Contribution of Vertical Methane Flux to Shallow Sediment Carbon Pools across Porangahau Ridge, New Zealand
Abstract
:1. Introduction
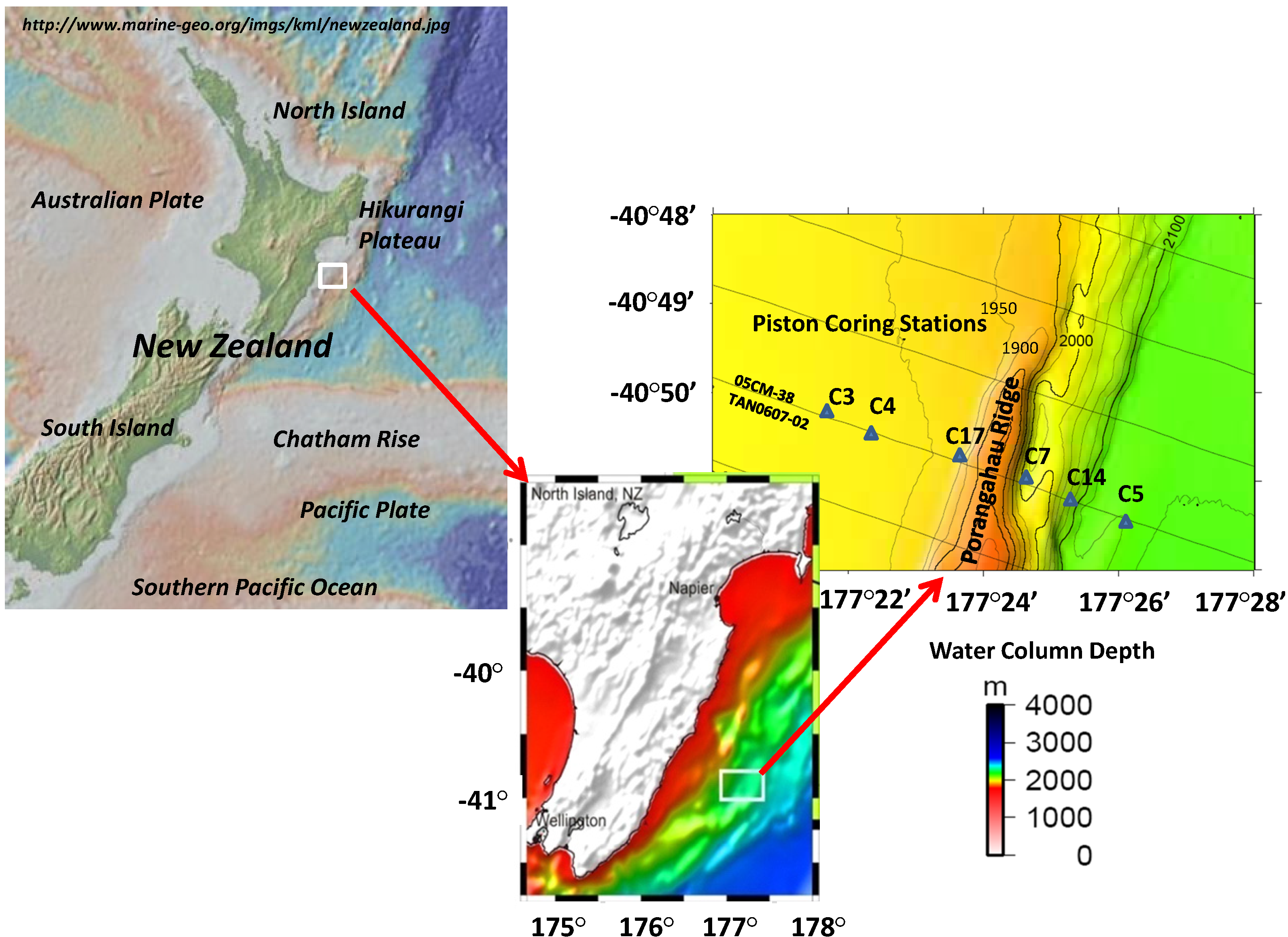
2. Methods
2.1. Regional Description
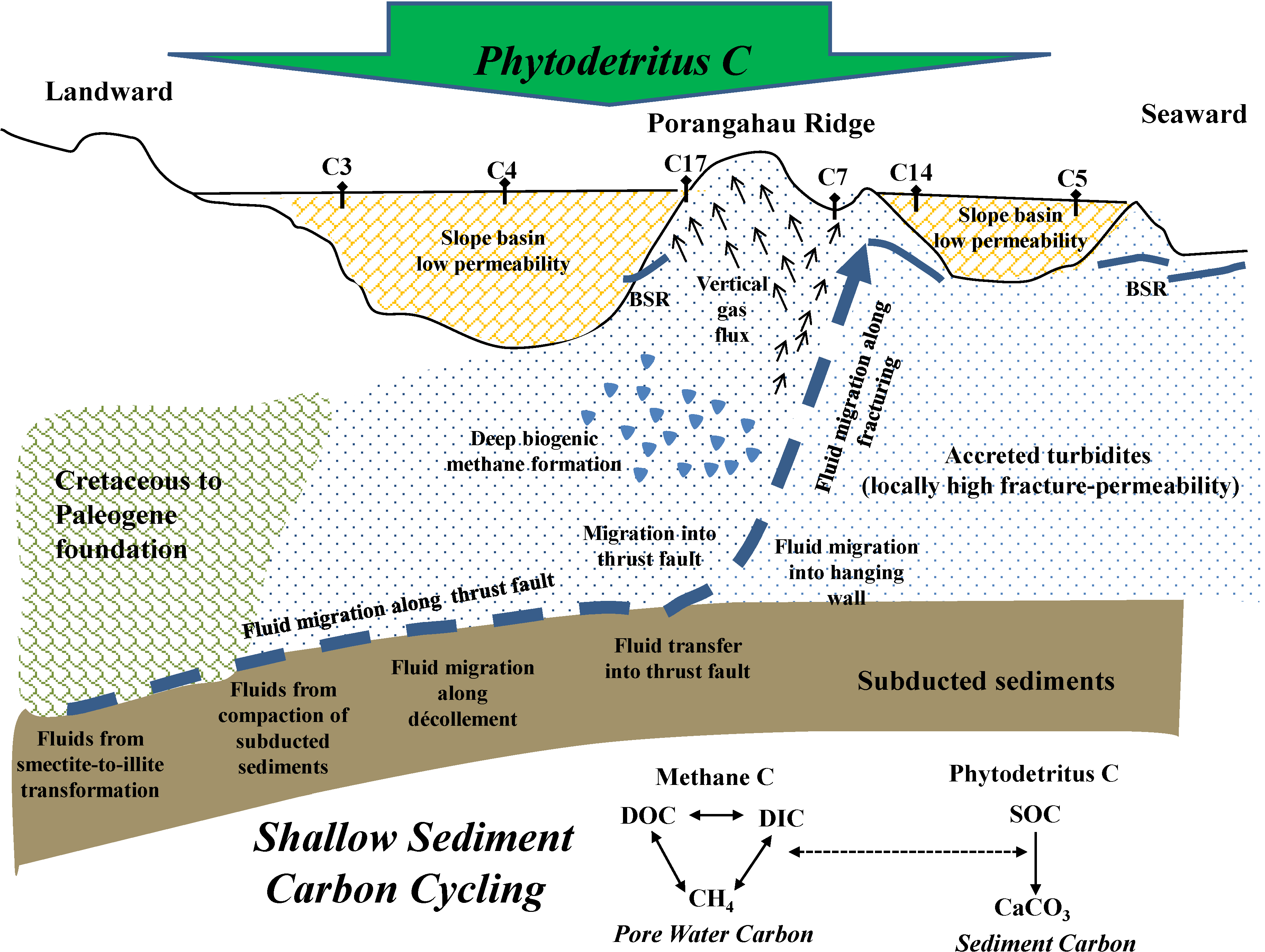
2.2. Sample Collection and Analysis
2.3. Carbon Pool Data Interpretation


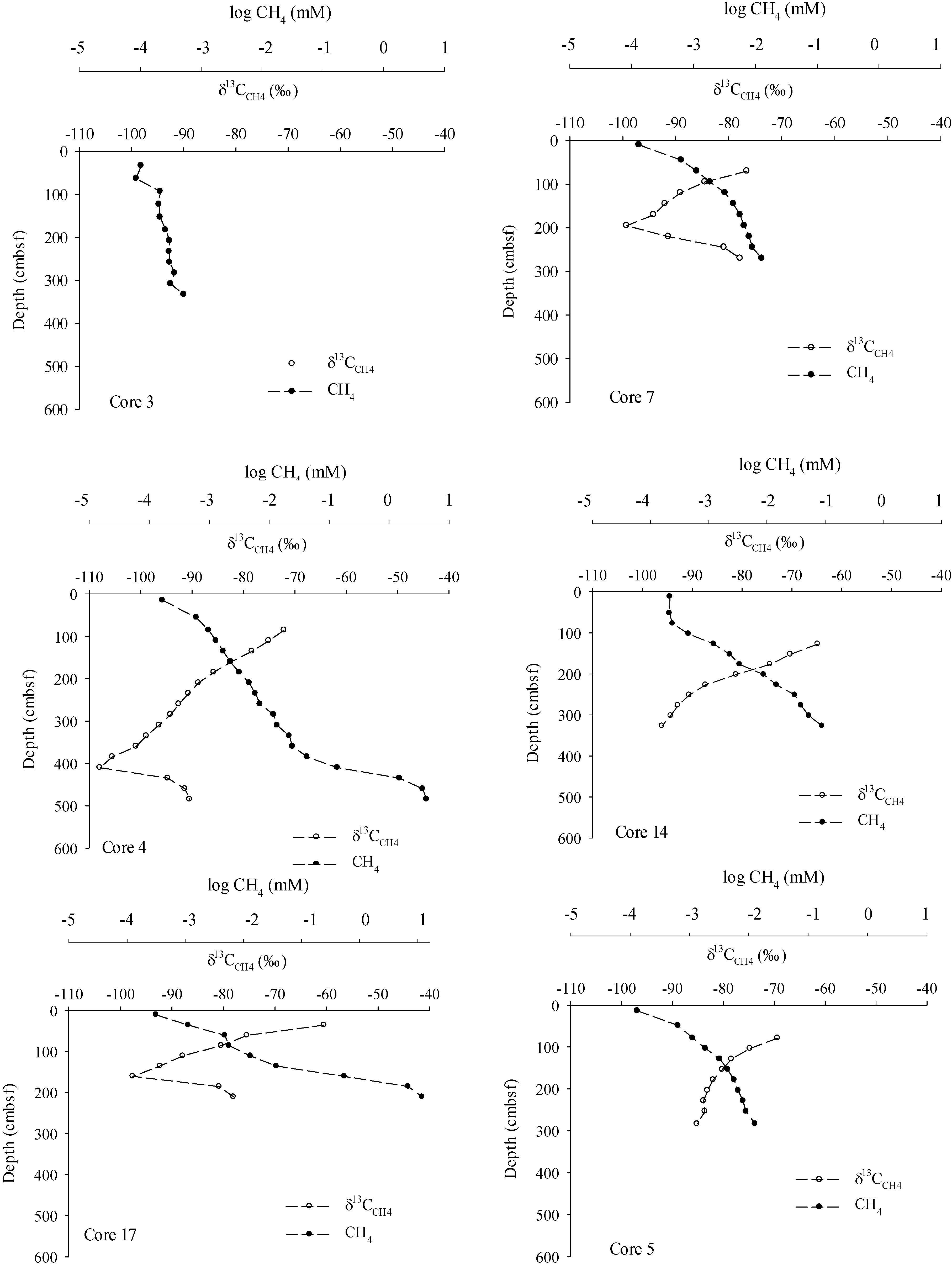
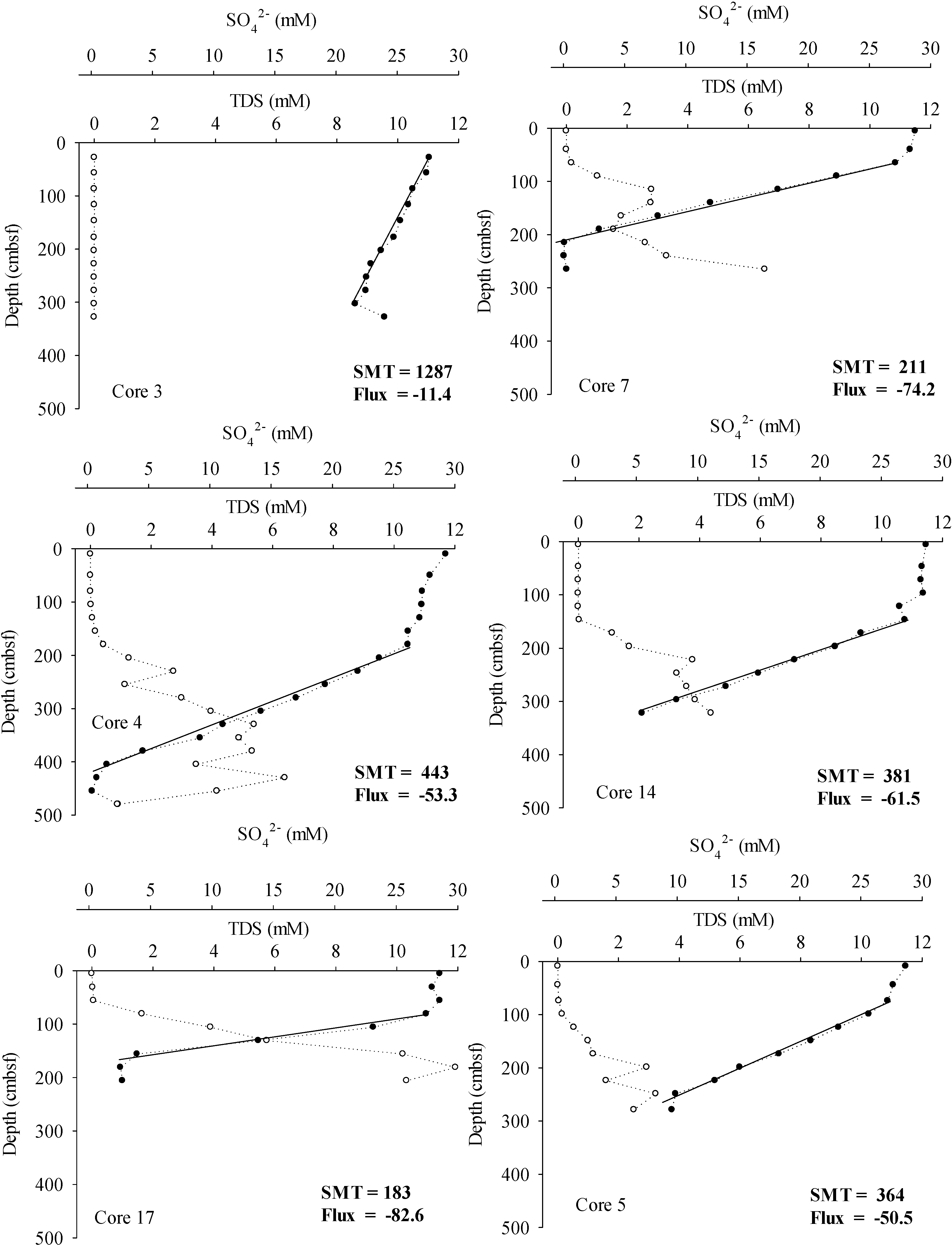
3. Results


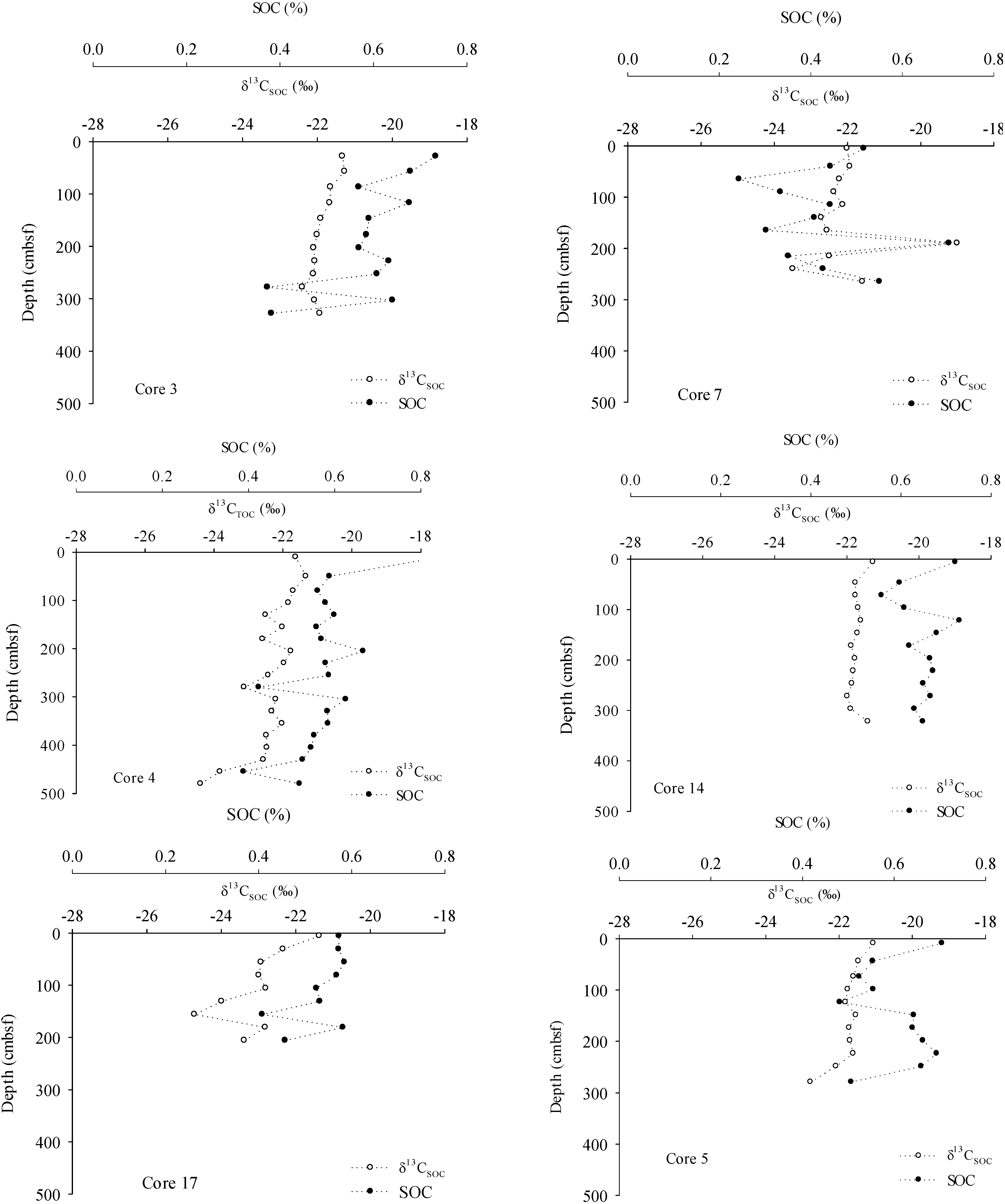
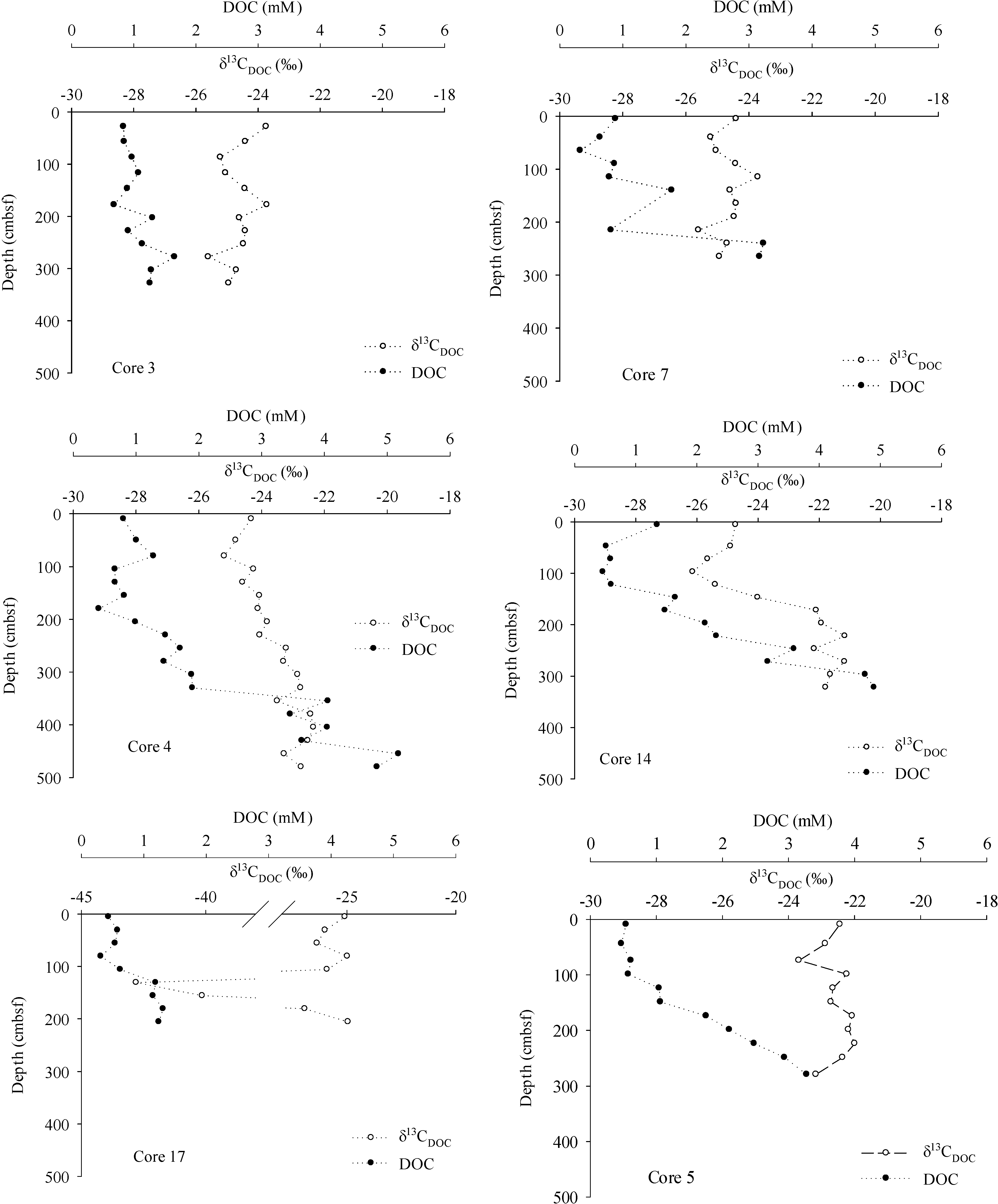
4. Discussion
4.1. Methane Source, Vertical Flux and Cycling
4.2. Inorganic Carbon Pools in Porangahau Ridge Sediments
4.3. Organic Carbon Pools in Porangahau Ridge Sediments
4.4. Methane Contribution to Shallow Sediment Carbon Pools
| Station | DIC | DOC | CaCO3 | SOC |
|---|---|---|---|---|
| δ13C∆ | ||||
| C3 | −31.4 | −26.5 | 0.0 | −20.8 |
| C4 A | −31.6 | −22.6 | 1.0 | −19.5 |
| B | −41.3 | −22.3 | 1.1 | −20.2 |
| C17 A | −48.8 | −36.5 | −56.6 | −17.8 |
| B | −49.2 | −40.9 | −58.0 | −17.1 |
| C7 A | −33.4 | −22.8 | −22.6 | −21.2 |
| B | −46.6 | −22.6 | −23.0 | −21.6 |
| C14 | −35.0 | −21.1 | 0.8 | −20.6 |
| C5 | −31.4 | −22.6 | 0.0 | −20.3 |
| Core | SOC | CaCO3 | DOC | DIC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| End members | Samples | End members | Samples | End members | Samples | End members | Samples | |||||
| PD | DIC * | SOC | PD | CH4 | TIC | PD | DIC * | DOC | PD | CH4 | DIC | |
| C4 | −21.7 | −41.5 | −24.4 | −0.3 | −83.3 | −2.7 | −21.7 | −41.5 | −23.3 | −21.7 | −83.3 | −41.5 |
| C17 | −21.7 | −47.6 | −24.7 | −0.3 | −83.3 | −43.9 | −21.7 | −47.6 | −42.8 | −21.7 | −83.3 | −47.6 |
| C7 | −21.7 | −38.5 | −23.4 | −0.3 | −83.3 | −15.0 | −21.7 | −38.5 | −26.6 | −21.7 | −83.3 | −38.5 |
| Core | DIC | DOC | CaCO3 | SOC |
|---|---|---|---|---|
| C4 | 32% | 7% | 3% | 12% |
| C17 | 47% | 71% | 55% | 8% |
| C7 | 33% | 24% | 19% | 8% |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Milliman, J. Flux and fate of fluvial sediment and water in coastal seas. In Ocean Margin Processes in Global Change; Mantoura, R.F.C., Martin, J., Wollast, R., Eds.; Wiley: New York, NY, USA, 1991; pp. 69–90. [Google Scholar]
- Goñi, M.A.; Ruttenberg, K.C.; Eglinton, T.I. Sources and contribution of terrigenous organic carbon to surface sediments in the Gulf of Mexico. Nature 1997, 389, 275–278. [Google Scholar]
- Mayer, L.M.; Schick, L.L.; Allison, M.A.; Rutthenberg, K.C.; Bentley, S.J. Marine vs. terrigenous organic matter in Louisiana coastal sediments: The uses of bromine:organic carbon ratios. Mar. Chem. 2007, 107, 244–254. [Google Scholar]
- Kelley, C.A.; Coffin, R.B.; Cifuentes, L.A. Stable isotope evidence for alternate carbon sources in the Gulf of Mexico. Limnol. Oceanogr. 1998, 43, 1962–1969. [Google Scholar]
- Wang, X.-C.; Chen, R.F.; Whelan, J.; Eglinton, T. Contribution of “old” carbon from natural marine hydrocarbon seeps to sedimentary and dissolved organic carbon pools in the Gulf of Mexico. Geophys. Res. Lett. 2001, 28, 3313–3316. [Google Scholar] [CrossRef]
- Joye, S.B.; Boetius, A.; Orcutt, B.N.; Montoya, J.P.; Schulz, H.N.; Erickson, M.J.; Lugo, S.K. The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chem. Geol. 2004, 205, 219–238. [Google Scholar] [CrossRef]
- Paull, C.K.; Ussler, W., III; Lorenson, T.; Winters, W.; Dougherty, J. Geochemical constraints on the distribution of gas hydrates in the Gulf of Mexico. Geol. Mar. Lett. 2005, 25, 273–280. [Google Scholar]
- Ruppel, C.; Dickens, G.R.; Castellini, D.G.; Gilhooly, W.; Lizarralde, D. Heat and salt inhibition of gas hydrate formation in the northern Gulf of Mexico. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Lapham, L.L.; Chanton, J.P.; Martens, C.S.; Sleeper, K.; Woolsey, J.R. Microbial activity in surficial sediments overlying acoustic wipeout zones at a Gulf of Mexico cold seep. Geochem. Geophy. Geosyst. 2008, 9. [Google Scholar] [CrossRef]
- Pohlman, J.W.; Bauer, J.E.; Waite, W.F.; Osburn, C.L.; Chapman, N.R. Methane hydrate-bearing seeps as a source of aged dissolved organic carbon to the oceans. Nat. Geosci. 2011, 4, 37–41. [Google Scholar] [CrossRef]
- McCarthy, M.D.; Beaupré, S.R.; Walker, B.D.; Voparil, I.; Guilderson, T.P.; Druffel, E.R.M. Chemosynthetic origin of 14C-depleted dissolved organic matter in a ridge-flank hydrothermal system. Nat. Geosci. 2011, 4. [Google Scholar] [CrossRef]
- Pohlman, J.W.; Kaneko, M.; Heuer, V.B.; Coffin, R.B.; Whiticar, M. Methane soucrces and production in the north Cascadia Margin gas hydrate system. Earth Planet. Sci. Lett. 2009, 287, 504–512. [Google Scholar] [CrossRef]
- Coffin, R.B.; Hamdan, L.; Plummer, R.; Smith, J.; Gardner, J.; Wood, W.T. Analysis of methane and sulfate flux in methane charged sediments from the Mississippi Canyon, Gulf of Mexico. Mar. Petrol. Geol. 2008, 25, 977–987. [Google Scholar] [CrossRef]
- Reitner, J.; Peckmann, J.; Reimer, A.; Schumann, G.; Thiel, V. Methane-derived carbonate build-ups and associated microbial communities at cold seeps on the lower Crimean shelf. (Black Sea). Facies 2005, 51, 66–79. [Google Scholar]
- Coffin, R.B.; Pohlman, J.W.; Gardner, J.; Downer, R.; Wood, W.; Hamdan, L.; Walker, S.; Plummer, R.; Gettrust, J.; Diaz, J. Methane hydrate exploration on the mid Chilean coast: A geochemical and geophysical survey. Am. Chem. Soc. Div. Pet. Chem. 2007, 56, 32–41. [Google Scholar]
- Coffin, R.B.; Hamdan, L.; Smith, J.P.; Plummer, R.; Millholland, L.; Larson, R. Spatial variation in shallow sediment methane source and cycling on the Alaskan Beaufort Sea. Mar. Pet. Geol. 2013, 45. [Google Scholar] [CrossRef]
- Borowski, W.S.; Paull, C.K.; Ussler, W., III. Marine porewater sulfate profiles indicate in situ methane flux from underlying gas hydrate. Geology 1996, 24, 655–658. [Google Scholar] [CrossRef]
- Borowski, W.S.; Paull, C.K.; Ussler, W., III. Global and local variations of interstitial sulfate gradients in the deep-water, continental margin sediments: Sensitivity to underlying methane and gas hydrates. Mar. Geol. 1999, 159, 131–154. [Google Scholar] [CrossRef]
- Orphan, V.J.; House, C.H.; Hinrichs, K.-U.; McKeegan, K.D.; DeLong, E.F. Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 2001, 293, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Valentine, D.L. Biogeochemistry and microbial ecology of methane oxidation in anoxic environments: A review. Antonie Leeuwenhoek 2002, 81, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Wehrmann, L.M.; Risgaard-Petersen, N.; Schrum, H.N.; Walsh, E.A.; Huh, Y.; Ikehara, M.; Pierre, C.; D’Hondt, S.; Ferdelman, R.G.; Ravelo, A.C.; et al. Couple organic and inorganic carbon cycling in the deep subseafloor sediment of the northeastern Bering Sea Slope (IODP Exp. 323). Chem. Geol. 2011, 284, 251–261. [Google Scholar]
- Lein, A.Y.; Savvichev, A.S.; Ivanov, M.V. Reservoir of dissolved methand in the water column of the sea of the Russian Arctic region. Doklady Earth Sci. 2011, 441, 1576–1578. [Google Scholar] [CrossRef]
- Treude, T.; Niggemann, J.; Kallmeyer, J.; Wintersteller, P.; Schubert, C.J.; Boetius, A.; Jørgensen, B.B. Anaerobic oxidation of methane and sulfate reduction along the Chilean continental margin. Geochim. Cosmochim. Acta 2005, 69, 2767–2779. [Google Scholar] [CrossRef]
- Kessler, J.D.; Reeburgh, W.S.; Valentine, D.L.; Kinnaman, F.S.; Peltzer, E.T.; Brewer, P.G.; Southon, J.; Tyler, S.C. A survey of methane isotope abundance (14C, 13C, 2H) from five nearshore marine basins that reveals unusual radiocarbon levels in subsurface waters. J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef]
- Savvichev, A.S.; Rusanov, I.I.; Pimenov, N.V.; Pimenov, E.E.; Zakharova, E.E.; Veslopolova, E.F.; Lein, A.Y.; Crane, K.; Ivanov, M.V. Microbial processes of the carbon and sulphur cycles in the Chukchi Sea. Microbiology 2007, 76, 603–613. [Google Scholar] [CrossRef]
- Broecker, W.; Barker, S.; Clark, E.; Hajdas, I.; Bonani, G.; Stott, L. Ventilation of the glacial deep pacific ocean. Science 2004, 306, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, R.F.; Gardner, G.B. Sources and transport of dissolved and particulate organic carbon in the Mississippi River estuary and adjacent coastal waters of the northern Gulf of Mexico. Mar. Chem. 2004, 89, 241–256. [Google Scholar] [CrossRef]
- Sassen, R.; MacDonald, I.R. Hydrocarbons of experimental and natural gas hydrates, Gulf of Mexico continental slope. Org. Geochem. 1997, 26, 289–293. [Google Scholar] [CrossRef]
- Milkov, A.V. Molecular and stable isotope compositions of natural gas hydrates: A revised global data set and basic interpretations in the context of geological settings. Org. Geochem. 2005, 36, 681–702. [Google Scholar] [CrossRef]
- Rodrigues, N.M.; Paull, C.K.; Borowski, W.S. Zonation of authigenic carbonates within gas hydrate-bearing sedimentary sections on the blake ridge: Offshore Southeastern North America. In Proceedings of the Ocean Drilling Program—Scientific Results; Paull, C.K., Matsumoto, R., Wallace, P.J., Dillon, W.P., Eds.; Ocean Drilling Program: College Station, TX, USA, 2000; Volume 164. [Google Scholar]
- Chen, F.; Zhang, L.; Yang, Y.; Zhang, D. Chemical and isotopic alteration of organic matter during early diagenesis: Evidence from the coastal area off-shore the Pearl River estuary, south China. J. Mar. Syst. 2008, 74, 372–384. [Google Scholar] [CrossRef]
- Heuer, V.B.; Pohlman, J.W.; Torres, M.E.; Elvert, M.; Hinrichs, K.-U. The stable isotope biogeochemistry of acetate and other dissolved carbon species in deep subseafloor sediments at the northern Cascadia Margin. Geochem. Cosmochim. Acta 2009, 73, 3323–3336. [Google Scholar] [CrossRef]
- DeMets, C.; Gordon, R.G.; Argus, D.F.; Stein, S. Effect of recent revisions to the geomagnetic reversal time scale on estimates of current plate motions. Geophys. Res. Lett. 1994, 21, 2191–2194. [Google Scholar] [CrossRef]
- Barnes, P.M.; Mercier de Lépinay, B. Rates and mechanics of rapid frontal acrretion along the very obliquely converget southern Hikurangi Margin, New Zealand. J. Geophys. Res. 1997, 102, 24931–24952. [Google Scholar] [CrossRef]
- Pecher, I.A.; Henrys, S.A.; Wood, W.T.; Kukowski, N.; Crutchley, G.J.; Fohrmann, M.; Kilner, J.; Senger, K.; Gorman, A.R.; Coffin, R.B.; et al. Focussed fluid flow on the Hikurangi Margin, New Zealand—Evidence from possible local upwarping of the base fo gas hydrate stability. Mar. Geol. 2010, 272, 99–113. [Google Scholar] [CrossRef]
- Schwalenberg, K.; Wood, W.; Pecher, I.; Hamdan, L.; Stuart, S.; Jegen, M.; Coffin, R. Preliminary interpretation of electromagnetic, heat flow, seismic and geochemistry data for gas hydrate distribution across the Porangahau Ridge, New Zealand. Mar. Geol. 2010, 272, 89–108. [Google Scholar] [CrossRef]
- Hamdan, L.J.; Gillevet, P.M.; Pohlman, J.P.; Sikaroodi, M.; Greinert, J.; Coffin, R.B. Diversity and biogeochemical structuring of bacterial communities across the Porangahau ridge accretionary prism, New Zealand. FEMS Microbiol. Ecol. 2011, 77, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.M.; Lamarche, G.; Bialas, J.; Henrys, S.A.; Pecher, I.A.; Netzeband, G.; Greinert, J.; Mountjoy, J.J.; Pedley, K.; Crutchley, G. Tectonic and geological framework for gas hydrates and cold seeps on the hikurangi subduction margin, New Zealand. Mar. Geol. 2010, 272, 26–48. [Google Scholar] [CrossRef]
- Katz, H.R. Probable gas hydrate in continental slope east of the North Island, New Zealand. J. Pet. Geol. 1981, 3, 315–324. [Google Scholar] [CrossRef]
- Pecher, I.A.; Henrys, S.A. Potential Gas Reserves in Gas Hydrate Sweet Spots on the Hikurangi Margin, New Zealand; Institute of Geological & Nuclear Sciences: Wellington, New Zealand, 2003. [Google Scholar]
- Lewis, K.B.; Pettinga, J.R. The emerging imbricate frontal wedge of the Hikurangi margin. In South Pacific Sedimentary Basins; Balance, P.F., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 225–250. [Google Scholar]
- Hoehler, T.M.; Borowski, W.S.; Alperin, M.J.; Rodriguez, N.M.; Paull, C.K. Model, stable isotope, and radiocarbon characterization of anaerobic methane oxidation in gas hydrate-bearing sediments of the Blake Ridge. In Proceedings of the Ocean Drilling Program, Scientific Results, College Station, TX, USA; 2000; Volume 164, pp. 79–85. [Google Scholar]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 1969, 14, 454–459. [Google Scholar] [CrossRef]
- Plummer, R.E.; Pohlman, J.W.; Coffin, R.B. Compound-specific stable carbon isotope analysis of low-concentration complex hydrocarbon mixtures from natural gas hydrate systems. In Proceedings of AGU Fall Meeting Abstracts, San Francisco, CA, USA, 5–9 December 2005.
- Osburn, C.L.; St-Jean, G. The use of wet chemical oxidation with high-amplification isotope ratio mass spectrometry (WCO-IRMS) to measure stable isotope values of dissolved organic carbon in seawater. Limnol. Oceanogr. Meth. 2007, 5, 296–308. [Google Scholar] [CrossRef]
- Borowski, W.S.; Paull, C.K.; Ussler, W., III. Carbon cycling within the upper methanogenic zone of continental rise sediments: An example from the methane-rich overlying the Blake Ridge gas hydrate deposits. Mar. Chem. 1997, 57, 299–311. [Google Scholar] [CrossRef]
- Aller, R.C.; Blair, N.E. Carbon remineralization in the Amazon-Guianas tropical mobile mudbelt: A sedimentary incinerator. Cont. Shelf Res. 2006, 26, 2241–2259. [Google Scholar] [CrossRef]
- Aller, R.C.; Blair, N.E.; Brunskill, G.J. Early diagenetic cycling, incineration, and burial of sedimentary organic carbon in the central Gulf of Papus (Papua New Guinea). J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef]
- Martin, W.R.; McNichol, A.P.; McCorkle, D.C. The radiocarbon age of calcite dissolving at the sea floor: Estimates from pore water data. Geochem. Cosmochim. Acta 2000, 64, 1391–1404. [Google Scholar] [CrossRef]
- Sayles, F.L.; Curry, W.B. δ13C, TCO2, and the metabolism of organic-carbon in deep-sea sediments. Geochem. Cosmochim. Acta 1988, 52, 2963–2978. [Google Scholar] [CrossRef]
- Macko, S.A.; Ostrom, N.E. Pollution studies using stable isotopes. In Stable Isotopes in Ecology and Environmental Science; Lajtha, K., Michener, R., Eds.; Blackwell Scientific Publications: Oxford, UK, 1994; pp. 45–62. [Google Scholar]
- Martens, C.S.; Berner, R.A. Methane production in interstitial waters of sulfate-depleted marine sediments. Science 1974, 185, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Whiticar, M. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gleske, A.; Amann, R.; Jørgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Meister, P.; Liu, B.; Ferdelman, T.G.; Jørgensen, B.B.; Khalili, A. Control of sulphate and methane distributions in marine sediments by organic matter reactivity. Geochim. Cosmochim. Acta 2013, 104, 183–193. [Google Scholar] [CrossRef]
- Berner, R.A. Sulfate reduction and the rate of deposition of marine sediments. Earth Planet. Sci. Lett. 1978, 37, 492–498. [Google Scholar] [CrossRef]
- Ussler, W., III; Paull, C.K. Rates of anaerobic oxidation of methane and authigenic carbonate mineralization in methane-rich deep-sea sediments inferred from models and geochemical profiles. Earth Planet. Sci. Lett. 2008, 266, 271–287. [Google Scholar] [CrossRef]
- Claypool, G.E.; Milkov, A.V.; Lee, Y.-J.; Torres, M.E.; Borowski, W.S.; Tomaru, H. Microbial Methane Generation and Gas Transport in Shallow Sediments of Accretionary Complex, Southern Hydrate Ridge (ODP Leg 204), Offshore Oregon, USA. Available online: http://ir.library.oregonstate.edu/xmlui/handle/1957/13714 (accessed on 12 August 2014).
- Claypool, G.E.; Kaplan, I.R. The origin and distribution of methane in marine sediments. In Natural Gases in Marine Sediments; Kaplan, I.R., Ed.; Plenum Press: New York, NY, USA, 1974; pp. 99–139. [Google Scholar]
- Malinverno, A.; Pohlman, J.W. Modeling sulfate reduction in methane hydrate-bearing continental margin sediments: Does a suflate-methane transition require anaerobic oxidation of methane? Geochem. Geophys. Geosyst. 2011, 12. [Google Scholar] [CrossRef]
- Boehme, S.E.; Blair, N.E.; Chanton, J.P.; Martins, C.S. A mass balance of 13C and 12C in an organic-rich methane-producing marine sediment. Geochim. Cosmochim. Acta 1996, 60, 3835–3848. [Google Scholar] [CrossRef]
- Peterson, B.J.; Howarth, R.W.; Garritt, R.H. Multiple stable isotopes used to trace the flow of organic matter in estuarine food webs. Science 1985, 277, 1361–1363. [Google Scholar] [CrossRef]
- Law, C.S.; Nodder, S.D.; Mountjoy, J.J.; Marriner, A.; Orpin, A.; Pilditch, C.A.; Franz, P.; Thompson, K. Geological, hydrodynamic and biogeochemical variability of a New Zealand deep-water methane cold seep during an integrated three-year time-series study. Mar. Geol. 2010, 272, 189–208. [Google Scholar] [CrossRef]
- Morse, J.W.; Beazley, M.J. Organic matter in deepwater sediments of the Northern Gulf of Mexico and its relationship to the distribution of benthic organisms. Deep-Sea Res. II 2008, 55, 2563–2571. [Google Scholar]
- Coffin, R.B.; Osburn, C.L.; Plummer, R.; Grabowski, K.S. Deep sediment methane incorporation into shallow sediment carbon pools in Atwater Valley, Texas-Louisiana Shelf, Gulf of Mexico. Energies 2014. submitted. [Google Scholar]
- Wegener, G.; Niewmann, H.; Elvert, M.; Hinrichs, K.-U.; Boetius, A. Assimilation of methane and inorganic carbon by microbial communities mediating the anaerobic oxidation of methane. Environ. Microbiol. 2008, 10, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Nauhaus, K.; Albrecht, M.; Elvert, M.; Boetius, A.; Widdel, F. In vitro cell growth of marine archaeal-bacterial consortia during anaerobic oxidation of methane with sulfate. Environ. Microbiol. 2007, 9, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Knittel, K.; Boetius, A. Anaerobic oxidation of methane: Progress with an unknown process. Annu. Rev. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Leak, D.J.; Dalton, H. Growth yields of methanotrophs. Appl. Microbiol. Biotechnol. 1986, 23, 470–476. [Google Scholar] [CrossRef]
- House, C.H.; Schopf, J.W.; Stetter, K.O. Carbon isotopic fractionation by Archaens and other thermophilic prokaryotes. Org. Geochem. 2003, 34, 345–356. [Google Scholar] [CrossRef]
- Zhang, C.L.; Fouke, B.W.; Bonheyo, G.T.; Peackock, A.D.; White, D.C.; Huang, Y.; Romanek, C.S. Lipid biomarkers and carbon-isotopes of modern travertine deposits (Yellowstone Natioinal Park, USA): Implications for biogeochemical dynamics in hot-spring systems. Geochem. Cosmochim. Acta 2004, 68, 3157–3169. [Google Scholar] [CrossRef]
- Quandt, L.; Gottschalk, G.; Ziegler, H.; Stichler, W. Isotope discrimination by photosynthetic bacteria. REMS Microbiol. Lett. 1977, 1, 125–128. [Google Scholar] [CrossRef]
- Preuß, A.; Schauder, R.; Fuchs, G.; Stichler, W. Carbon isotope fractionation by autotrophic bacteria with three different CO2 fixation pathways. Z. Naturforsch. J. Biosci. 1989, 44, 397–402. [Google Scholar]
- Manske, A.K.; Glaeser, J.; Kuypers, M.M.M.; Overmann, J. Physiology and phylogeny of green sulfur bacteria forming a monospecific phototrophic assemblage at a depth of 100 meters in the Black Sea. Appl. Environ. Microbiol. 2005, 71, 8049–8060. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, F.; Nunoura, T.; Nakagawa, S.; Teske, A.; Lever, M.; Lauer, A.; Suzuki, M.; Takai, K.; Delwiche, M.; Colwell, F.S. Biogeographical distribution and diversity of microbes in the methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc. Natl. Acad. Sci. 2006, 103, 2815–2820. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, M.; Igarashi, Y. A novel oxalosuccinate-forming enzyme involved in the reductive carboxylation of 2-oxoglutarate in Hydrogenbacter thermophiles TK-6. Mol. Microbiol. 2006, 62, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Goñi, M.A.; Ruttenberg, K.C.; Eglinton, T.I. A re-assessment of the sources and importance of land-derived organic matter in surface sediment from the Gulf of Mexico. Geochem. Cosmochim. Acta 1998, 62, 3055–3075. [Google Scholar]
- Gordon, E.S.; Goñi, M.A. Controls on the distribution and accumulation of terrigenous organic matter in sediments from the Mississippi and Atchafalaya river margin. Mar. Chem. 2004, 92, 331–352. [Google Scholar] [CrossRef]
- Szynkiewicz, A.M.; Jedrysek, O.; Kurasiewicz, M. Carbon isotope effects during precipitation of barium carbonate: Implications for environmental studies. Environ. Chem. Lett. 2006, 4, 29–35. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Coffin, R.B.; Hamdan, L.J.; Smith, J.P.; Rose, P.S.; Plummer, R.E.; Yoza, B.; Pecher, I.; Montgomery, M.T. Contribution of Vertical Methane Flux to Shallow Sediment Carbon Pools across Porangahau Ridge, New Zealand. Energies 2014, 7, 5332-5356. https://doi.org/10.3390/en7085332
Coffin RB, Hamdan LJ, Smith JP, Rose PS, Plummer RE, Yoza B, Pecher I, Montgomery MT. Contribution of Vertical Methane Flux to Shallow Sediment Carbon Pools across Porangahau Ridge, New Zealand. Energies. 2014; 7(8):5332-5356. https://doi.org/10.3390/en7085332
Chicago/Turabian StyleCoffin, Richard B., Leila J. Hamdan, Joseph P. Smith, Paula S. Rose, Rebecca E. Plummer, Brandon Yoza, Ingo Pecher, and Michael T. Montgomery. 2014. "Contribution of Vertical Methane Flux to Shallow Sediment Carbon Pools across Porangahau Ridge, New Zealand" Energies 7, no. 8: 5332-5356. https://doi.org/10.3390/en7085332





