid heating and quenching rate, and high temperatures [11]. Hydrothermal liquefaction (HTL) or solvolysis, on the other hand, is preferred over pyrolysis for processing feedstock with significant moisture content because the process does not consume energy in the removal of water, either through pre-drying or in-process evaporation. Moreover, the reaction of these substances with water or other hydrogen donor solvents facilitates separation of the oily product stream from the more polar by-product stream [12].
Hydrothermal liquefaction (HTL) produces liquid bio-crude through treatment of biomass at high pressures of 50–200 atm and high temperatures of 250–400 °C [13]. HTL exploits the properties of superheated fluids to reduce mass transfer resistances [12]. The high pressure also enables higher penetration of the solvent into the biomass structure to facilitate fragmentation of biomass molecules [14]. The nature of the process allows for feedstock with high moisture content, therefore a wide range of material can be subjected to HTL to produce bio-crude. Studies liquefying wood [15,16,17,18], forest residues [19,20,21], agricultural residues [14,16,17,20,22,23], municipal wastes [24,25], sewage sludge [26,27], manure [28,29], and algae [30,31,32,33,34,35] have been published.
The choice of feedstock is contingent on many factors such as availability [36] and ease of transportation; however from a processing perspective, it is important to know the composition of the material. Lignocellulosic materials such as wood, forest and agricultural residues contain varying levels of cellulose, hemicellulose and lignin [12]. Under HTL, these complex biopolymers break into a complex mixture of chemicals consisting mostly of carbon, hydrogen and oxygen [13]. Municipal wastes and sewage sludge contain a significant amount of nitrogen due to the protein content derived from human wastes [25,27]. Algae contain carbohydrates, lipids and proteins [31,35], which break down to various organic chemicals, some of which contain nitrogen from deamination of amino acids from proteins [12].
HTL bio-crudes are semi-liquid [6], viscous, dark-coloured and have a smoke-like smell [35]. The typical viscosity of bio-crudes is 10–10,000 times higher than that of diesel and biodiesel [16,21,25,30,32,35]. Moreover, heating values are not comparable with conventional fuels and biodiesel. These properties make HTL bio-crude difficult to use as transportation fuels, apart from marine applications. Nabi et al. [37] blended wood powder HTL bio-crude with conventional diesel fuel and studied fuel properties, emissions, and engine performance. The investigation concluded that the blended fuel doesn’t cause significant changes in engine performance. It was observed that particulate matter mass (PM) and particulate number (PN) were lower while total unburnt hydrocarbon (UHC) and nitric oxide emissions were higher. While this study demonstrated the feasibility of directly using HTL bio-crudes by blending with diesel, the blend was still predominantly fossil fuel. Therefore, there is an opportunity to maximise the benefits of using a totally-renewable fuel by improving the properties of the HTL bio-crude through upgrading.
Upgrading refers to processing oils in order to improve their physical and chemical properties to values given in existing fuel standards. As shown in Figure 1, upgrading processes follow HTL, with a general objective to produce fuel with standard properties.
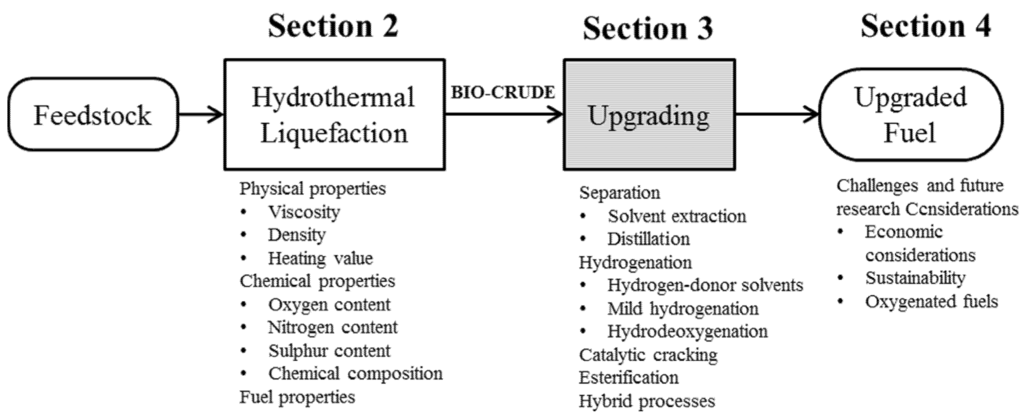
Figure 1.
Thermochemical process conceptual diagram and outline of the article.
Figure 1 shows the organisation of the topics discussed in this article. In the next section, physical and chemical properties of bio-crude are examined. In Section 3, upgrading processes that have been investigated and prospective processes that can be applied to upgrade HTL bio-crude are reviewed. Finally, in Section 4, a discussion on challenges of HTL bio-crude upgrading and considerations for prospective research are also discussed. In this article, products of pyrolysis will be referred to as bio-oil, while HTL products will be referred to as bio-crude.
2. Bio-Crude Properties
Physical properties are indicative of the characteristics and interactions of the mixture of chemicals that comprise bio-crudes. Chemical composition of bio-crude depends on HTL reaction conditions such as temperature, solvent, solvent density, reaction time, and gas used as reaction atmosphere, but the composition of the biomass fed into the liquefaction process has the most significant effect [38]. The use of different feedstocks greatly affects bio-crude properties. A comparison of physical and chemical properties of bio-crudes obtained from various HTL studies with diesel and biodiesel standards are summarised in Table 1, showing findings for bio-crude viscosity, density, heating value, hydrogen-carbon (H/C) and oxygen carbon (O/C) ratios.
In this review, physical properties of bio-crude will be compared with diesel or biodiesel standards, since these have been well-studied and regulated in many jurisdictions. Chemical properties, such as composition will be discussed independently or where appropriate, compared to similar chemicals or substances used as fuel.

Table 1.
Bio-crude produced from various feedstock and their properties.
| Feedstock Type | Feedstock | Composition a | Viscosity, mPa·s | Density, kg/L | Heating Value, MJ/kg | H/C b | O/C b | References |
|---|---|---|---|---|---|---|---|---|
| Liquefaction bio-crudes | ||||||||
| Lignocellulosics | Beech wood | C: 44.2%; H: 33.5%; L: 21.8% | - | 1.1 | 35 | 1.11 | 0.16 | [16] |
| Bagasse | C: 49.2%; H: 25.8%; L: 19.5% | 6.7 × 105 | - | 31 | 1.12 | 0.21 | [21] | |
| Bagasse/black liquor | C: 41.3%; H: 23.7%; L: 25.6% c | - | - | 28 | 1.35 | 0.39 | [39] | |
| Coconut husk | C: 30.6%; H: 25.9%; L: 38.8% | 1.3 × 106 | - | 30 | 1.00 | 0.21 | [21] | |
| Corn stalk | C: 42.4%; H: 25.8%; L: 21.7% | 1.6 × 106 | - | 30 | 1.01 | 0.21 | [21] | |
| Garbage | Carb: 55%; Prot: 18.4%; Fat: 5.3% | 53,000 | - | 36 | 1.48 | 0.13 | [25] | |
| Microalgae | Dunaliella tertiolecta | Carb: 14.7%; Prot: 63.6%; Fat: 20.5% | 15–330, 50 °C | - | 36 | 1.36 | 0.09 | [30] |
| Botryococcus braunii | 98% organic content; 50% hexane soluble | 64–160, 50 °C | - | 48 | 2.42 | 0.02 | [32] | |
| Spirulina platensis | Carb: 30.2%; Prot: 48.4%; Fat: 13.3% | 189.80, 40 °C | 0.97 | 34 | 1.44 | 0.1 | [35] | |
| Scenedesmus sp. | - | 3.27–3.75, 25 °C | 0.97–1.04 d | 30 | 1.60 | 0.1 | [40] | |
| Reference fuels | ||||||||
| Fuel | Diesel | 1.1–3.5, 40 °C | 0.85 | 45.1 | 1.79 | 0 | [41] | |
| Biodiesel | 1.7–5.3, 40 °C | 0.88 | 40.5 | 1.87 | 0.11 | [41] | ||
Note: a. Cellulose, Hemicellulose, Lignin; Carbohydrate, Protein, Fat; b. Molar ratio; c. Bagasse; d. Calculated.
2.1. Physical Properties
2.1.1. Viscosity
Viscosity is a measure of flow behaviour of a fluid and an important quantity in many fluid flow calculations. For an organic compound its viscosity is related to its chemical structure. Boelhouwer et al. [42] concluded that straight chain hydrocarbons have higher viscosities than branched hydrocarbons, and alcohol or acid groups have more effect on viscosity compared to esters and ketones.
Kinematic viscosity is more commonly used for fuels. High-viscosity fuel will not be well-atomised, leading to poor combustion [43], increased engine deposits, and higher energy requirements for fuel pumping [44]. Moreover, higher fuel viscosity has been observed to increase carbon monoxide (CO) and UHC [45]. In contrast, very low fuel viscosity leads to poor lubrication of fuel injection pumps, causing leaks and increased wear [46]. This results in biodiesel standards having upper and lower limits in kinematic viscosity.
2.1.2. Density
In fuels, density is related to the energy content for a given volume. Since the engine injection system measures the fuel by volume, a higher density fuel will have a greater power output from combustion of a larger fuel mass [44]. Density has also been correlated with increases in nitrogen oxides (NOx) [47,48], PM [48], CO, and UHC [49] in emissions. The heating value and cetane number are also both related to density [50]. In literature and in legislated standards, specific gravity is sometimes reported instead of density.
2.1.3. Heating Value
The fuel heating value is a common criterion for evaluating a liquefaction process. The heating value is a quantitative representation of the bio-crude’s energy content [51], which can be used to evaluate efficiency of converting feedstock to fuel. This quantity also gives the energy density of the fuel, which dictates how much energy is released with each volume of fuel injected into the combustion chamber. Heating value can be presented as a higher heating value (HHV) or a lower heating value (LHV). The HHV takes into account the heat of vaporisation of water during combustion, while the LHV does not. In fuels, HHV has been correlated with chemical composition given by ultimate [52] and proximate [53] analyses. Recently, this approach has been applied for HTL bio-crudes. Correlations state that heating value is directly proportional with the elemental composition, with carbon and hydrogen increasing heating value and oxygen and nitrogen having a negative effect [54]. However, it is the experience of the authors that traditional correlations do not closely match experimental data for HTL bio-crudes [39,40] and so existing correlations should be modified. While HHV quantity is not regulated, it is prudent to produce biofuels with heating values similar to conventional fuels to ensure minimal modifications to engines, particularly in injection technology.
2.2. Chemical Properties
2.2.1. Oxygen Content
Liquefaction bio-crudes have significant oxygen content resulting from the depolymerisation of biomass components (i.e., cellulose, hemicellulose and lignin). These oxygenated compounds take the form of organic acids, alcohols, ketones, aldehydes, sugars, furans, phenols, guaiacols, syringols, and other oxygenates [13]. In crude oil refining, oxygen is removed to prevent poisoning of catalysts in the reforming process [55]. Studies correlating oxygen content to fuel properties, engine operation and performance have been done on biodiesel. Lower CO emissions [56] and PM [57] have been observed for relatively highly oxygenated fuels such as biodiesel.
2.2.2. Nitrogen Content
Nitrogen in fuel may interact with degradation products and form solid deposits [58]. Nitrogen content is not regulated by diesel or biodiesel standards, although in crude oil refining, nitrogen content is reduced through hydrotreatment to minimise catalyst deactivation and improve diesel stability [55].
Bio-crude from HTL of lignocellulosic materials usually has low levels of nitrogen with a maximum of 2% [14,16,21,22]. Higher levels of nitrogen have been reported for bio-crudes produced from garbage, wastewater sludge, and algae (up to 10%) due to the protein content of the feedstock [25,27,30,31,32].
2.2.3. Sulphur Content
The sulphur content of fuel is a regulated quantity as burning sulphur in fuel produces sulphur oxides [55] and sulphate particles that contribute to PM emissions [59]. Moreover, sulphur can cause increased cylinder wear and deposit formation [59]. ASTM D975 [58] and D6751 [60] limits sulphur content in diesel and biodiesel, respectively, to 15 ppm.
Lignocellulosic materials and algae have very minimal sulphur content. Bio-crude has been produced with only 0.1–1.3 wt % sulphur [22,31,33,35]. Biochar, on the other hand, has a higher sulphur content [13,61], which may mean reactions in liquefaction favour sulphur binding into compounds in the solid fraction.
2.2.4. Chemical Composition
Diesel is mainly composed of alkanes, alkenes and aromatics [62], while biodiesel is more oxygenated, comprised of fatty acid methyl/ethyl esters [63]. HTL bio-crude, on the other hand, is a complex mixture of oxygenated organic chemicals [13,64], aliphatics, sugars, oligomers, nitrogenous aliphatics, and nitrogenous aromatics [65]. Table 2 shows the main chemical groups for bio-crude.
The chemical composition of bio-crudes is usually determined through gas chromatography-mass spectrometry (GC-MS). However, the vast amount of components and high complexity of the bio-crude prevent effective chromatographic separation, resulting in broad background signals [66]. More recent studies have used nuclear magnetic resonance (NMR) spectroscopy [66] and Fourier transform ion cyclotron resonance-mass spectrometry (FTICR-MS) to perform analyses with higher resolution and accuracy [67].

Table 2.
Groups of chemicals of hydrothermal liquefaction bio-crude.
| Main Components | Area% * Range | References |
|---|---|---|
| Phenolics | 6%–65% | [14,20,39] |
| Esters | 2%–44% | [14,27,39] |
| Aromatics and heterocyclics | 6%–35% | [14,39] |
| Aldehydes | 0%–18% | [14,20] |
| Carboxylic acids | 2%–40% | [20,27,35] |
| Ketones | 0%–38% | [20,27,35,40] |
| Alkanes | 9%–13% | [35,40] |
| Nitrogenates | 12%–23% | [35,40] |
Note: *. Area % from gas chromatography-mass spectrometry results.
The effects of varying compositions on the physical properties of diesel and biodiesel have been studied, while for HTL bio-crudes these relationships have not been elucidated. Table 3 shows the properties of various groups in diesel and their effect on fuel properties. In biodiesels, chain length and unsaturation of fatty acids are usually correlated to properties. Increasing chain length increases cetane number (an indication of ignition quality; Section 3), heating value and viscosity, while increasing unsaturation in fatty acids decreases viscosity and cetane number, but increases density and volumetric heating value [68]. Although these relationships are for diesel and biodiesel they provide an idea of the potential effects chemical composition may have on the physical properties of HTL bio-crude.

Table 3.
Properties of various chemical groups and their effect on diesel properties [62].
| Group | Ignition Quality | Heating Value | Density |
|---|---|---|---|
| n-Alkanes | Good | Low | Low |
| Isoalkanes | Low | Low | Low |
| Alkenes | Low | Low | Low |
| Cycloalkanes | Moderate | Moderate | Moderate |
| Aromatics | Poor | High | High |
2.3. Key Fuel Properties
These final fuel properties may not be directly influenced by upgrading processes; however, some consideration should also be given to improving them when processing bio-crude. Brief discussions of some key fuel properties to be considered are provided here.
2.3.1. Cetane Number
The Cetane Number (CN) is related to the fuel ignition delay time. Dorn et al. [69] determined the relationship between fuel components and CN. Normal alkanes increase cetane number the most, followed by branched alkanes, normal alkenes, branched alkenes, cycloalkanes, and aromatics. A high CN signifies good ignition quality, good cold start properties, minimal white smoke in exhaust [46], and low UHC [45] and CO emissions [45,48]. On the other hand, a low CN is related to a longer ignition delay time, which leads to higher amounts of injected fuel mixed prior to combustion. This then causes high rates of combustion and pressure rise that manifests as diesel knock. This also brings about premixed burning that leads to high combustion temperatures and increased NOx [45,70].
2.3.2. Vapour Pressure
Total vapour pressure of the fuel is dependent on the interactions of components within the mixture. Vapour pressure of a mixture can be estimated through the use of activity coefficients and thermodynamic models [71]. These models demonstrate the dependence of vapour pressure on fuel chemical composition. As a fuel property, vapour pressure affects performance of fuels, especially during cold start conditions [59]. However, a high vapour pressure is a concern due to higher fuel evaporation that contributes to increased hydrocarbon emissions [71].
2.3.3. Oxidation Stability
Oxidation stability describes the resistance to oxidation of fuel during storage. Biodiesel is degraded more easily than diesel due to the presence of double bonds in ester chains [46]. In HTL bio-crude the oxidation stability of upgraded fuels has not been investigated, however, stability of pyrolysis and HTL products has been observed. This is further discussed in Subsection 3.2.
The physical and chemical properties dictate how appropriate the fuel is for combustion in transportation engines. A number of studies, such as those referred to earlier in this section have discussed effects of biodiesel properties to diesel engine operation. Fundamentally, molecular weight and branching of organic molecules affect intermolecular attractions and subsequently physical properties. The presence of aromatic rings, nitrogen and oxygen also affect physical properties. These properties inform the selection of pathways to upgrade bio-crude to transportation fuels.
3. Upgrading Processes
Due to the similarity of pyrolysis and HTL, recent research on HTL bio-crude upgrading has so far focused on upgrading technologies that had previously been studied for pyrolysis bio-oils. The upgrading processes for pyrolysis bio-oils were themselves inspired by petroleum refining technologies. Although the authors draw on learnings from the pyrolysis literature, we have applied the understanding to HTL conversion. Upgrading processes discussed in this section are shown in Figure 2.
3.1. Separation
Products of HTL are usually a gas phase portion, a liquid oily fraction, a liquid aqueous fraction and solid residue. Studies on HTL and pyrolysis of biomass use a number of physical separation methods, mainly done as part of the work-up to segregate product fractions for analysis. These methods can realistically carry out separations to isolate high-value products or facilitate further processing to produce fuel and high-value products. Removal of water content in the oil fraction is specifically important for suitability for upgrading processes, as water may cause catalyst inactivity [72].
3.1.1. Solvent Extraction
Addition of a solvent to the two-phase product can enhance separation and extraction. The liquid product can be decanted to separate aqueous and oil portions. This crude separation results in an oil fraction with a moisture content of around 5% [12]. The choice of solvent will primarily be based on its immiscibility with water to facilitate separation, and its efficiency to extract the organic components and maximise yield. Selection of an appropriate solvent can be done through a number of methods. One such method is the use of the Robbins’ chart of solute-solvent interactions, which describes effect of functional groups on solubility based on hydrogen bonding and electron donor-acceptor interactions [73]. Use of this chart will be beneficial when targeting extraction of chemicals with specific functional groups. However, due to the complexity of bio-crude as a mixture, many researchers have investigated various chemicals to determine the most appropriate solvent. The most efficient solvent is somewhat dependent on the composition of the bio-crude and hence, the original feedstock.
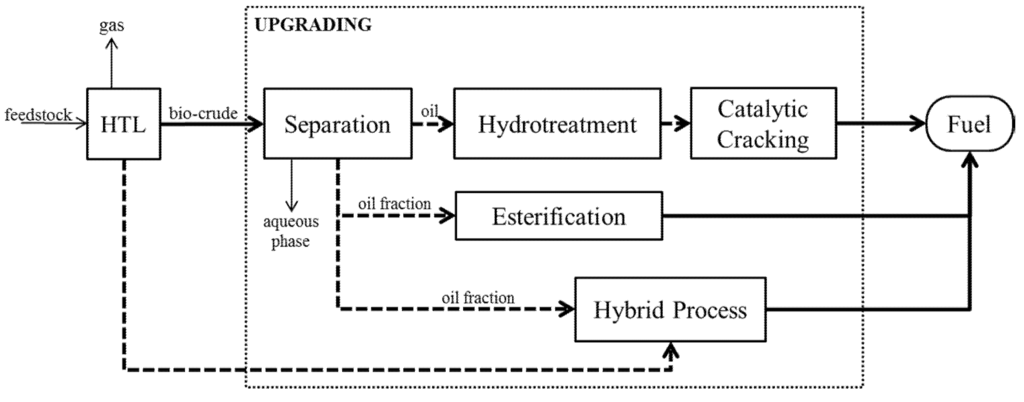
Figure 2.
Block diagram of upgrading processes discussed in this section.
Solvent polarity is a key parameter for consideration in choosing appropriate solvents. With the abundance of polar compounds in bio-crude and bio-oil, polar solvents are often more appropriate for extraction. This was confirmed by Garcia-Perez et al. [74] by performing successive extractions of bagasse pyrolysis bio-oil using solvents of increasing polarity. The solvents used were, in this order, pentane, benzene, dichloromethane, ethyl acetate, and methanol. The fractions that were extracted in ethyl acetate and dichloromethane were the two largest, owing to the high polarity of the bio-oil components. Another fractionation method, performed by Chum et al. [75] used ethyl acetate to separate a phenol fraction from pyrolysis bio-oil.
In liquefaction studies, oils for product characterisation are usually extracted using polar solvents such as acetone [14,16,17,20,31], tetrahydrofuran (THF) [22], ethanol [27], chloroform [76], or dichloromethane (DCM) [25,29,30,32,33,40,77].
On the other hand, microalgae bio-crude contains significant amounts of alkanes [35,40], which means non-polar solvents may be more effective. Valdez et al. [78] studied the use of nonpolar solvents hexadecane, decane, hexane, and cyclohexane, and polar solvents methoxycyclopentane, dichloromethane, and chloroform to separate bio-crude from products of algae liquefaction. Higher yield was obtained from nonpolar solvents due to the similarity of the long carbon chain solvents to chemicals in bio-crude, as confirmed by GCMS analysis. On the other hand, polar solvents extracted oil with higher carbon content, with the researchers posing that these solvents recovered carbon-rich compounds akin to resins and asphaltenes. Table 4 shows the oil fraction yields of HTL of different feedstock at the optimum condition reported, without use of catalysts or pretreatment, where the bio-crude was extracted from the liquid product with a solvent.

Table 4.
Yields of solvent extraction of HTL bio-crude from the liquid fraction using polar and non-polar solvents.
| Feedstock | Solvent | Yield | References |
|---|---|---|---|
| Acacia mangium wood | Acetone | 32% | [21] |
| Ailanthus wood | Acetone | 29% | [17] |
| Bagasse | Acetone | 59% | [14] |
| Acetone | 31% | [21] | |
| Bagasse pith | Acetone | 30% | [21] |
| Banana stem | Acetone | 21% | [21] |
| Beech wood | Acetone | 28% | [16] |
| Acetone | 34% | [17] | |
| Botryococcus braunii microalgae | Dichloromethane | 58% a | [32] |
| Cattle manure | Dichloromethane | 49% b,c | [29] |
| Chaetomorpha linum macroalgae | Dichloromethane | 17% | [33] |
| Chlorella microalgae | Dichloromethane | 42% | [77] |
| Cladophora coelothrix macroalgae | Dichloromethane | 20% | [33] |
| Cladophora vagabunda macroalgae | Dichloromethane | 28% | [33] |
| Coconut husk | Acetone | 28% | [21] |
| Coconut shell | Acetone | 34% | [21] |
| Corn stalk | Acetone | 28% | [21] |
| Corncob | Acetone | 76% | [17] |
| Cypress wood | Diethyl ether | 15% | [18] |
| Derbesia tenuissima macroalgae | Dichloromethane | 33% | [33] |
| Dunaliella tertiolecta microalgae | Dichloromethane | 37% | [30] |
| Dunaliella tertiolecta cake | Chloroform | ~22% | [76] |
| Garbage | Dichloromethane | ~22% | [25] |
| Hazelnut seedcoat | Acetone | 22% | [17] |
| Hazelnut shell | Acetone | 22% | [16] |
| Acetone | 28% | [17] | |
| Kenaf | Acetone | 28% | [21] |
| Metroxylon sp. petioles | Acetone | 23% | [21] |
| Metroxylon sp. stem | Acetone | 29% | [21] |
| Nannochloropsis salina microalgae | Acetone | 46% | [31] |
| Nannochloropsis sp. microalgae | Chloroform | 35% | [78] |
| Dichloromethane | 30% | [78] | |
| Methoxycyclopentane | 32% | [78] | |
| Hexane | 32% | [78] | |
| Hexadecane | 38% | [78] | |
| Decane | 39% | [78] | |
| Cyclohexane | 34% | [78] | |
| Oedogonium sp. macroalgae | Dichloromethane | 36% | [33] |
| Oil-palm empty fruit bunch | Acetone | 33% | [21] |
| Oil-palm fruit press fiber | Diethyl ether | 19% | [23] |
| Oil-palm husk | Acetone | 27% | [21] |
| Oil-palm petioles | Acetone | 23% | [21] |
| Oil-palm shell | Acetone | 36% | [21] |
| Olive husk | Acetone | 23% | [17] |
| Pine bark | Acetone | 21% | [17] |
| Pineapple leaf | Acetone | 24% | [21] |
| Rice husk | Diethyl ether | 1.7% | [20] |
| Acetone | 29% | [21] | |
| Rice straw | Acetone | 23% | [21] |
| Tetrahydrofuran | 40% | [22] | |
| Rubber tree | Acetone | 31% | [21] |
| Scenedesmus sp. microalgae | Dichloromethane | 34% | [41] |
| Hexane | 31% | [41] | |
| Sewage sludge | Ethanol | ~55% | [27] |
| Spirulina platensis | Acetone | 38% | [31] |
| Spruce wood | Acetone | 26% | [16] |
| Acetone | 26% | [17] | |
| Tea waste | Acetone | 23% | [16] |
| Ulva ohnoi macroalgae | Dichloromethane | 30% | [33] |
| Wheat straw | Acetone | 40% | [17] |
Note: a. Organic content basis; no run without catalyst; b. Volatile content basis; c. NaOH was used as catalyst.
There are very few studies that have used more than one solvent to fractionate the oil product. Karagöz et al. [20] separated the liquid product of liquefaction of sawdust and rice husk with diethyl ether and ethyl acetate. The solid fraction was also washed with acetone to obtain adhering oils. The largest oil fraction was obtained from the acetone wash, containing mostly phenolic compounds. The fraction obtained from extraction with diethyl ether has the second highest yield and is also comprised of mostly phenolics.
3.1.2. Distillation
Another separation process that can be applied is distillation. Its ubiquity in petroleum refining makes it a likely candidate for industrial scale bio-crude fractionation. There are various methods of distillation, and process selection depends on physical and chemical characteristics of the feed and the range of its components. Fractional distillation separates components using differences in boiling points. Vacuum distillation operates at reduced pressures, lowering the boiling point and enabling separation of components in less severe temperatures, preventing cracking or decomposition of components. Steam distillation uses steam to lower the partial pressure of the mixture, reducing the boiling point of components. Molecular distillation employs pressures below 1 Pa to separate components without the pressure exerted by the gaseous phase [51], thus the separation relies on differences of mean free paths of each component [79]. A typical continuous industrial scale distillation set-up is shown in Figure 3.
Distillation has been used in studies that characterise pyrolysis bio-oils. The temperatures used ranged from 100–250 °C for atmospheric distillation and 80–230 °C for vacuum distillation [79,80,81,82]. The ranges can be attributed to the varying tendency of bio-oil for cracking and polymerisation [83] when it reaches a certain temperature. Vacuum and molecular distillation allow for separation at lower temperatures to minimise thermal degradation. Removal of moisture is also a key result of distillation, to a resulting moisture content of 0.49% to 6.46% in middle and heavy fractions [79,80,81].
Figure 3.
Continuous Binary Fractional Distillation [84].
Fractional distillation of corn stover pyrolysis bio-oil was performed by Capunitan and Capareda [80] at both atmospheric and reduced pressures. The bio-oil was separated into three fractions. In atmospheric distillation, 84% of the bio-oil was recovered and at 500 mbar, 73% was recovered. Dramatic reduction of moisture was reported for the middle and heavy fractions, as well as a reduction of total acid number of the heavy fraction. These results were attributed to separation of water to the light fraction and of acidic components to the middle fraction.
Vacuum distillation and two stages of molecular distillation of pyrolysis bio-oil in series were conducted by Guo et al. [79]. Vacuum distillation was performed only as a pre-treatment step to remove water and light hydrocarbons. The product of vacuum distillation was fed into the first molecular distillation at 1600 Pa and the process yielded 26% bio-oil. The product of the first molecular distillation was fed into the second molecular distillation at 340 Pa and yielded 23%. It was observed that viscosity of the light fractions was less than the original bio-oil since lower molecular weight compounds were separated into these fractions. Furthermore, the separations conducted and a detailed analysis of chemicals in each fraction using GCMS led the researchers to conclude that acids and ketones are easier to separate, compared with aldehydes and phenols, while diphenols and sugars cannot be removed by distillation.
Steam distillation was used by Murwanashyaka et al. [82] to obtain a phenol-rich fraction and separate syringol from wood pyrolysis bio-oil at 105 °C. While this study was geared towards isolation and purification of valuable chemicals, this might also be a pathway to remove unwanted compounds or concentrate compounds with heteroatoms for more focused processing.
At this time, there are limited studies on distillation of HTL products before hydrotreatment, since separation is not necessary to analyse products.
3.2. Hydrogenation
As discussed in the previous section, hydrogen and oxygen content is directly correlated to the bio-crude’s heating value. A way to improve heating value is to increase hydrogen content and remove oxygen content [13]. Hydrogenation is a process used in petroleum refining to increase saturation of hydrocarbons and remove sulphur, nitrogen, and oxygen. This is done to prevent catalyst deactivation in further processing, to minimise coking, and to improve fuel characteristics [55].
Another issue is the unstable nature of pyrolysis and liquefaction products due to polymerisation or degradation of components. Adjaye et al. [61] studied the stability of wood HTL bio-oil and observed an increase in viscosity and the amount of residue collected after distillation. Jena et al. [35] observed an increase of 73% in viscosity of algae HTL bio-crude over 90 days. This suggests reactions of aldehydes and organic acids and an increase in the amount of higher molecular weight compounds due to polymerisation and condensation [85]. These potential reactions in bio-crude can be addressed by hydrogenation. Several hydrogenation methods and processes that have been investigated are discussed in this section. Typical reactions are shown in Figure 4.
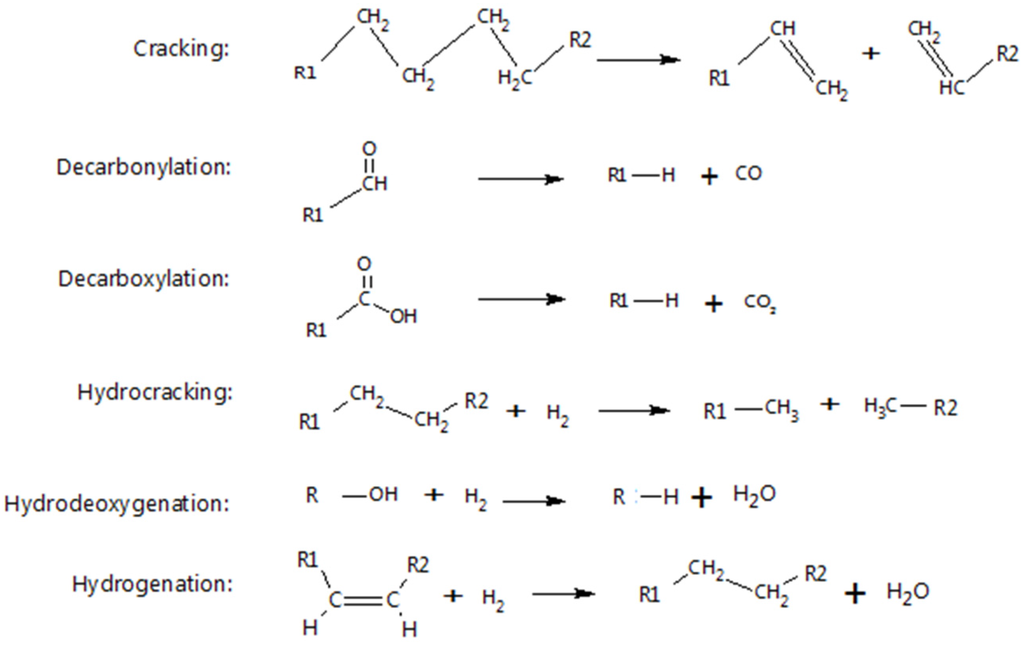
Figure 4.
Typical reactions in hydrogenation and cracking processes [86].
3.2.1. Hydrogen-Donor Solvents
Addition of a hydrogen-donor solvent improved stability of pyrolysis and liquefaction oils and prepared the bio-oil for further upgrading processes. Studies in catalytic hydrodeoxygenation (HDO) observed thermal decomposition, production of coke and decrease in catalyst activity [87,88] when processing pyrolysis bio-oils.
Solvents such as tetralin, methanol, and ethanol [85] have been used to arrest free radical polymerisation. Adjaye et al. [61] added tetralin to wood HTL bio-crude and observed stable viscosities over 31 days. It was also important to note that the amount of oil decreased and the amount of residue increased in samples without tetralin, compared to a stable composition in samples treated with tetralin. Rezzoug and Capart [89] added tetralin to wood liquefaction bio-crude prior to catalytic hydrogenation and obtained increasing light fractions with increasing tetralin/oil ratio. Diebold and Czernik [90] studied ethyl acetate, ethanol, acetone, methanol, a mixture of methyl isobutyl ketone and a mixture of methanol and acetone to wood pyrolysis bio-oil and observed the effect of each additive over accelerated aging at 90 °C. Of all the solvents, the sample with 10% methanol had the slowest aging rate, measured as change in viscosity over time. It was further inferred that the low aging rate was caused by molecular dilution and formation of intermediate products that hinder polymerisation.
3.2.2. Mild Hydrogenation
Mild hydrogenation prior to a more severe hydrogenation process was proposed in several studies of pyrolysis bio-oils due to reports of coking during the severe HDO step [85]. This initial step uses catalysts similar to a typical hydrogenation process, but with less severe conditions. Processes are run with temperatures below 300 °C, and lower hydrogen pressure is required. This process aims to stabilise the bio-oil and reduce reactive oxygen sites that can produce char [72]. In several studies, mild hydrogenation resulted in higher thermal stability and reduced char formation [91] due to hydrogenation of aldehydes and ketones in the initial step [72]. It can then be inferred that lower coking has decreased catalyst inactivity and resulted in a higher oil yield [87].
A review of mild hydrogenation studies by Diebold [85] revealed reduction in oxygen content but an increase in viscosity of bio-oil. Furthermore, a study by Elliot and Baker [91] observed that HTL bio-oil can be directly subjected to HDO, unlike pyrolysis bio-oils which need to undergo low temperature treatment. This posits that mild hydrogenation prior to a more severe hydrogenation is an option for HTL bio-crude that has properties similar to pyrolysis bio-oil, such as high oxygen content and high amounts of carbonyl-containing groups.
3.2.3. Hydrodeoxygenation (HDO) with Metal Catalysts
Hydrodeoxygenation, which consists of hydrogenation and oxygen-removal processes, is done to improve the properties of the HTL product and bring it as close as possible to petroleum fuels or biodiesel. HDO of bio-crude involves high temperature, high hydrogen pressure, and the use of a catalyst to provide the right conditions for the hydrogenation process to proceed. Oxygenated components mentioned in Section 2 are the target chemicals in this process. Ease of oxygen removal depends on bonding of the heteroatom and steric effects [86]. A study of HDO of model compounds in a cobalt-molybdenum (CoMo) catalyst proposed a low-temperature reactivity ranking of the various categories of components in pyrolysis bio-oil. It was concluded that ketones have high reactivity, followed by carboxylic acids, phenols and furans. It was also postulated that saturation of double bonds and HDO of alcohols and ethers will occur at a lower temperature than what is required for a ketone [92]. These conclusions were based on observed activation energies, temperature of identical conversions and hydrogen consumption of the HDO of the model chemicals, which are shown in Table 5. A study by Laurent and Delmon [93] also observed a similar trend in activity of groups in HDO of pyrolysis bio-oil. They further observed that decarboxylation occurs alongside the HDO of carboxylic groups, and carbon is being converted to CO2.

Table 5.
Activation energies, iso-reactive temperature and hydrogen consumption of hydrodeoxygenation of model compounds with a CoMo catalyst, presented by Grange et al. [92].
| Chemical/Group | Activation Energy (KJ/mol) | Iso-Reactive Temperature (°C) | Hydrogen Consumption |
|---|---|---|---|
| Ketone | 50 | 203 | 2 H2/group |
| Carboxylic acid | 109 | 283 | 3 H2/group |
| Methoxyphenol | 113 | 301 | ~6 H2/molecule |
| 4-Methylphenol | 141 | 340 | ~4 H2/molecule |
| 2-Ethylphenol | 150 | 367 | ~4 H2/molecule |
| Dibenzofuran | 143 | 417 | ~8 H2/molecule |
The use of CoMo and nickel catalysts is common for hydroprocessing in oil refineries. The selection of appropriate catalysts depended on the properties of crude oil, including metal, nitrogen, and sulphur content [94]. Therefore, application of oil refining processes and selection of appropriate catalysts for the hydrotreatment of bio-crude will have different considerations, some of which have been investigated in various studies.
An early study by the Pacific Northwest National Laboratory [91] investigated catalytic hydrotreatment of HTL bio-crude by using model chemicals, bio-crude distillates, and whole bio-crude. The study concluded that sulphided CoMo and Ni catalysts are the most effective for HDO since these two catalysts have high specificity, compared to other metallic catalysts tested. Furthermore, when sulphided CoMo was used, there was less saturation of aromatic compounds, which was desired for producing fuel similar to gasoline.
A study of 12 catalysts and eight hydrogen donors in hydrotreatment of wood HTL bio-crude was conducted by Grlic et al. [95]. It was observed that use of sulphided NiMo/Al2O3 catalysts resulted in the highest yield, lowest viscosity and high gross calorific value. The highest gross calorific value was obtained using an oxide form of the NiMo/Al2O3 catalyst. Of the solvents, use of tetralin contributed to a high product calorific value and low amounts of residue. It should be noted, however, that products of HDO with oxided NiMo/Al2O3 had very high viscosity, with oils sticking to reactor parts. In another study of the same group [96], effects of process conditions were observed in upgrading wood HTL bio-crude over a NiMo/Al2O3 catalyst. Temperature was the most influential factor: an increase in the heating value with an increase in the reaction temperature.
Several HDO studies observed that products from processes that use sulphided catalysts have some amount of sulphur [91,97]. To prevent adding sulphur into the HDO product, the use of noble metal catalysts can be an alternative to the more conventional catalysts already discussed. Noble metals such as platinum and rhenium were used in reforming processes to increase the octane number of fuels. However, the presence of sulphur in the feed poisons the catalyst [55]. On the other hand, use of noble metals to upgrade bio-crude is possible because of its low sulphur. A study using Ru/C and Pd/C in hydrotreatment of corn stover bio-oil observed 25.5% deoxygenation and a product H/C ratio of 1.47 at 300 °C using Ru/C [98].
Metal catalysts containing Rh, Rh-Co, Ni and Ni-Cu and using SiO2, Al2O3, ZiO2, CeO2, and CeO2-ZrO2 supports were investigated by Yakolev, et al. [99]. Anisole and biodiesel (esters) were used as model reactants. In this study, it was concluded that the Ni-Cu catalysts were most effective in HDO, having a degree of deoxygenation of 60%–100%. Using CeO2 as support achieved 100% HDO with Ni–Cu and 94.6% HDO with Rh, while using ZrO2 achieved 60% HDO with Ni–Cu and 90.8% HDO with Rh. The oxide forms provided adequate oxygen vacancy on the support surface, allowing more oxygen removal.
Subcritical water with metallic catalysts in HDO was also studied. Zhang, et al [100] upgraded duckweed HTL bio-crude in subcritical water at 350 °C using Ru/C, Pd/C, Pt/C, Pt/γ-Al2O3, Pt/C-sulfide, Rh/γ-Al2O3, activated carbon, MoS2, Mo2C, Co–Mo/γ-Al2O3, and zeolite. Since the duckweed bio-crude had small amounts of sulphur and nitrogen, removal of sulphur and nitrogen was also considered in upgrading. The researchers observed that Ru/C had activity for HDO, desulphurisation, and denitrogenation, resulting to a product with the lowest sulphur, highest hydrocarbon content, and highest heating value. Conversely, Pt/C was observed to have the best HDO performance.
Another pathway for hydrogenation and reduction of carbonyl groups into methylene groups is the use of zero valent metals such as Fe, Zn, Al and Mg. Liu et al. [101] used Zn to upgrade pyrolysis bio-oil at ambient temperature and pressure. The experiment resulted in a minor change in % C, % H and % O; however it was observed through NMR spectroscopy that alcohols and ethers (C–O) increased, while ketones, aldehydes and carboxyls (C=O) decreased. GCMS results also confirmed a reduction in carboxylic acids and aldehydes.
3.3. Catalytic Cracking
While hydrodeoxygenation aims to remove oxygen atoms from the bio-crude, cracking processes aim to produce lighter products with improved properties. Thermal cracking, which was used in the early 1900s to produce gasoline from gas oil [55], is not considered to be a viable alternative for cracking bio-crudes. The highly-oxygenated bio-crudes have high coking potential [102]. Catalytic cracking, on the other hand, has better selectivity and can be performed with milder conditions, decreasing production of undesirable side-products like gases and coke [102]. Using hydrogen in cracking processes is termed hydrocracking, which is a hydrogen addition process with more severe conditions, compared to hydrotreatment [55]. Catalysts used in cracking are natural clay materials, synthetic amorphous silica-alumina, and synthetic crystalline zeolites [55]. One example of a synthetic zeolite is the ZSM-5, shown in Figure 5. The catalytic cracking process carries out dehydration, decarbonylation, dehydrogenation, hydrogenation, and hydrogen-transfer reactions [103]. There have been several studies on cracking pyrolysis bio-oils with zeolites [104,105] but only a few for HTL bio-crudes [106,107,108,109].
A continuous, downflow, fixed bed reactor with HZSM-5 at 330–410 °C and atmospheric pressure was used by Adjaye and Bakhshi [106] to upgrade wood powder HTL bio-crude. This process used cracking catalysts to upgrade bio-oil without the use of H2. The study compared results of using HZSM-5, H-mordenite, H-Y, silicalite, and silica-alumina. Volatile “organic distillate” fraction yields ranged from 40%–67%, with optimum yield obtained from using H-mordenite and H-Y at 370 °C. However, the organic fraction still contained 10.7%–36.5% oxygenated compounds. Furthermore, 4.4%–20.5% weight of coke was produced. The researchers also studied reactions of bio-crude model compounds with the HZSM-5 catalyst. The study observed minimal coke formation and increase in cracking of non-volatile components in low feed concentration and low temperature. However, low conversion was also observed [107,108]. Furthermore, cracking, deoxygenation, aromatisation and polymerisation were proposed as the main reactions with this catalyst, which is similar to the proposal of Corma et al. [103].
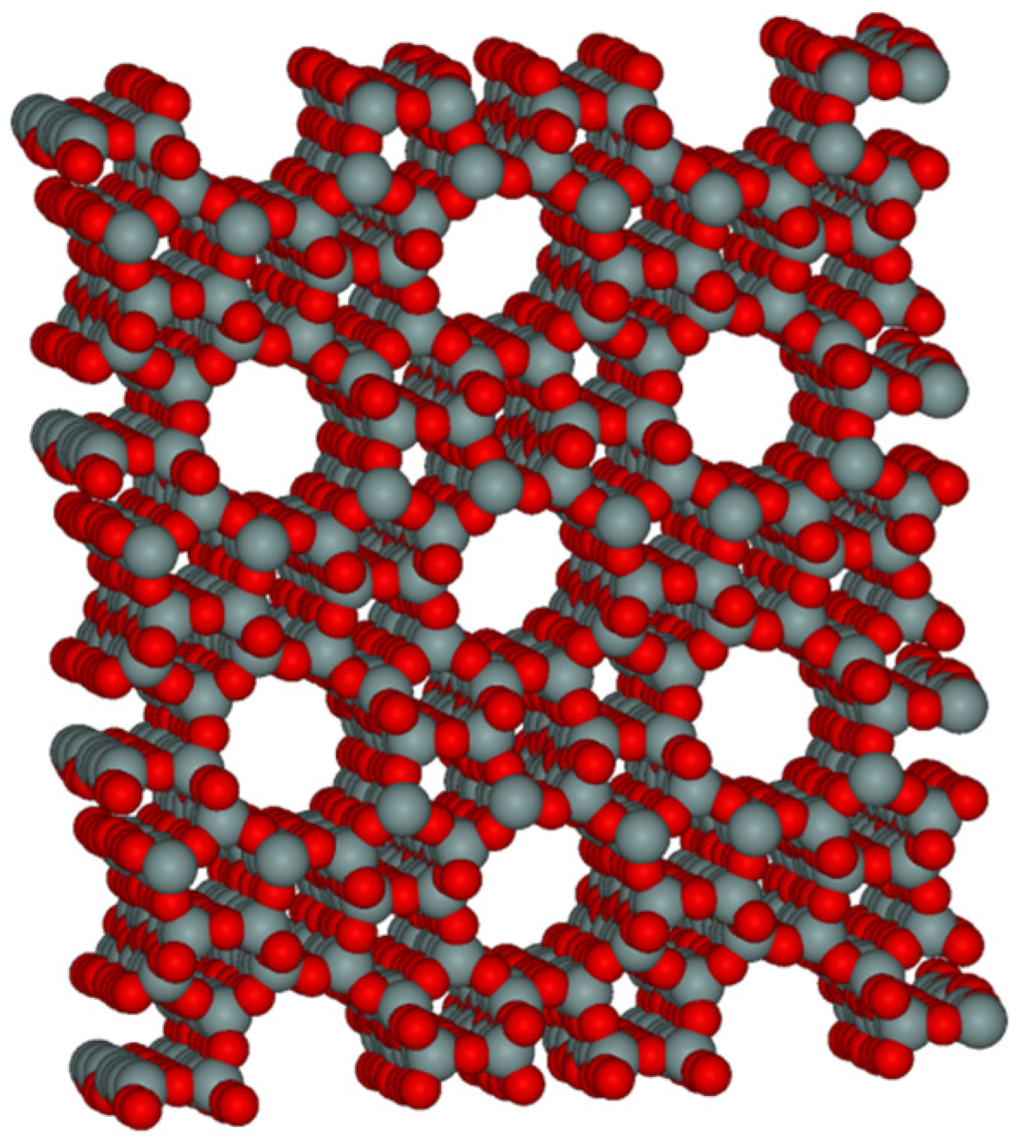
Figure 5.
Microporous molecular structure of ZSM-5.
Thermal cracking and catalytic cracking were compared by Gevert and Otterstedt [109] using alpha alumina, EKZ-4 (containing rare earth zeolite Y), and EKZ-2 (commercial equilibrium catalyst). Better yield was achieved at 500 °C for thermal cracking, compared to a higher temperature, owing to the sensitivity to thermal cracking of components of the hydroprocessed bio-crude. Catalytic cracking achieved better liquid product yield when compared to thermal cracking at the same temperature. Furthermore, it was observed that lower catalyst to oil ratios resulted in better oil yield and lower gas and coke production.
3.4. Esterification
Adding alcohols to bio-crude is another method for changing chemical composition and improving physical properties. The added alcohol reacts with the organic acids to form esters, similar to chemicals that comprise biodiesel. The esterification reaction is shown in Figure 6. Using ethanol to upgrade pyrolysis bio-oils has been investigated as an alternative to hydrogen. Zhang et al. [110] reacted rice husk pyrolysis bio-oil with ethanol over solid acid 40SiO2/TiO2-SO42− and solid base 30K2CO3/Al2O3-NaOH catalysts. It was observed that esterified bio-oils had vast improvements in viscosity, density and calorific value. The amount of esters increased 20-fold with the acidic catalyst, while also producing acetals.
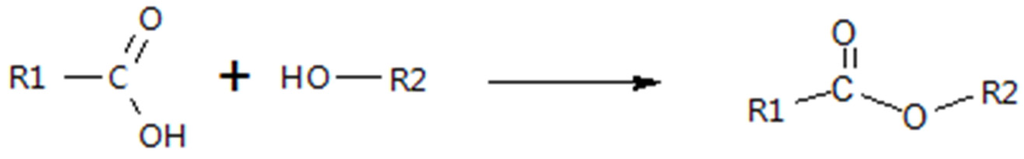
Figure 6.
Esterification reaction.
Use of these alcohols in the supercritical regime has also been considered, exploiting superior fluid properties, the same rationale for hydrothermal liquefaction. Peng et al. [111] compared the subcritical and supercritical upgrading of rice husk pyrolysis bio-oil with ethanol and HZSM-5 and reported the supercritical process as being more effective. The researchers observed that residue after vacuum distillation of unprocessed bio-oil and upgraded product was reduced from 38% to 15%.
The use of supercritical ethanol with Pt/C, Pd/C, Ru/C and Ru/HZSM catalysts to upgrade rice husk pyrolysis bio-oil was studied by Chen et al. [112]. The heating value of the bio-oil increased from 21.45 to 30 MJ/kg. It was also observed that the relative amount of desired products was achieved when Ru/C was used. The amount of acids and methyl esters decreased, while ethyl acetate increased. The amount of phenols also decreased, while cyclic ketones and alcohols increased.
3.5. Hybrid Processes
Due to the resulting low yields and high levels of coking in some of the upgrading processes discussed in previous subsections, there has also been research around combining features of multiple processes into a single process. These hybrid processes aim to encourage desired reactions and inhibit undesired reactions.
A combined reaction-distillation process can be used to simultaneously alter and separate bio-crude components. A reactive distillation process was studied by Mahfud et al. [113] by mixing pyrolysis bio-oil with high boiling alcohols and an acid catalyst. Organic acids and aldehydes reacted with alcohols to form esters and acetals, improving physical properties of the bio-oil. The reactions used a number of alcohols and acid catalysts at 50–80 °C, while distilling at a reduced pressure of 5 kPa. The study compared alcohols and observed that n-butanol and ethylene glycol perform similarly to carry out esterification of the organic acids. Liquid sulphuric acid was the best catalyst; however the solid acid catalyst used had significant results as well. Reduction of moisture content to up to a sixth of the initial value was achieved in reduced-pressure distillation. Different solid catalysts and solvents were used by Xu et al. [114]. Zirconium-containing mesoporous molecular sieve SO42−/Zr-MCM-41 as a solid acid catalyst, and ethanol and hydrogen peroxide as solvents were used in the reactive rectification study. The process resulted in a yield of 21% of product with improved density, water content, heating value and pH.
A one-step upgrading process involving hydrotreatment, esterification and cracking at the same time was proposed by Tang et al. [115]. The process used supercritical ethanol, a hydrogen atmosphere and a Pd/SO42−/ZrO2/SBA-15 catalyst to upgrade rice husk pyrolysis bio-oil. It was observed that properties of the bio-oil, such as viscosity, density, pH and heating values improved, with the process generating trace amounts of tar. This was attributed to conversion of large molecular weight compounds and esterification of acids.
Another “one-pot” conversion process using Ni/ZrO2 in supercritical cyclohexane at 300 °C was conducted by Shi et al. [116] to upgrade cornstalk HTL bio-crude, obtaining 81.6% carbon yield with 90% diesel and jet fuel hydrocarbons. The upgraded oil had 0.75% oxygen, from 26.79% in the feed bio-crude, and a HHV of 46.86 MJ/kg.
Each upgrading process aims to improve the properties of the bio-crude to make it an acceptable fuel. Table 6 summarises the information discussed in this section and shows the effect of each process to bio-crude properties.
4. Challenges and Future Research Prospects
As remarked earlier, there is a far larger quantum of research on upgrading pyrolysis bio-oils compared to HTL bio-crude. This has been demonstrated in the range of technologies that have been investigated for pyrolysis bio-oil upgrading. Taking into account the advantages of liquefaction over pyrolysis, there is adequate imperative to advance research in upgrading HTL bio-crude to transportation fuels. However, there are still major hurdles in development of a commercially-competitive integrated process, starting from the significant capital needed for the HTL process alone [116]. Challenges in upgrading HTL bio-crude can be translated to opportunities for researchers to develop cost-efficient and sustainable technologies.
4.1. Economic Considerations
Production of second-generation fuels uses low-cost feedstock but incurs significant capital costs. For liquefaction, a study estimated that using the current state of technology (SOT), the minimum selling price of woody biomass HTL fuel is US$ 4.44/ gallon gasoline equivalent (GGE) [117]. This is assuming a two-stage hydrotreatment process. In 2050, it is projected that the cost of production will be around US$ 3.03–3.79/GGE (0.80–1.00 per litre), depending on development of more cost-effective technology and process improvements [9]. In the same techno-economic study, Zhu et al. [117] obtained the goal case minimum selling price at US$ 2.52/GGE, assuming less organics lost to the water phase and a hydrocracking unit added to a single-step hydrotreatment process. The HTL and upgrading units comprise 61% of total installed costs for the SOT case and 49% in the goal case. Other costs such as those related to consumption of hydrogen and catalysts, waste treatment and disposal also differ according to efficiency of processes. Adding a hydrocracker for the goal case and increasing product yield eliminates costs associated with selling the heavy oil produced in HTL as a by-product, and offsets capital costs in installing hydrocracking equipment.
In another techno-economic study by another group led by Zhu [118], the minimum fuel selling price of products of HTL of lipid-extracted algae (LEA) was between US$ 2.07–7.11/GGE. The study included an HTL process, a hydrotreatment unit, a hydrocracking unit, and three separation columns. The highest capital cost was determined to be the hydrotreatment process, which was 39% of the total installed cost. The feedstock price affects price sensitivity the most, followed by product yield and upgrading equipment cost.
In these studies, it can be observed clearly that upgrading processes impact immensely on product price. As technologies improve, product yield and quality improves, translating to better revenues, offsetting installation costs. Moreover, cheaper and more efficient technologies can also reduce installation and operating costs. Lastly, hydrogen requirements and waste treatment and disposal costs may also be reduced.

Table 6.
Upgrading processes and their effect on physical and chemical properties. Direct influence of processes to bio-crude property towards standard values.
| Upgrading Process | Upgrading Mechanism | Viscosity | Density | Heating Value | O-Content | N-Content | S-Content | Chemical Composition | References |
|---|---|---|---|---|---|---|---|---|---|
| Solvent Extraction | Separation from water; increasing organic yield. | √ | √ | √ | [14,16,17,20,21,22,25,27,29,30,31,32,33,35,40,76,77,78] | ||||
| Distillation | Removal of water; separation of light from heavy components. | √ | √ | √ | [79,80,81,82,112,113] | ||||
| Addition of hydrogen-donor solvents | Provision of hydrogen in liquid phase for stability. | √ | √ | √ | √ | √ | [61,85,89,90] | ||
| Mild hydrogenation | Provision of hydrogen in gas, hydrogenation reaction in mild conditions. | √ | √ | [72,85,91,92] | |||||
| HDO/HDN/HDS | Hydrogenation in severe conditions to remove heteroatoms. | √ | √ | √ | √ | √ | [86,92,93,94,95,96,97,98,99,100,101,115] | ||
| Cracking | Cleavage of high molecular weight compounds. | √ | √ | √ | √ | √ | [102,103,104,105,106,107,108,109,115] | ||
| Esterification | Conversion of organic acids to esters. | √ | √ | √ | [110,111,112,113,114,115] |
4.2. Sustainability
Research in biofuels has been advanced under the banner of sustainable energy. Biofuels are sustainable in that they are produced from renewable sources [119]. Furthermore, production of fuels through HTL emits less greenhouse gases than production from fossil fuels [120,121]. Liu et al. [121] determined that the energy return on investment (EROI) of producing fuels through HTL of algae has a value of approximately 1, i.e., the energy output of a fuel produced by HTL is almost equal to the energy input, using current state-of-the-art technologies. Opportunities to improve EROI are identified in production of upstream nutrients; however, upgrading will also be a significant energy burden [121]. There is a possibility to use upgrading technologies that use less energy than conventional refining as algae bio-crude has lower sulphur and heavy metal content, reducing the need for additional heteroatom removal processes [121]. In a life cycle analysis by Frank et al. [122], energy use in upgrading is significantly affected by hydrogen gas consumption in deoxygenation and denitrogenation, which was determined using the Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation (GREET) model. These findings emphasise that energy consumption in upgrading is affected by the quality of the bio-crude being upgraded, or the efficiency of hydrogenation processes.
Other emissions of the biofuel production process are wastewater and residues. Zhu et al. [117] proposed a scenario of effective separation of organics from water after HTL that reduces wastewater treatment costs by 50%.
While offgas from HTL and upgrading units can be processed to be used in hydrogenation processes, the hydrogen consumption will depend on oxygen content of bio-crude, and effectiveness of hydrogenation. Deficit in H2 from these processes can be supplemented by a hydrogen plant, which uses natural gas as feed [117]. This demonstrates a process which is still dependent on a fossil fuel.
Further research on HTL and upgrading technologies can be directed towards improving process and separation efficiencies to minimise wastes and emissions, decrease hydrogenation burden, and improve conversion and use of by-products through a biorefinery approach that advances sustainable biofuel production [123].
4.3. Oxygenated Biofuels
As mentioned in Section 2.4, high oxygen fuels have been linked to better emissions. There have been numerous studies on emissions of biodiesels and other oxygenated fuels. However, research on the effects of fuels from HTL or pyrolysis on emissions and engine performance is just emerging. Zhou et al. [124] studied the overall combustion performance of cellulose and lignin derivatives di-n-butyl ether and anisole, as blended with diesel. The study observed a decrease in PM; however, the concentration of anisole was not beneficial to overall performance and emissions. Results seem promising and further research can be geared towards understanding the effects of upgraded bio-crudes on performance and emissions. Consequently, this presents an opportunity for different technologies for upgrading to be investigated, focusing less on reducing oxygen content, and more on other properties.
Author Contributions
This work was developed and written by J.A. Ramirez. It was conceived with T.J. Rainey and R.J. Brown who both provided major editorial contributions and guidance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dlugokencky, E.; Tans, P. Trends in Atmospheric Carbon Dioxide: Recent Global CO2. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed on 6 May 2015).
- Arvizu, D.; Bruckner, T.; Chum, H.; Edenhofer, O.; Estefen, S.; Faaij, A.; Fischedick, M.; Hansen, G.; Hiriart, G.; Hohmeyer, O.; Edenhofer, O.; et al. Technical Summary. In IPCC Special Report on Renewable Energy Sources and Climate Change Mitigation; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Seyboth, K., Matschoss, P., Kadner, S., Zwickel, T., Eickemeier, P., Hansen, G., Schlömer, S., et al., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Chum, H.; Faaij, A.; Moreira, J.; Berndes, G.; Dhamija, P.; Dong, H.; Gabrielle, B.; Goss Eng, A.; Lucht, W.; Mapako, M.; Edenhofer, O.; et al. Bioenergy. In IPCC Special Report on Renewable Energy Sources and Climate Change Mitigation; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Seyboth, K., Matschoss, P., Kadner, S., Zwickel, T., Eickemeier, P., Hansen, G., Schlömer, S., et al., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- The Secretary-General’s High-level Group on Sustainable Energy for All. Sustainable Energy for All: A Framework for Action. Available online: http://www.se4all.org/wp-content/uploads/2013/09/SE_for_All_-_Framework_for_Action_FINAL.pdf (accessed on 13 February 2015).
- White, L.P.; Plaskett, L.G. Biomass as Fuel; Academic Press London: New York, NY, USA, 1981. [Google Scholar]
- Berl, E. Production of oil from plant material. Science 1944, 99, 309–312. [Google Scholar] [CrossRef] [PubMed]
- International Renewable Energy Agency. REmap 2030: A Renewable Energy Roadmap, Summary of Findings; International Renewable Energy Agency: Abu Dhabi, UAE, 2014. [Google Scholar]
- Nakada, S.; Saygin, D.; Gielen, D. Global Bioenergy Supply and Demand Projections; International Renewable Energy Agency: Abu Dhabi, UAE, 2014. [Google Scholar]
- O’Connell, D.; Batten, D.; O’Connor, M.; May, B.; Raison, J.; Keating, B.; Beer, T.; Braid, A.; Haritos, V.; Begley, C. Biofuels in Australia—Issues and Prospects; Rural Industries Research and Development Corporation: Barton, Australia, 2007. [Google Scholar]
- Bridgwater, A.V.; Toft, A.J.; Brammer, J.G. A techno-economic comparison of power production by biomass fast pyrolysis with gasification and combustion. Renew. Sustain. Energy Rev. 2002, 6, 181–246. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Chumpoo, J.; Prasassarakich, P. Bio-oil from hydro-liquefaction of bagasse in supercritical ethanol. Energy Fuels 2010, 24, 2071–2077. [Google Scholar] [CrossRef]
- Ogi, T.; Yokoyama, S.Y.; Koguchi, K. Direct liquefaction of wood by alkali and alkaline earth salt in an aqueous phase. Chem. Lett. 1985, 14, 1199–1202. [Google Scholar] [CrossRef]
- Demirbaş, A. Thermochemical conversion of biomass to liquid products in the aqueous medium. Energy Source 2005, 27, 1235–1243. [Google Scholar] [CrossRef]
- Demirbaş, A. Effect of lignin content on aqueous liquefaction products of biomass. Energy Convers. Manag. 2000, 41, 1601–1607. [Google Scholar] [CrossRef]
- Liu, H.M.; Wang, F.Y.; Liu, Y.L. Alkaline pretreatment and hydrothermal liquefaction of cypress for high yield bio-oil production. J. Anal. Appl. Pyrolysis 2014, 108, 136–142. [Google Scholar] [CrossRef]
- Araya, P.E.; Droguett, S.E.; Neuburg, H.J.; Badilla-Ohlbaum, R. Catalytic wood liquefaction using a hydrogen donor solvent. Can. J. Chem. Eng. 1986, 64, 775–780. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Comparative studies of oil compositions produced from sawdust, rice husk, lignin and cellulose by hydrothermal treatment. Fuel 2005, 84, 875–884. [Google Scholar] [CrossRef]
- Minowa, T.; Kondo, T.; Sudirjo, S.T. Thermochemical liquefaction of Indonesian biomass residues. Biomass Bioenergy 1998, 14, 517–524. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Li, H.; Zeng, G.M.; Tong, J.Y.; Xie, W. Sub- and supercritical liquefaction of rice straw in the presence of ethanol-water and 2-propanol-water mixture. Energy 2007, 32, 2081–2088. [Google Scholar] [CrossRef]
- Mazaheri, H.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Subcritical water liquefaction of oil palm fruit press fiber for the production of bio-oil: Effect of catalysts. Bioresour. Technol. 2010, 101, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Benn, F.R.; McAuliffe, C.A. Conversion of Municipal Waste to Fuel. U.S. Patent 4,618,736, 21 October 1986. [Google Scholar]
- Minowa, T.; Murakami, M.; Dote, Y.; Ogi, T.; Yokoyama, S.-Y. Oil production from garbage by thermochemical liquefaction. Biomass Bioenergy 1995, 8, 117–120. [Google Scholar] [CrossRef]
- Bosetti, A.; Bianchi, D.; Franzosi, G. Integrated Process for the Production of Bio-Oil from Sludge Coming from a Wastewater Purification Plant. U.S. Patent Application 14/002,321, 2012. [Google Scholar]
- Li, H.; Yuan, X.; Zeng, G.; Huang, D.; Huang, H.; Tong, J.; You, Q.; Zhang, J.; Zhou, M. The formation of bio-oil from sludge by deoxy-liquefaction in supercritical ethanol. Bioresour. Technol. 2010, 101, 2860–2866. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.W.; Stiller, A.H. Liquid Fuel from Agricultural Waste which may be Animal Manure, Includes Admixing Agricultural Waste with at Least One Material Selected from the Group Consisting of Hydrogen Gas and Water, at Elevated Pressure Heating a Mixture. U.S. Patent Application 10/239,256, 2001. [Google Scholar]
- Yin, S.; Dolan, R.; Harris, M.; Tan, Z. Subcritical hydrothermal liquefaction of cattle manure to bio-oil: Effects of conversion parameters on bio-oil yield and characterization of bio-oil. Bioresour. Technol. 2010, 101, 3657–3664. [Google Scholar] [CrossRef] [PubMed]
- Minowa, T.; Yokoyama, S.Y.; Kishimoto, M.; Okakura, T. Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel 1995, 74, 1735–1738. [Google Scholar] [CrossRef]
- Toor, S.S.; Reddy, H.; Deng, S.; Hoffmann, J.; Spangsmark, D.; Madsen, L.B.; Holm-Nielsen, J.B.; Rosendahl, L.A. Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresour. Technol. 2013, 131, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Dote, Y.; Sawayama, S.; Inoue, S.; Minowa, T.; Yokoyama, S.Y. Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction. Fuel 1994, 73, 1855–1857. [Google Scholar] [CrossRef]
- Neveux, N.; Yuen, A.K.L.; Jazrawi, C.; Magnusson, M.; Haynes, B.S.; Masters, A.F.; Montoya, A.; Paul, N.A.; Maschmeyer, T.; de Nys, R. Biocrude yield and productivity from the hydrothermal liquefaction of marine and freshwater green macroalgae. Bioresour. Technol. 2014, 155, 334–341. [Google Scholar] [CrossRef] [PubMed]
- López Barreiro, D.; Prins, W.; Ronsse, F.; Brilman, W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C. Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae. Energy Fuels 2011, 25, 5472–5482. [Google Scholar] [CrossRef]
- Kosinkova, J.; Doshi, A.; Maire, J.; Ristovski, Z.; Brown, R.J.; Rainey, T.J. Measuring the regional availability of biomass for biofuels and the potential for microalgae. Renew. Sustain. Energy Rev. 2015, 49, 1271–1285. [Google Scholar] [CrossRef]
- Nabi, M.N.; Rahman, M.M.; Islam, M.A.; Hossain, F.M.; Brooks, P.; Rowlands, W.N.; Tulloch, J.; Ristovski, Z.D.; Brown, R.J. Fuel characterisation, engine performance, combustion and exhaust emissions with a new renewable licella biofuel. Energy Convers. Manag. 2015, 96, 588–598. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Kosinkova, J.; Ramirez, J.A.; Hossain, F.M.; Nguyen, J.; Ristovski, Z.; Brown, R.J.; Lind, C.S.K.; Chang, J.; Rainey, T.J. Hydrothermal liquefaction of bagasse using ethanol and black liquor as solvents. Biofuels Bioprod. Biorefing 2015. submitted. [Google Scholar]
- Hossain, F.M.; Kosinkova, J.; Brown, R.J.; Ristovski, Z.; Stephens, E.; Hankamer, B.; Ramirez, J.A.; Rainey, T.J. The chemical-physical properties of bio-crude derived from the hydrothermal liquefaction of microalgae. Algal Res. 2015. submitted. [Google Scholar]
- National Renewable Energy Laboratory. Biodiesel Handling and Use Guide, 4th ed.; National Renewable Energy Laboratory: Golden, CO, USA, 2009. [Google Scholar]
- Boelhouwer, J.W.M.; Nederbragt, G.W.; Verberg, G. Viscosity data of organic liquids. Appl. Sci. Res. 1951, 2, 249–268. [Google Scholar] [CrossRef]
- Lee, S.W.; Tanaka, D.; Kusaka, J.; Daisho, Y. Effects of diesel fuel characteristics on spray and combustion in a diesel engine. JSAE Rev. 2002, 23, 407–414. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Determination of the density and the viscosities of biodiesel-diesel fuel blends. Renew. Energy 2008, 33, 2623–2630. [Google Scholar] [CrossRef]
- Ng, J.H.; Ng, H.K.; Gan, S. Development of emissions predictor equations for a light-duty diesel engine using biodiesel fuel properties. Fuel 2012, 95, 544–552. [Google Scholar] [CrossRef]
- Jahirul, M.; Brown, R.; Senadeera, W.; O’Hara, I.; Ristovski, Z. The use of artificial neural networks for identifying sustainable biodiesel feedstocks. Energies 2013, 6, 3764–3806. [Google Scholar] [CrossRef]
- Watanabe, H.; Tahara, T.; Tamanouchi, M.; Iida, J. Study of the effects on exhaust emissions in direct injection diesel engines: Effects of fuel injection system, distillation properties and cetane number. JSAE Rev. 1998, 19, 21–26. [Google Scholar] [CrossRef]
- Karonis, D.; Lois, E.; Zannikos, F.; Alexandridis, A.; Sarimveis, H. A neural network approach for the correlation of exhaust emissions from a diesel engine with diesel fuel properties. Energy Fuels 2003, 17, 1259–1265. [Google Scholar] [CrossRef]
- Hochart, N.; Raux, S.; Montagne, X.; Belot, G.; Delage, A.; Faucon, R.; Petit, A.; Michon, S. Present Day Diesel Engine Pollutant Emissions: Proposed Model for Refinery Mases Impact; SAE Technical Paper No. 2000-01-1852; SAE International: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Tat, M.; Van Gerpen, J. The specific gravity of biodiesel and its blends with diesel fuel. J. Am. Oil Chem. Soc. 2000, 77, 115–119. [Google Scholar] [CrossRef]
- Schaschke, C.A. Dictionary of Chemical Engineering; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.A.; Ghosal, G.K. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Demirbas, A.; Gullu, D.; Çaglar, A.; Akdeniz, F. Estimation of calorific values of fuels from lignocellulosics. Energy Sources 1997, 19, 765–770. [Google Scholar] [CrossRef]
- Alfke, G.; Irion, W.W.; Neuwirth, O.S. Oil Refining. In Handbook of Fuels: Energy Sources for Transportation; Elvers, B., Ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Kegl, B. Influence of biodiesel on engine combustion and emission characteristics. Appl. Energy 2011, 88, 1803–1812. [Google Scholar] [CrossRef]
- Rahman, M.M.; Pourkhesalian, A.M.; Stevanovic, S.; Islam, M.; Wang, H.; Miljevic, B.; Phamxuan, P.; Brown, R.J.; Masri, A.; Ristovski, Z.D. Biodiesel with Controlled Physicochemical Properties, a Means to Further Reduce Diesel Engine Particle Emissions. In Proceedings of the European Aerosol Conference, Prague, Czech Republic, 1–6 September 2013.
- American Society for Testing and Materials International. Standard Specification for Diesel Fuel Oils; ASTM Standard D975-15. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2015. Available online: http://www.astm.org/Standards/D975.htm (accessed on 5 May 2015).
- Westbrook, S.R.; LaCren, R. Automotive Diesel and Non-Aviation Gas Turbine Fuels. In Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing; Totten, G.E., Westbrook, S.R., Shah, R.J., Eds.; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- American Society for Testing and Materials International. Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels; ASTM Standard D6751-15. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2015. Available online: http://www.astm.org/Standards/D6751.htm (accessed on 5 May 2015).
- Adjaye, J.D.; Sharma, R.K.; Bakhshi, N.N. Characterization and stability analysis of wood-derived bio-oil. Fuel Process. Technol. 1992, 31, 241–256. [Google Scholar] [CrossRef]
- Dabelstein, W.; Reglitzky, A.; Schütze, A.; Reders, K. Automotive Fuels. In Handbook of Fuels: Energy Sources for Transportation; Elvers, B., Ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Demirbas, A. Competitive liquid biofuels from biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Mørup, A.J.; Christensen, P.R.; Aarup, D.F.; Dithmer, L.; Mamakhel, A.; Glasius, M.; Iversen, B.B. Hydrothermal liquefaction of dried distillers grains with solubles: A reaction temperature study. Energy Fuels 2012, 26, 5944–5953. [Google Scholar] [CrossRef]
- Leonardis, I.; Chiaberge, S.; Fiorani, T.; Spera, S.; Battistel, E.; Bosetti, A.; Cesti, P.; Reale, S.; de Angelis, F. Characterization of bio-oil from hydrothermal liquefaction of organic waste by NMR spectroscopy and FTICR mass spectrometry. ChemSusChem 2013, 6, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Chiaberge, S.; Leonardis, I.; Fiorani, T.; Cesti, P.; Reale, S.; Angelis, F.D. Bio-oil from waste: A comprehensive analytical study by soft-ionization FTICR mass spectrometry. Energy Fuels 2014, 28, 2019–2026. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Dorn, P.; Mourao, A.M.; Herbstman, S. The Properties and Performance of Modern Automotive Fuels; SAE Technical Paper 861178; SAE International: Washington, DC, USA, 1986. [Google Scholar] [CrossRef]
- Ryan, T.W., III. Diesel Fuel Combustion Characteristics. In Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing; Totten, G.E., Westbrook, S.R., Shah, R.J., Eds.; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2003; pp. 717–728. [Google Scholar]
- Pumphrey, J.A.; Brand, J.I.; Scheller, W.A. Vapour pressure measurements and predictions for alcohol-gasoline blends. Fuel 2000, 79, 1405–1411. [Google Scholar] [CrossRef]
- Venderbosch, R.H.; Ardiyanti, A.R.; Wildschut, J.; Oasmaa, A.; Heeres, H.J. Stabilization of biomass-derived pyrolysis oils. J. Chem. Technol. Biotechnol. 2010, 85, 674–686. [Google Scholar] [CrossRef]
- Frank, T.C.; Dahuron, L.; Holden, B.S.; Prince, W.D.; Seibert, A.F.; Wilson, L.C. Liquid-Liquid Extraction and Other Liquid-Liquid Operations and Equipmen. In Perry’s Chemical Engineering Handbook, 8th ed.; Green, D.W., Perry, R.H., Eds.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Garcia-Perez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Characterization of bio-oils in chemical families. Biomass Bioenergy 2007, 31, 222–242. [Google Scholar] [CrossRef]
- Chum, H.; Diebold, J.; Scahill, J.; Johnson, D.; Black, S. Biomass Pyrolysis Oil Feedstocks for Phenolic Adhesives. In Adhesives from Renewable Resources; Hemingway, R.W., Conner, A.H., Branham, S.J., Eds.; American Chemical Society: Washington, DC, USA, 1989; pp. 135–151. [Google Scholar]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Kaleem, I.; Chun, L.; Tong, J. Production and characterization of bio-oil from hydrothermal liquefaction of microalgae dunaliella tertiolecta cake. Energy 2010, 35, 5406–5411. [Google Scholar] [CrossRef]
- Jazrawi, C.; Biller, P.; Ross, A.B.; Montoya, A.; Maschmeyer, T.; Haynes, B.S. Pilot plant testing of continuous hydrothermal liquefaction of microalgae. Algal Res. 2013, 2, 268–277. [Google Scholar] [CrossRef]
- Valdez, P.J.; Dickinson, J.G.; Savage, P.E. Characterization of product fractions from hydrothermal liquefaction of Nannochloropsis sp. and the influence of solvents. Energy Fuels 2011, 25, 3235–3243. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, S.; Gu, Y.; Xu, G.; Li, X.; Luo, Z. Separation characteristics of biomass pyrolysis oil in molecular distillation. Sep. Purif. Technol. 2010, 76, 52–57. [Google Scholar] [CrossRef]
- Capunitan, J.A.; Capareda, S.C. Characterization and separation of corn stover bio-oil by fractional distillation. Fuel 2013, 112, 60–73. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Y.; Liu, Q.; Yao, Y.; Guo, Z.; Luo, Z.; Cen, K. Separation of bio-oil by molecular distillation. Fuel Process. Technol. 2009, 90, 738–745. [Google Scholar] [CrossRef]
- Murwanashyaka, J.N.; Pakdel, H.; Roy, C. Seperation of syringol from birch wood-derived vacuum pyrolysis oil. Sep. Purif. Technol. 2001, 24, 155–165. [Google Scholar] [CrossRef]
- Boucher, M.E.; Chaala, A.; Roy, C. Bio-oils obtained by vacuum pyrolysis of softwood bark as a liquid fuel for gas turbines. Part I: Properties of bio-oil and its blends with methanol and a pyrolytic aqueous phase. Biomass Bioenergy 2000, 19, 337–350. [Google Scholar] [CrossRef]
- Padleckas, H. Continuous Binary Fractional Distillation. Available online: https://commons.wikimedia.org/wiki/File:Continuous_Binary_Fractional_Distillation.PNG (accessed on 6 May 2015).
- Diebold, J.P. A Review of the Chemical and Physical Mechanisms of the Storage Stability of Fast Pyrolysis Bio-Oils; National Renewable Energy Laboratory: Golden, CO, USA, 2000. [Google Scholar]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Elliott, D.C.; Oasmaa, A. Catalytic hydrotreating of black liquor oils. Energy Fuels 1991, 5, 102–109. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Phillips, C.B. Renewable fuels via catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2011, 397, 1–12. [Google Scholar] [CrossRef]
- Rezzoug, S.-A.; Capart, R. Liquefaction of wood in two successive steps: Solvolysis in ethylene-glycol and catalytic hydrotreatment. Appl. Energy 2002, 72, 631–644. [Google Scholar] [CrossRef]
- Diebold, J.P.; Czernik, S. Additives to lower and stabilize the viscosity of pyrolysis oils during storage. Energy Fuels 1997, 11, 1081–1091. [Google Scholar] [CrossRef]
- Elliott, D.C.; Baker, E.G. Catalytic Hydrotreating of Biomass Liquefaction Products to Produce Hydrocarbon Fuels: Interim Report; Report No. PNL-5844; Pacific Northwest Laboratory: Richland, WA, USA, 1986. [Google Scholar]
- Grange, P.; Laurent, E.; Maggi, R.; Centeno, A.; Delmon, B. Hydrotreatment of pyrolysis oils from biomass: Reactivity of the various categories of oxygenated compounds and preliminary techno-economical study. Catal. Today 1996, 29, 297–301. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Study of the hydrodeoxygenation of carbonyl, carboxylic and guaiacyl groups over sulfided CoMo/γ-Al2O3 and NiMo/γ-Al2O3 catalysts: I. Catalytic reaction schemes. Appl. Catal. A Gen. 1994, 109, 77–96. [Google Scholar] [CrossRef]
- Furimsky, E. Selection of catalysts and reactors for hydroprocessing. Appl. Catal. A Gen. 1998, 171, 177–206. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, J. Hydrodeoxygenation and hydrocracking of solvolysed lignocellulosic biomass by oxide, reduced and sulphide form of NiMo, Ni, Mo and Pd catalysts. Appl. Catal. B Environ. 2014, 150–151, 275–287. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, J. Hydrotreatment of solvolytically liquefied lignocellulosic biomass over NiMo/Al2O3 catalyst: Reaction mechanism, hydrodeoxygenation kinetics and mass transfer model based on FTIR. Biomass Bioenergy 2014, 63, 300–312. [Google Scholar] [CrossRef]
- Furimsky, E. Hydroprocessing challenges in biofuels production. Catal. Today 2013, 217, 13–56. [Google Scholar] [CrossRef]
- Capunitan, J.A.; Capareda, S.C. Hydrotreatment of corn stover bio-oil using noble metal catalysts. Fuel Process. Technol. 2014, 125, 190–199. [Google Scholar] [CrossRef]
- Yakovlev, V.A.; Khromova, S.A.; Sherstyuk, O.V.; Dundich, V.O.; Ermakov, D.Y.; Novopashina, V.M.; Lebedev, M.Y.; Bulavchenko, O.; Parmon, V.N. Development of new catalytic systems for upgraded bio-fuels production from bio-crude-oil and biodiesel. Catal. Today 2009, 144, 362–366. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, P.; Xu, Y.; Wang, B.; Wang, F.; Zhang, L. Catalytic upgrading of duckweed biocrude in subcritical water. Bioresour. Technol. 2014, 166, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Zhang, X.S.; Qv, Y.C.; Jiang, H.; Yu, H.Q. Bio-oil upgrading at ambient pressure and temperature using zero valent metals. Green Chem. 2012, 14, 2226–2233. [Google Scholar] [CrossRef]
- Melero, J.A.; García, A.; Iglesias, J. 7-Biomass catalysis in conventional refineries. In Advances in Clean Hydrocarbon Fuel Processing; Khan, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 199–240. [Google Scholar]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; O’Connor, P. Processing biomass-derived oxygenates in the oil refinery: Catalytic cracking (FCC) reaction pathways and role of catalyst. J. Catal. 2007, 247, 307–327. [Google Scholar] [CrossRef]
- Hew, K.L.; Tamidi, A.M.; Yusup, S.; Lee, K.T.; Ahmad, M.M. Catalytic cracking of bio-oil to organic liquid product (OLP). Bioresour. Technol. 2010, 101, 8855–8858. [Google Scholar] [CrossRef] [PubMed]
- Bertero, M.; Sedran, U. Conversion of pine sawdust bio-oil (raw and thermally processed) over equilibrium FCC catalysts. Bioresour. Technol. 2013, 135, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Adjaye, J.D.; Bakhshi, N.N. Upgrading of a wood-derived oil over various catalysts. Biomass Bioenergy 1994, 7, 201–211. [Google Scholar] [CrossRef]
- Adjaye, J.D.; Bakhshi, N.N. Catalytic conversion of a biomass-derived oil to fuels and chemicals I: Model-compound studies and reaction pathways. Biomass Bioenergy 1995, 8, 131–149. [Google Scholar] [CrossRef]
- Adjaye, J.D.; Bakhshi, N.N. Catalytic conversion of a biomass-derived oil to fuels and chemicals II: Chemical kinetics, parameter estimation and model predictions. Biomass Bioenergy 1995, 8, 265–277. [Google Scholar] [CrossRef]
- Gevert, B.S.; Otterstedt, J.-E. Upgrading of directly liquefied biomass to transportation fuels: Catalytic cracking. Biomass 1987, 14, 173–183. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.J.; Xu, Y. Upgrading bio-oil over different solid catalysts. Energy Fuels 2006, 20, 2717–2720. [Google Scholar] [CrossRef]
- Peng, J.; Chen, P.; Lou, H.; Zheng, X. Catalytic upgrading of bio-oil by HZSM-5 in sub- and super-critical ethanol. Bioresour. Technol. 2009, 100, 3415–3418. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, Z.; Yu, C.; Li, G.; Yang, Y.; Zhang, H. Upgrading of bio-oil in supercritical ethanol: Catalysts screening, solvent recovery and catalyst stability study. J. Supercrit. Fluids 2014, 95, 387–393. [Google Scholar] [CrossRef]
- Mahfud, F.H.; Melin-Cabrera, I.; Manurung, R.; Heeres, H.J. Biomass to fuels: Upgrading of flash pyrolysis oil by reactive distillation using a high boiling alcohol and acid catalysts. Process. Saf. Environ. Prot. 2007, 85, 466–472. [Google Scholar] [CrossRef]
- Xu, J.M.; Jiang, J.C.; Sun, Y.J.; Lu, Y.J. A novel method of upgrading bio-oil by reactive rectification. J. Fuel Chem. Technol. 2008, 36, 421–425. [Google Scholar] [CrossRef]
- Tang, Z.; Lu, Q.; Zhang, Y.; Zhu, X.; Guo, Q. One step bio-oil upgrading through hydrotreatment, esterification, and cracking. Ind. Eng. Chem. Res. 2009, 48, 6923–6929. [Google Scholar] [CrossRef]
- Shi, W.; Gao, Y.; Song, S.; Zhao, Y. One-pot conversion of bio-oil to diesel- and jet-fuel-range hydrocarbons in supercritical cyclohexane. Ind. Eng. Chem. Res. 2014, 53, 11557–11565. [Google Scholar] [CrossRef]
- Zhu, Y.; Biddy, M.J.; Jones, S.B.; Elliott, D.C.; Schmidt, A.J. Techno-economic analysis of liquid fuel production from woody biomass via hydrothermal liquefaction (HTL) and upgrading. Appl. Energy 2014, 129, 384–394. [Google Scholar] [CrossRef]
- Zhu, Y.; Albrecht, K.O.; Elliott, D.C.; Hallen, R.T.; Jones, S.B. Development of hydrothermal liquefaction and upgrading technologies for lipid-extracted algae conversion to liquid fuels. Algal Res. 2013, 2, 455–464. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Kampman, B.; den Boer, L.; Croezen, H. Biofuels Under Development. An Analysis of Currently Available and Future Biofuels, and a Comparison with Biomass Application in Other Sectors; Netherlands Petroleum Industry Association: Delft, The Netherlands, 2005. [Google Scholar]
- Liu, X.; Saydah, B.; Eranki, P.; Colosi, L.M.; Greg Mitchell, B.; Rhodes, J.; Clarens, A.F. Pilot-scale data provide enhanced estimates of the life cycle energy and emissions profile of algae biofuels produced via hydrothermal liquefaction. Bioresour. Technol. 2013, 148, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Elgowainy, A.; Han, J.; Wang, Z. Life cycle comparison of hydrothermal liquefaction and lipid extraction pathways to renewable diesel from algae. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 137–158. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Heuser, B.; Boot, M.; Kremer, F.; Pischinger, S. Performance and Emissions of Lignin and Cellulose Based Oxygenated Fuels in a Compression-Ignition Engine; SAE Technical Paper 2015-01-0910; SAE International: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).