Online Internal Temperature Estimation for Lithium-Ion Batteries Based on Kalman Filter
Abstract
:1. Introduction
2. Online Internal Temperature Estimation Based on a KF
2.1. Internal Temperature Estimation Model
2.2. State Space Model
2.3. Parameters Identification
2.3.1. Thermal Parameter Identification
| Parameters | Ru (K/W) | Rc (K/W) | Cc (J/K) | Cs (J/K) |
|---|---|---|---|---|
| ±40 A current pulse (1.5 h) | 0.260 | 0.864 | 1067 | 545.3 |
| ±60 A current pulse (1.5 h) | 0.255 | 0.873 | 1069 | 548.1 |
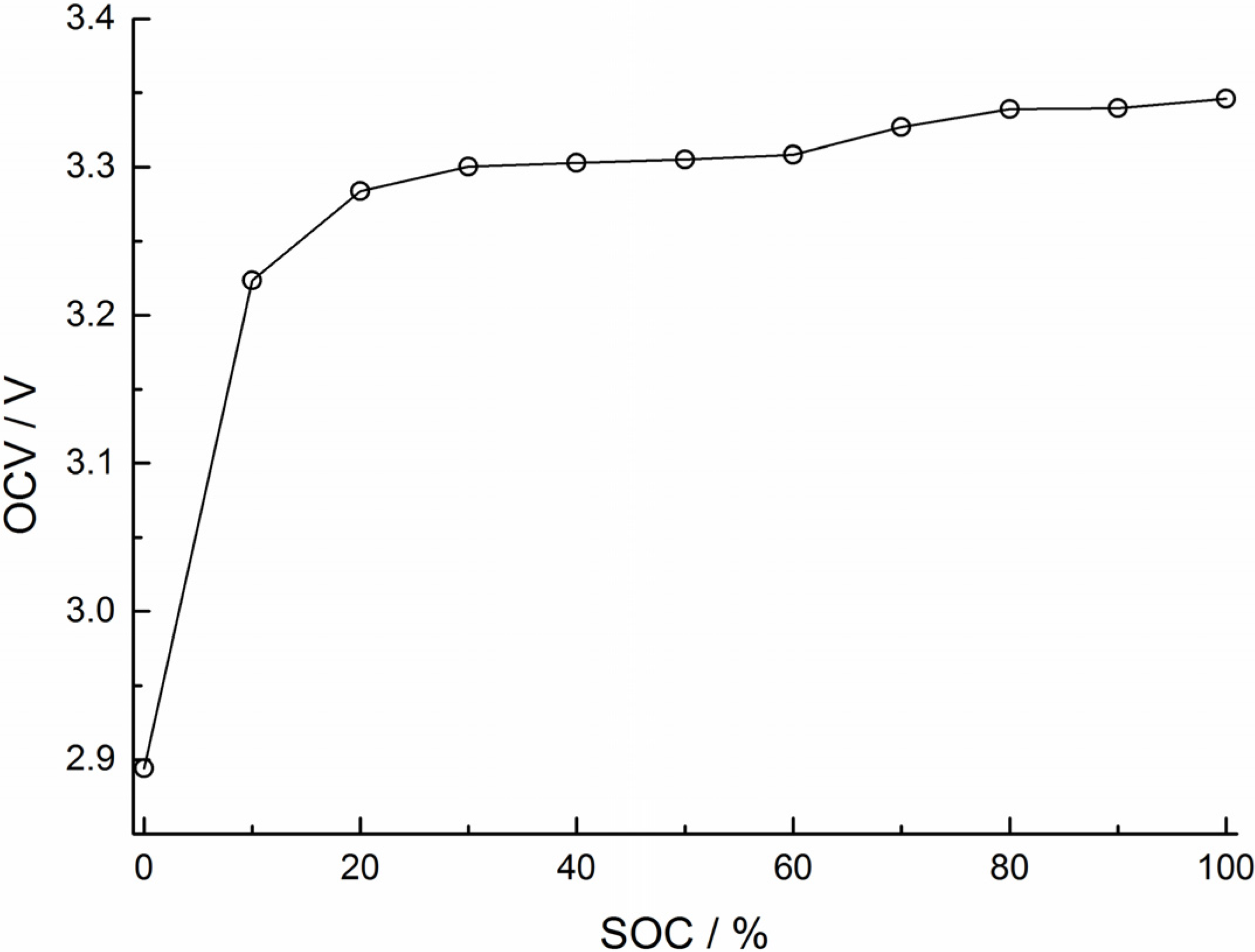
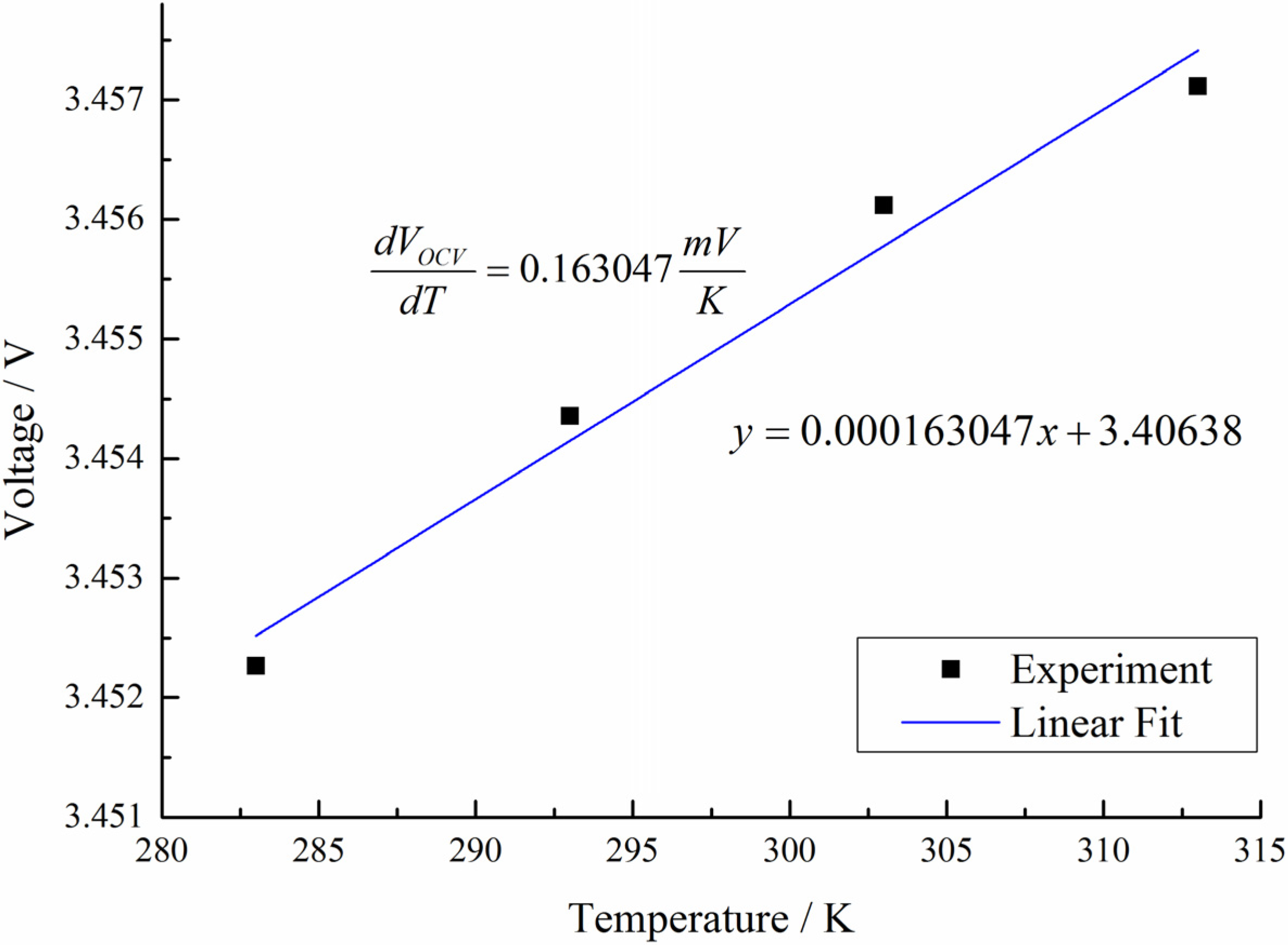
2.3.2. Electrical parameters identification

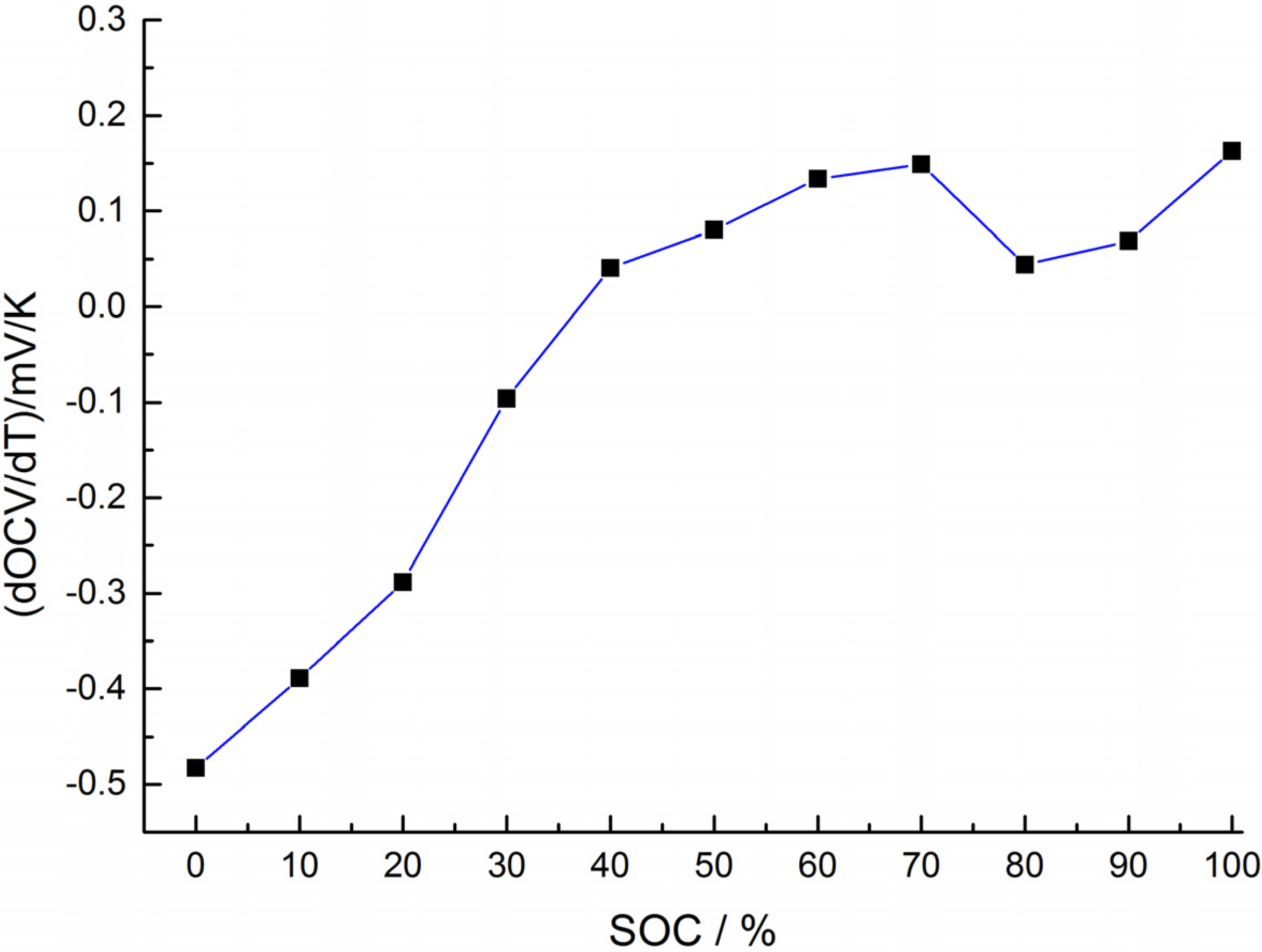
3. KF-Based Internal Temperature Estimation
4. Experiment Design
4.1. Battery Charge/Discharge Cycle Test
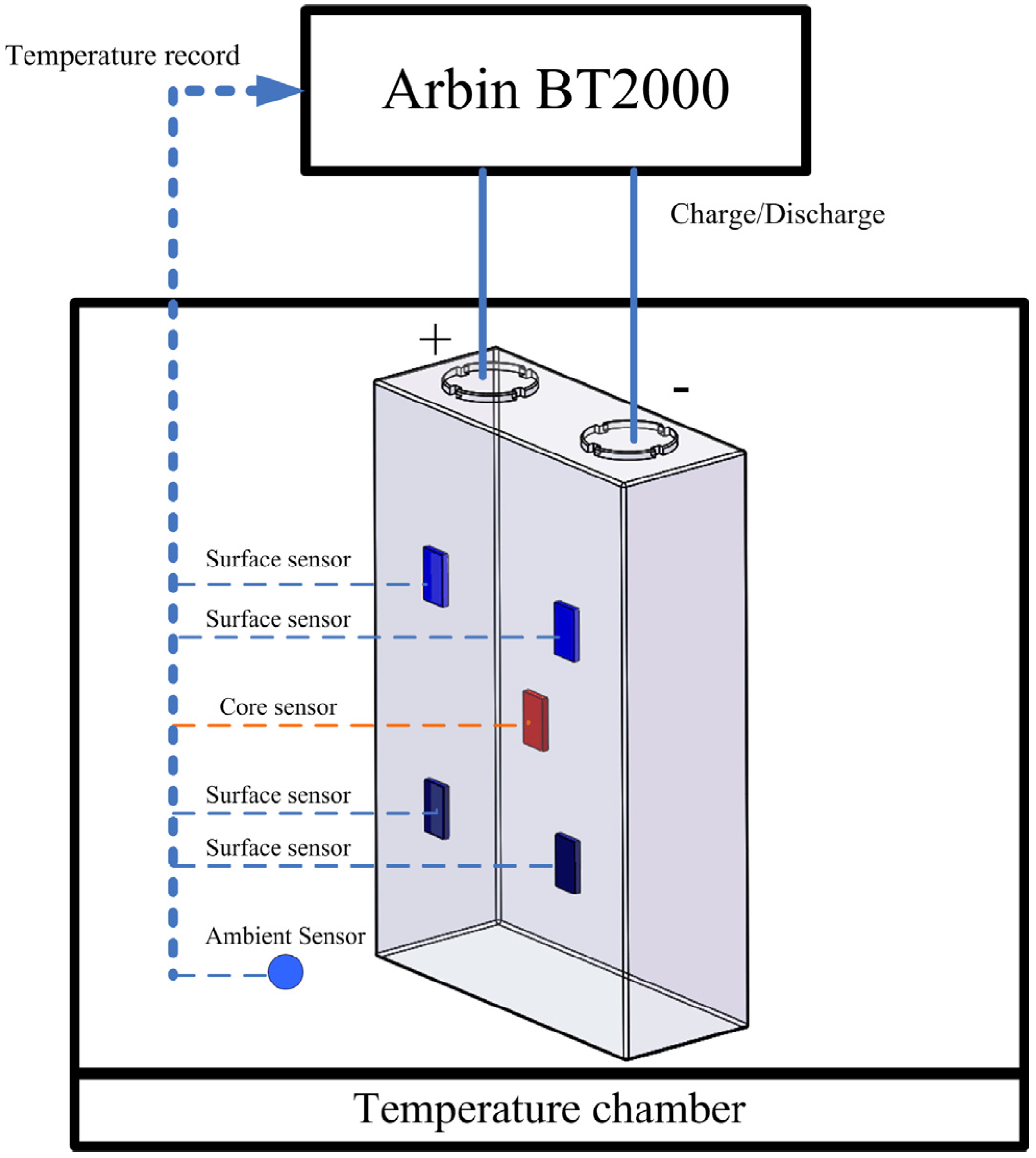
4.2. Measurement Equipment
4.3. Experiment Object
5. Results and Discussion
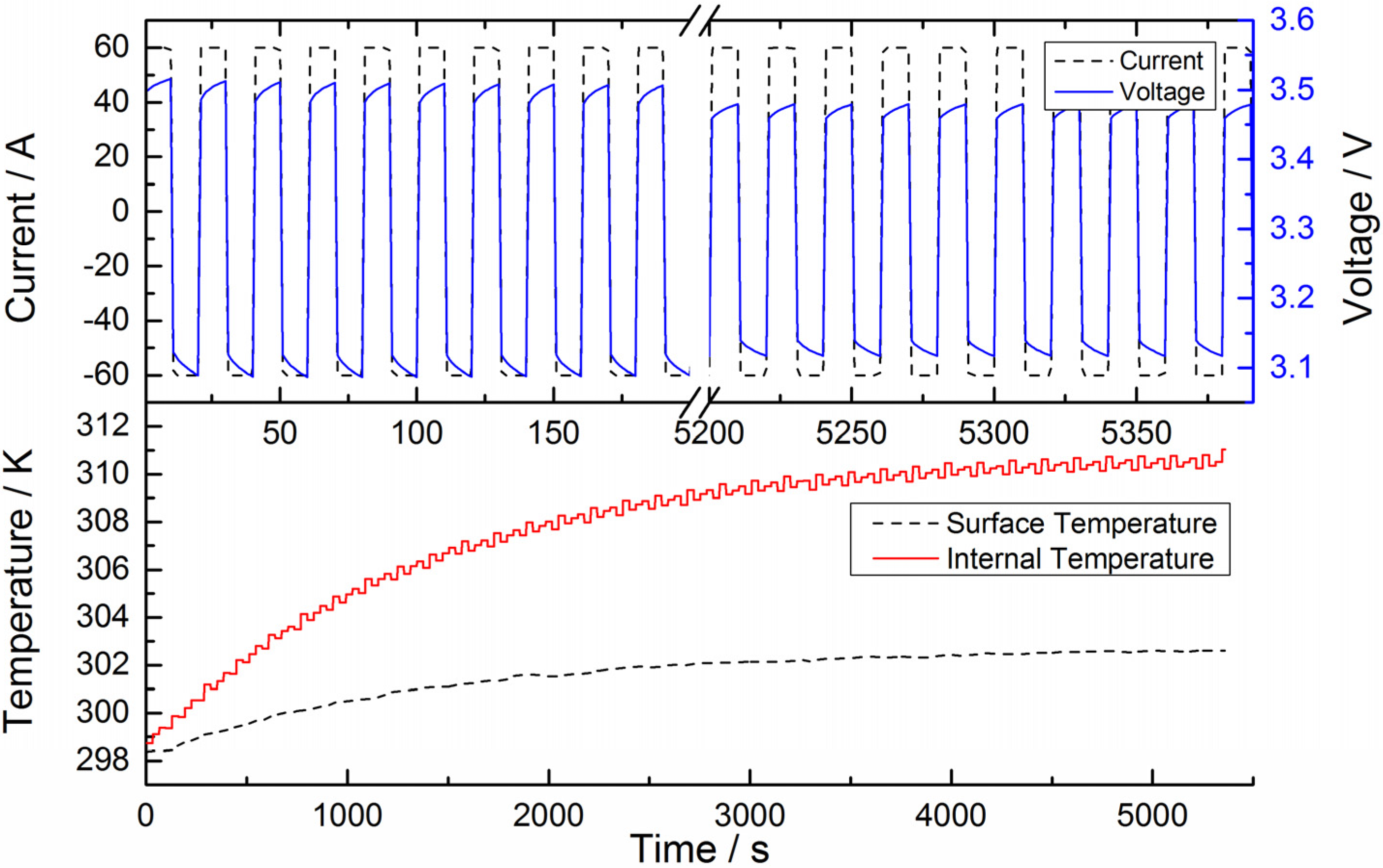
5.1. Heat Generation Analysis
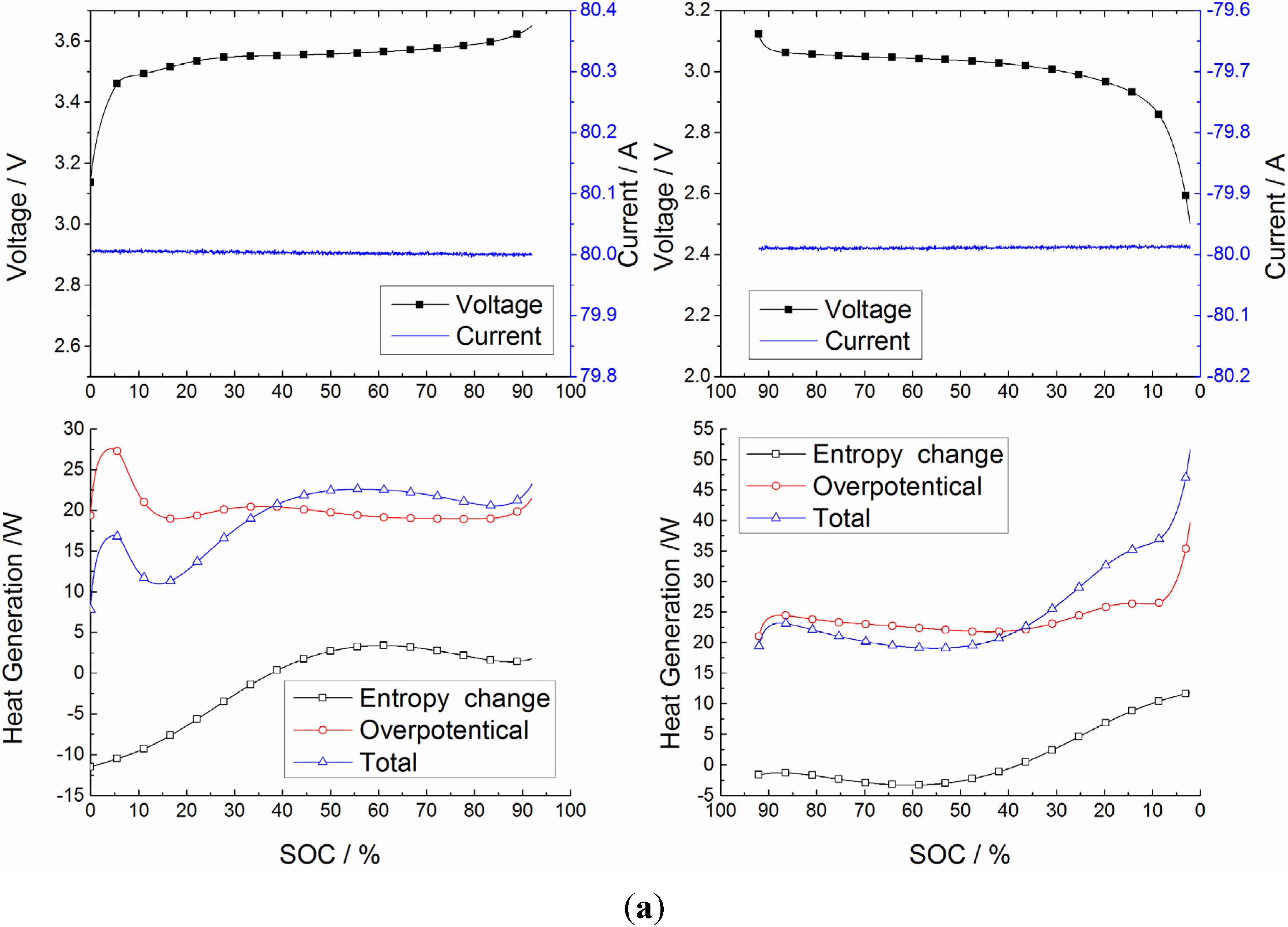

5.2. Validation of Battery Internal Temperature Estimation
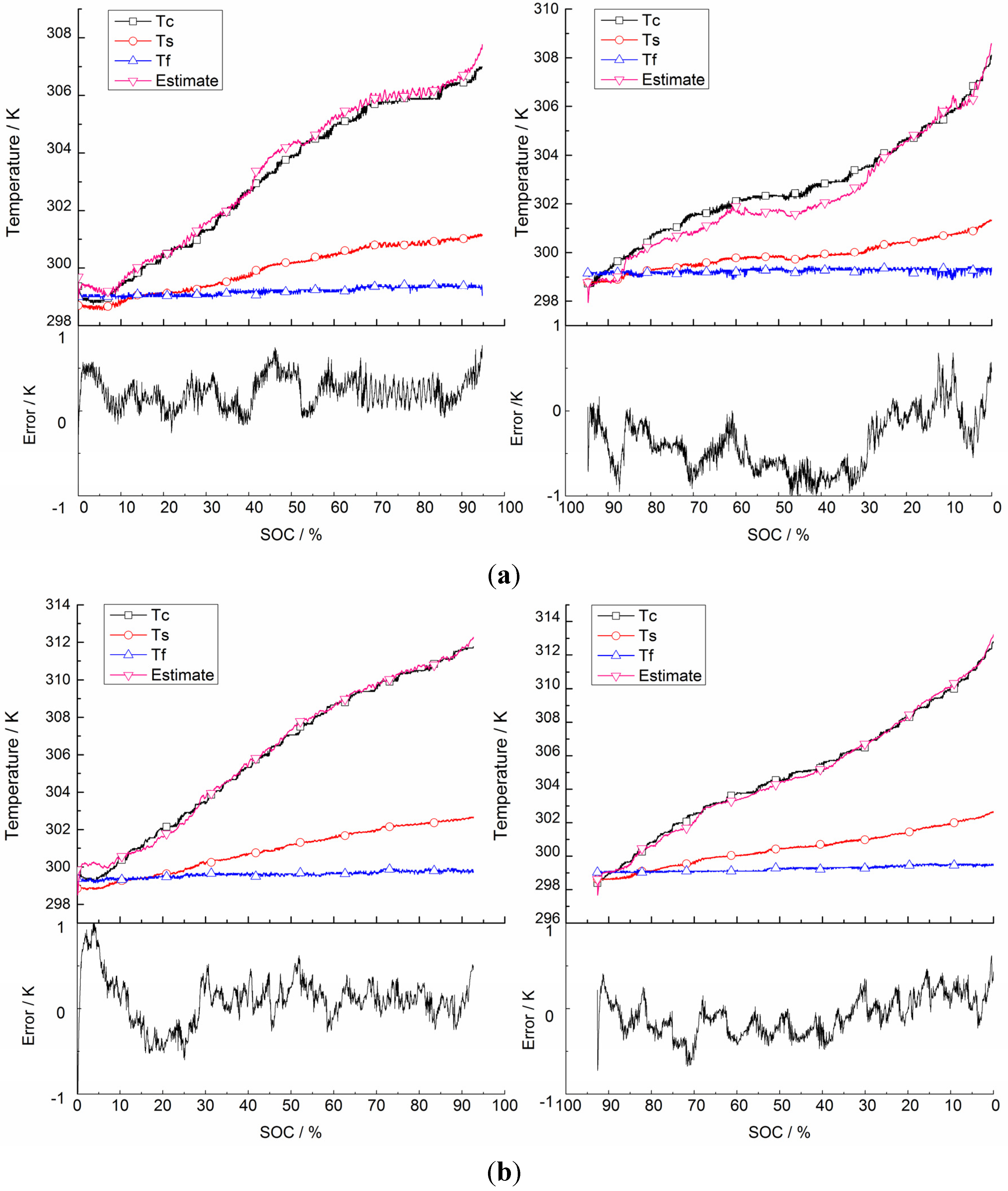
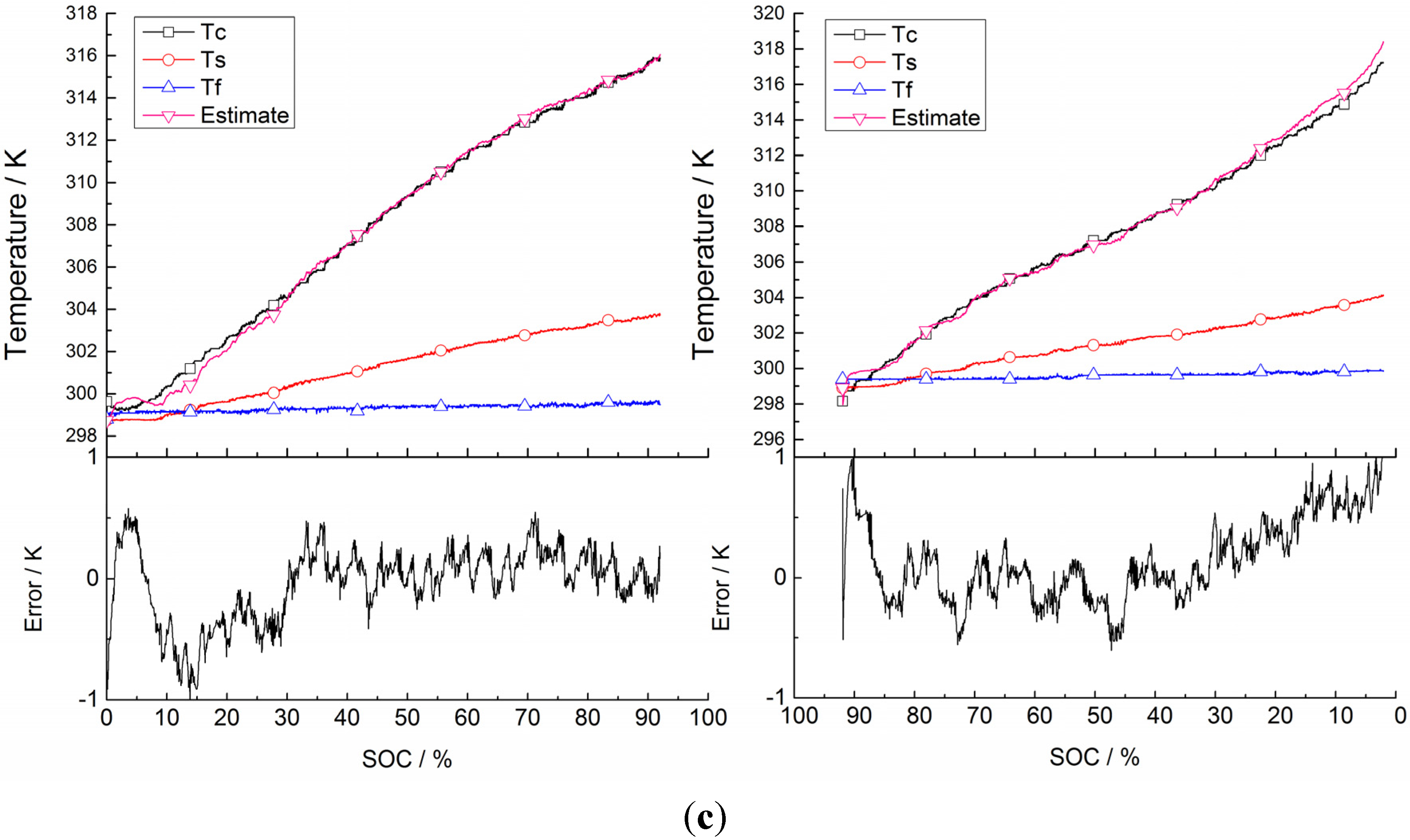
| Current | Current Direction | RMSE |
|---|---|---|
| 40 A | Charge/Discharge | 0.287145/0.502787 |
| 60 A | Charge/Discharge | 0.294537/0.243265 |
| 80 A | Charge/Discharge | 0.301420/0.362020 |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Richardson, R.R.; Ireland, P.T.; Howey, D.A. Battery internal temperature estimation by combined impedance and surface temperature measurement. J. Power Sources 2014, 265, 254–261. [Google Scholar] [CrossRef]
- Forgez, C.; Do, D.V.; Friedrich, G.; Morcrette, M.; Delacourt, C. Thermal modeling of a cylindrical lifepo4/graphite lithium-ion battery. J. Power Sources 2010, 195, 2961–2968. [Google Scholar] [CrossRef]
- Debert, M.; Colin, G.; Bloch, G.; Chamaillard, Y. An observer looks at the cell temperature in automotive battery packs. Control Eng. Pract. 2013, 21, 1035–1042. [Google Scholar] [CrossRef]
- Lin, X.; Perez, H.E.; Siegel, J.B.; Stefanopoulou, A.G.; Li, Y.; Anderson, R.D.; Ding, Y.; Castanier, M.P. Online parameterization of lumped thermal dynamics in cylindrical lithium ion batteries for core temperature estimation and health monitoring. IEEE Trans. Control Syst. Technol. 2013, 21, 1745–1755. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, R1–R25. [Google Scholar] [CrossRef]
- Dong, H.J.; Seung, M.B. Thermal modeling of cylindrical lithium ion battery during discharge cycle. Energy Convers. Manag. 2011, 52, 2973–2981. [Google Scholar] [CrossRef]
- Rad, M.S.; Danilov, D.L.; Baghalha, M.; Kazemeini, M.; Notten, P.H.L. Adaptive thermal modeling of Li-ion batteries. Electrochim. Acta 2013, 102, 183–195. [Google Scholar] [CrossRef]
- Samba, A.; Omar, N.; Gualous, H.; Firouz, Y.; van den Bossche, P.; van Mierlo, J.; Boubekeur, T.I. Development of an advanced two-dimensional thermal model for large size lithium-ion pouch cells. Electrochim. Acta 2014, 117, 246–254. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Ramadass, P.; Zhang, J.Z. Analysis of internal short-circuit in a lithium ion cell. J. Power Sources 2009, 194, 550–557. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.B.; Wu, B.; Huang, J.; Nie, Z.H.; Sun, Y.; An, F.Q.; Wu, N.N. Examining temporal and spatial variations of internal temperature in large-format laminated battery with embedded thermocouples. J. Power Sources 2013, 241, 536–553. [Google Scholar] [CrossRef]
- Mutyala, M.; Zhao, J.Z.; Li, J.Y.; Pan, H.G.; Yuan, C.; Li, X.C. In-situ temperature measurement in lithium ion battery by transferable flexible thin film thermocouples. J. Power Sources 2014, 260, 43–49. [Google Scholar] [CrossRef]
- Thomas, K.E.; Newman, J. Thermal modeling of porous insertion electrodes. J. Electrochem. Soc. 2003, 150, A176–A192. [Google Scholar] [CrossRef]
- Lin, X.F.; Perez, H.E.; Mohan, S.; Siegel, J.B.; Stefanopoulou, A.G.; Ding, Y.; Castanier, M.P. A lumped-parameter electro-thermal model for cylindrical batteries. J. Power Sources 2014, 257, 1–11. [Google Scholar] [CrossRef]
- Pei, L.; Wang, T.; Lu, R.; Zhu, C. Development of a voltage relaxation model for rapid open-circuit voltage prediction in lithium-ion batteries. J. Power Sources 2014, 253, 412–418. [Google Scholar] [CrossRef]
- Williford, R.E.; Viswanathan, V.V.; Zhang, J.-G. Effects of entropy changes in anodes and cathodes on the thermal behavior of lithium ion batteries. J. Power Sources 2009, 189, 101–107. [Google Scholar] [CrossRef]
- Plett, G.L. Extended kalman filtering for battery management systems of LiPB-based HEV battery packs: Part 1. Background. J. Power Sources 2004, 134, 252–261. [Google Scholar] [CrossRef]
- Pei, L.; Zhu, C.; Wang, T.; Lu, R.; Chan, C.C. Online peak power prediction based on a parameter and state estimator for lithium-ion batteries in electric vehicles. Energy 2014, 66, 766–778. [Google Scholar] [CrossRef]
- Kalman, R.E. A new approach to linear filtering and prediction problems. Trans. ASME J. Basic Eng. 1960, 82, 35–45. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wei, G.; Pei, L.; Lu, R.; Song, K.; Wu, C.; Zhu, C. Online Internal Temperature Estimation for Lithium-Ion Batteries Based on Kalman Filter. Energies 2015, 8, 4400-4415. https://doi.org/10.3390/en8054400
Sun J, Wei G, Pei L, Lu R, Song K, Wu C, Zhu C. Online Internal Temperature Estimation for Lithium-Ion Batteries Based on Kalman Filter. Energies. 2015; 8(5):4400-4415. https://doi.org/10.3390/en8054400
Chicago/Turabian StyleSun, Jinlei, Guo Wei, Lei Pei, Rengui Lu, Kai Song, Chao Wu, and Chunbo Zhu. 2015. "Online Internal Temperature Estimation for Lithium-Ion Batteries Based on Kalman Filter" Energies 8, no. 5: 4400-4415. https://doi.org/10.3390/en8054400
APA StyleSun, J., Wei, G., Pei, L., Lu, R., Song, K., Wu, C., & Zhu, C. (2015). Online Internal Temperature Estimation for Lithium-Ion Batteries Based on Kalman Filter. Energies, 8(5), 4400-4415. https://doi.org/10.3390/en8054400






