Affects of Mechanical Milling and Metal Oxide Additives on Sorption Kinetics of 1:1 LiNH2/MgH2 Mixture
Abstract
:1. Introduction






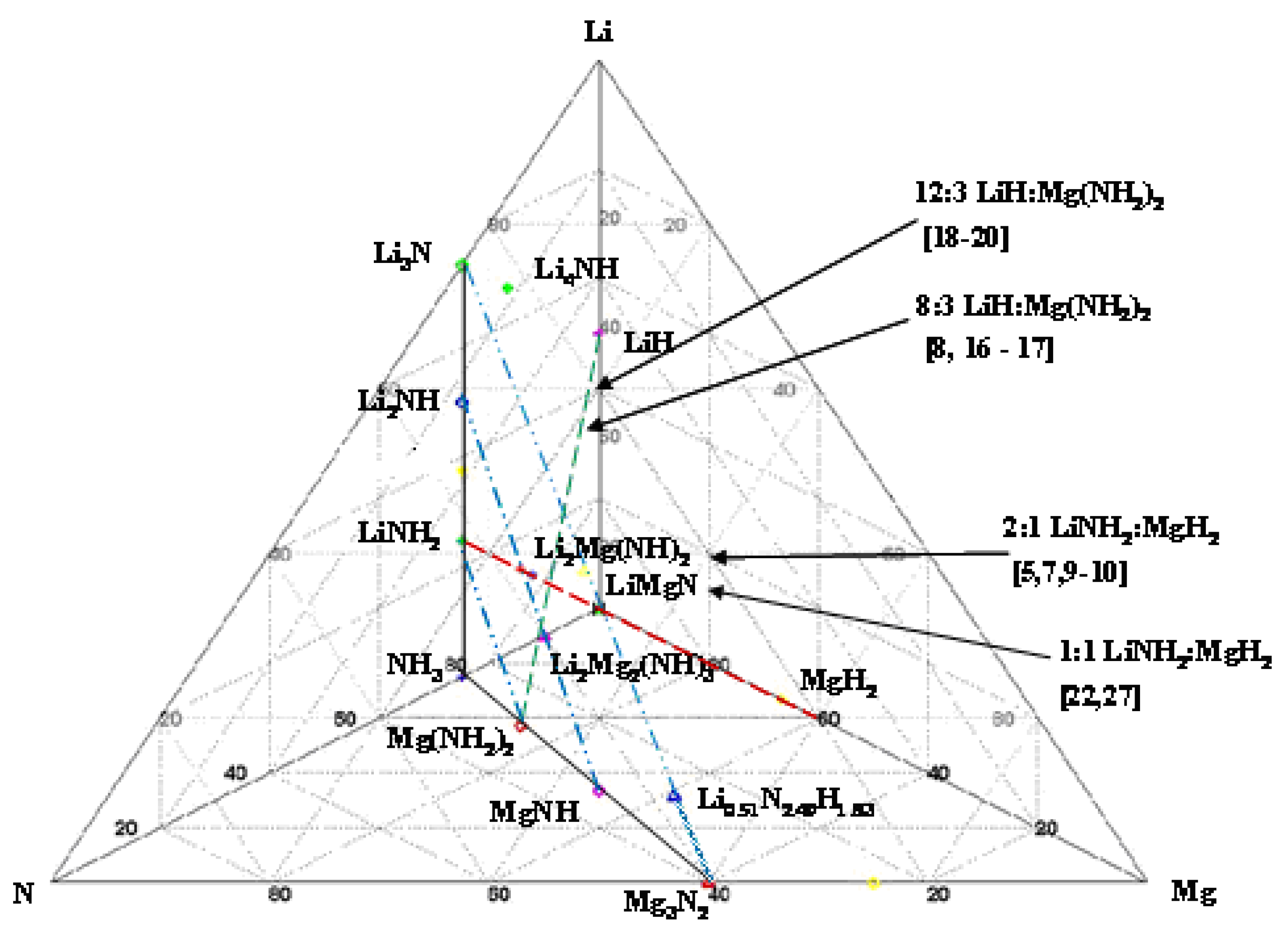
 = 128.5 and 200.7 kJ/mol H2 [28], and not to give up further hydrogen at temperatures of potential utilization below 200 °C [5,7,9]. These structures have yet to be indentified experimentally. Similarly, with no Mg present, the terminal phase is the Li2NH. However, with the additional of MgH2, at the Li:Mg ratio of 1:1, these amine phases can be avoided resulting in the terminal LiMgN and complete hydrogen release [23]. This decomposition has been shown to occur at temperatures as low at 120 °C and can be rehydrogenated under 138 bar [23]
= 128.5 and 200.7 kJ/mol H2 [28], and not to give up further hydrogen at temperatures of potential utilization below 200 °C [5,7,9]. These structures have yet to be indentified experimentally. Similarly, with no Mg present, the terminal phase is the Li2NH. However, with the additional of MgH2, at the Li:Mg ratio of 1:1, these amine phases can be avoided resulting in the terminal LiMgN and complete hydrogen release [23]. This decomposition has been shown to occur at temperatures as low at 120 °C and can be rehydrogenated under 138 bar [23]2. Results and Discussion
2.1. Unmodified System
2.1.1. Characterization of Unmodified As-Milled Material
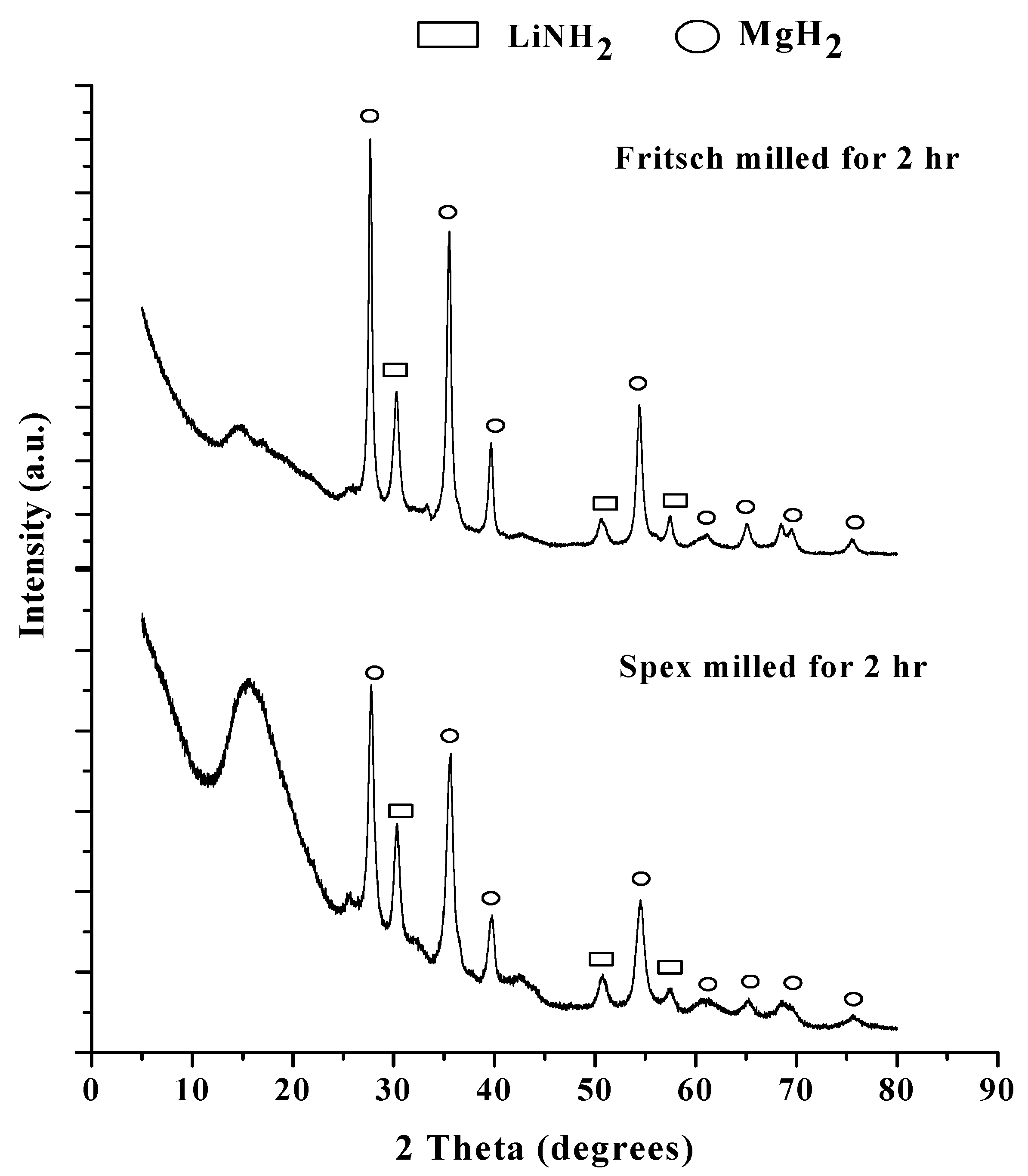
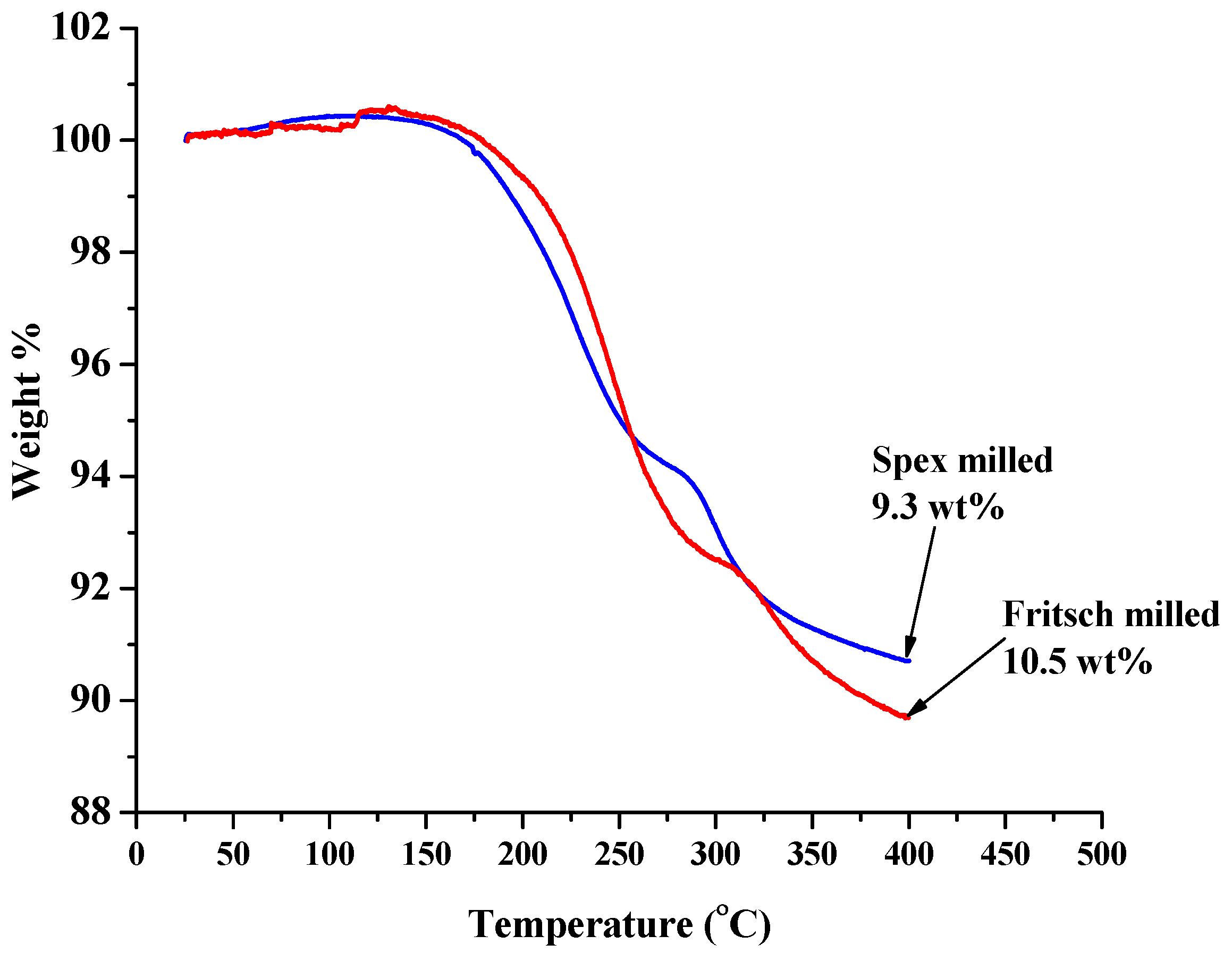
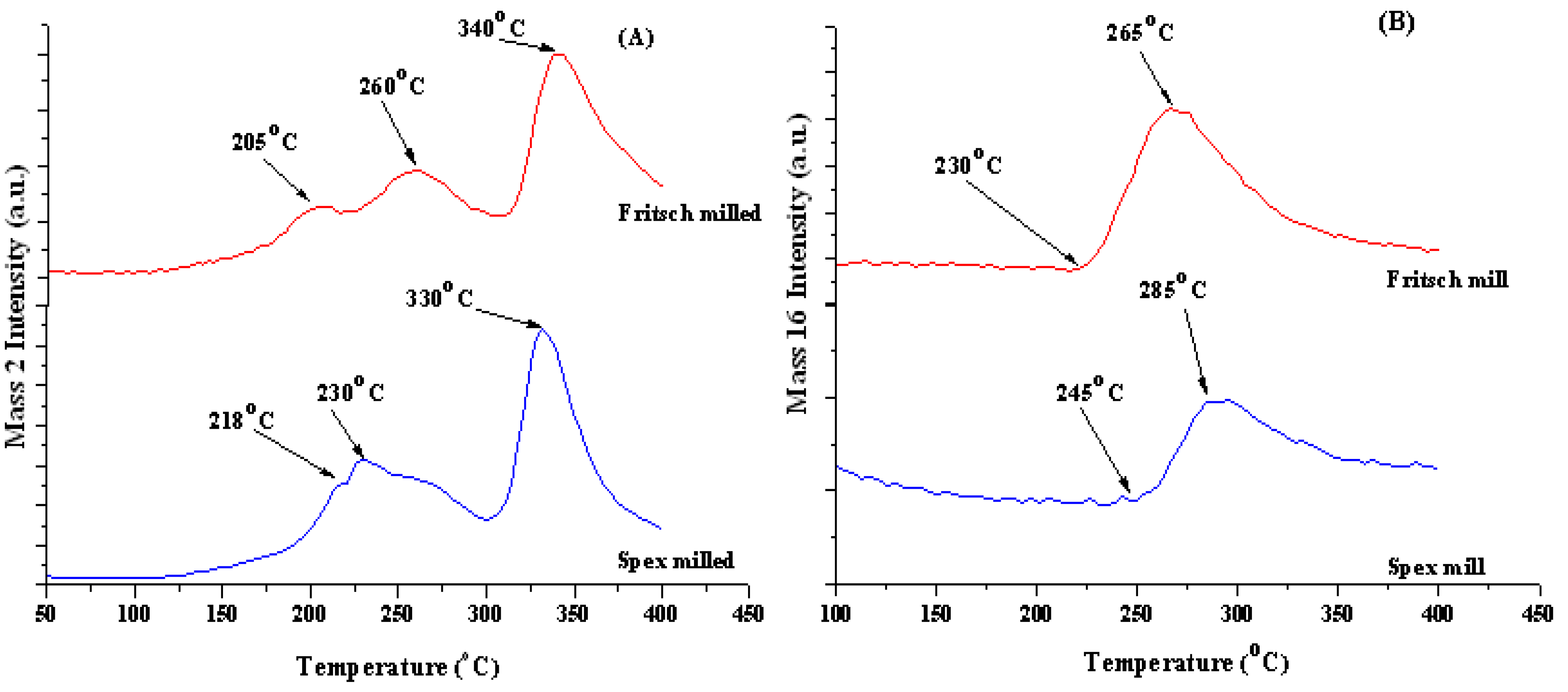
2.1.2. Decomposition Behavior of Unmodified As-Milled Material
2.1.3. Isothermal Dehydrogenation/Hydrogenation Cycling of Unmodified System
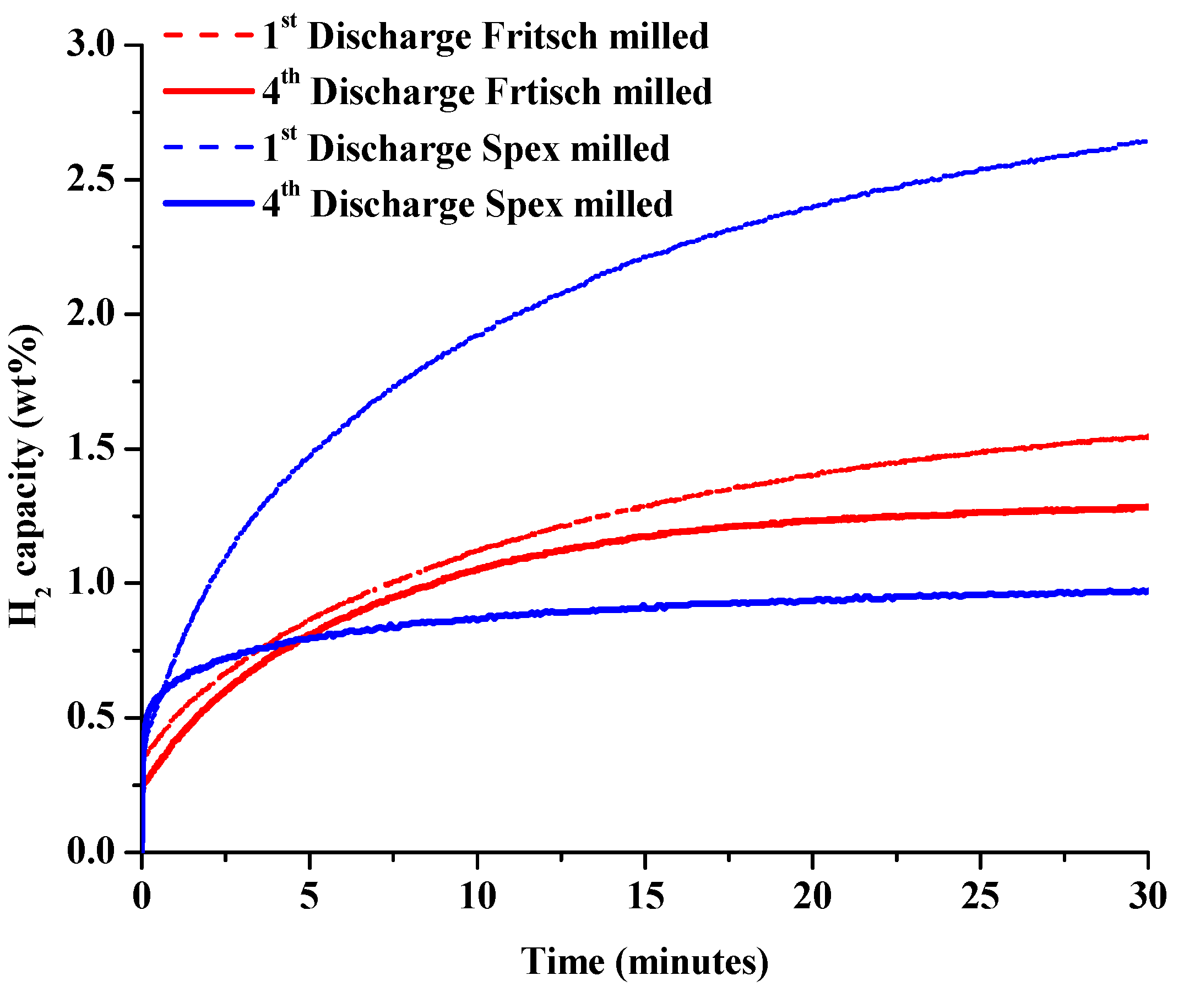
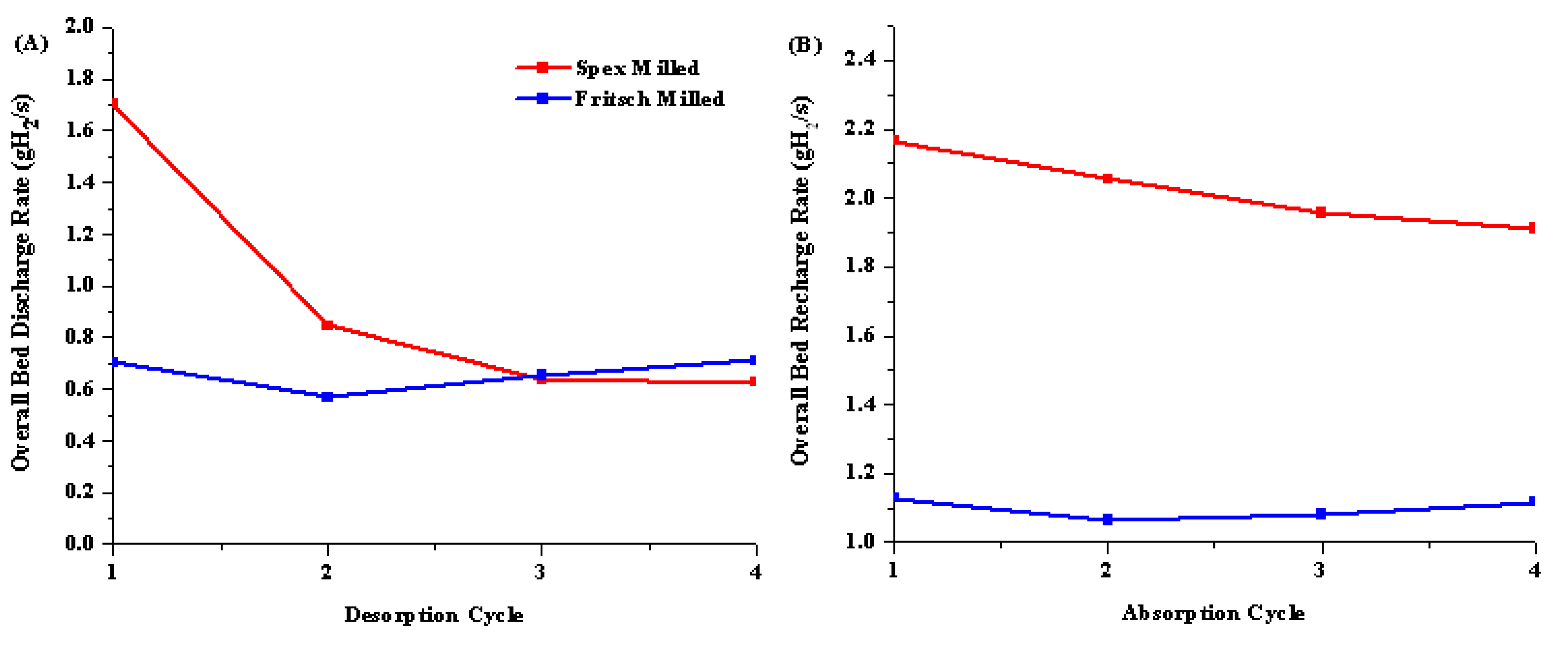
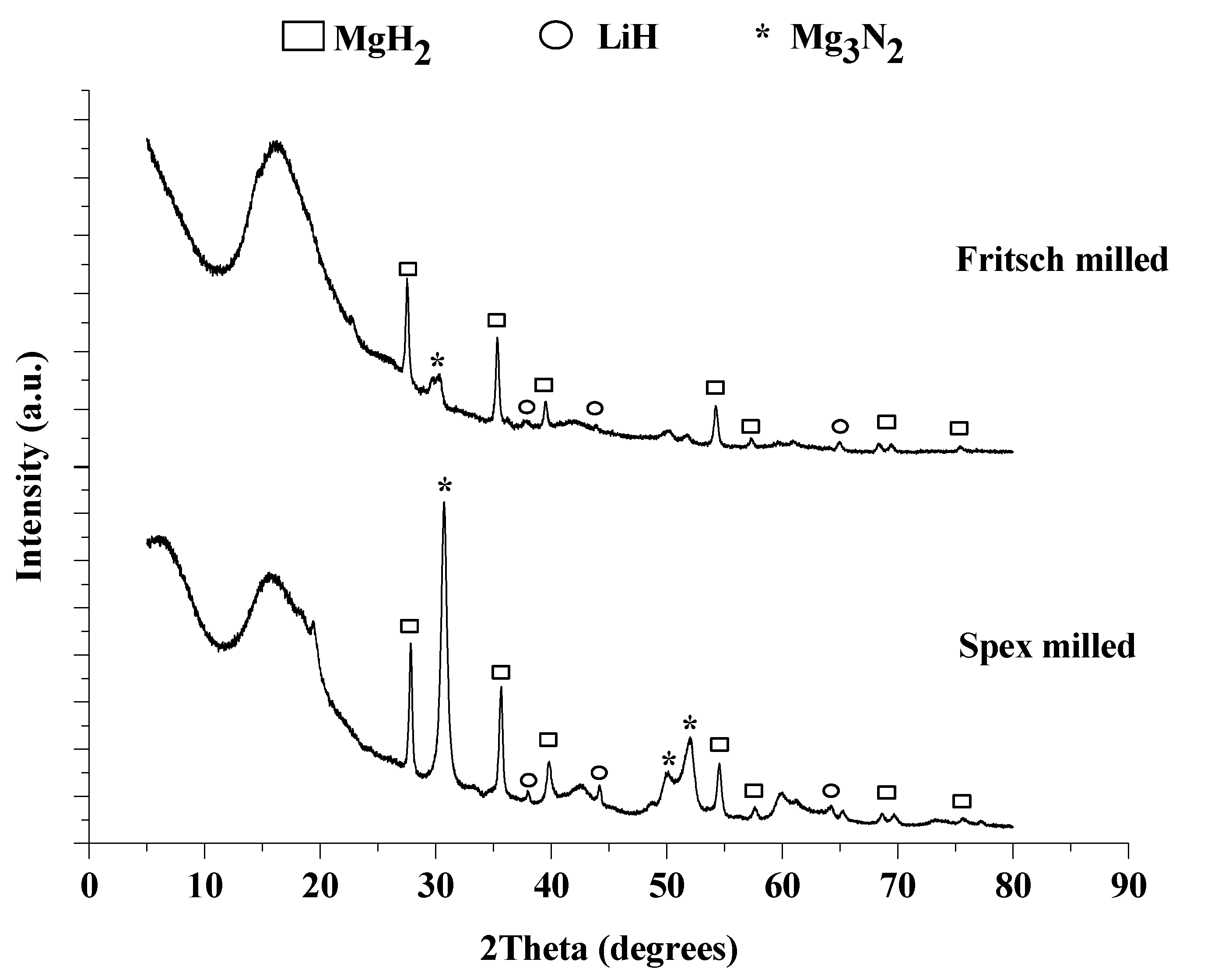
2.1.4. Phase Identification

| Milling Conditions | Average MgH2 Particle Size before Cycling (nm) | Average MgH2 Particle Size after Cycling (nm) |
|---|---|---|
| Spex | 23.6 | 43.5 |
| Fritsch | 35.3 | 49.8 |
2.2. Modified 1:1 LiNH2:MgH2 Systems
2.2.1. Characterization of As-Milled Modified Material


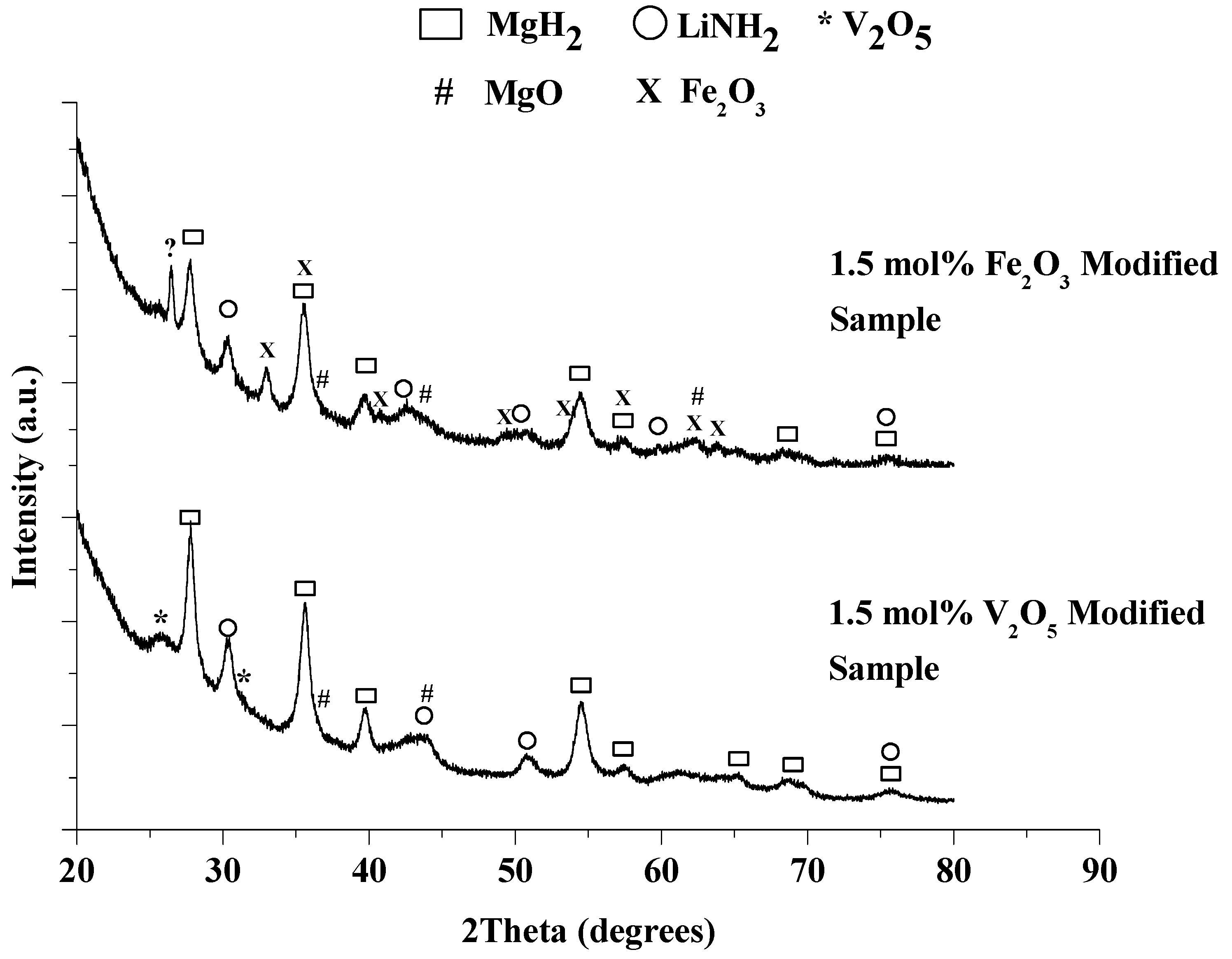
2.2.2. Decomposition Behavior of As-Milled Modified Material
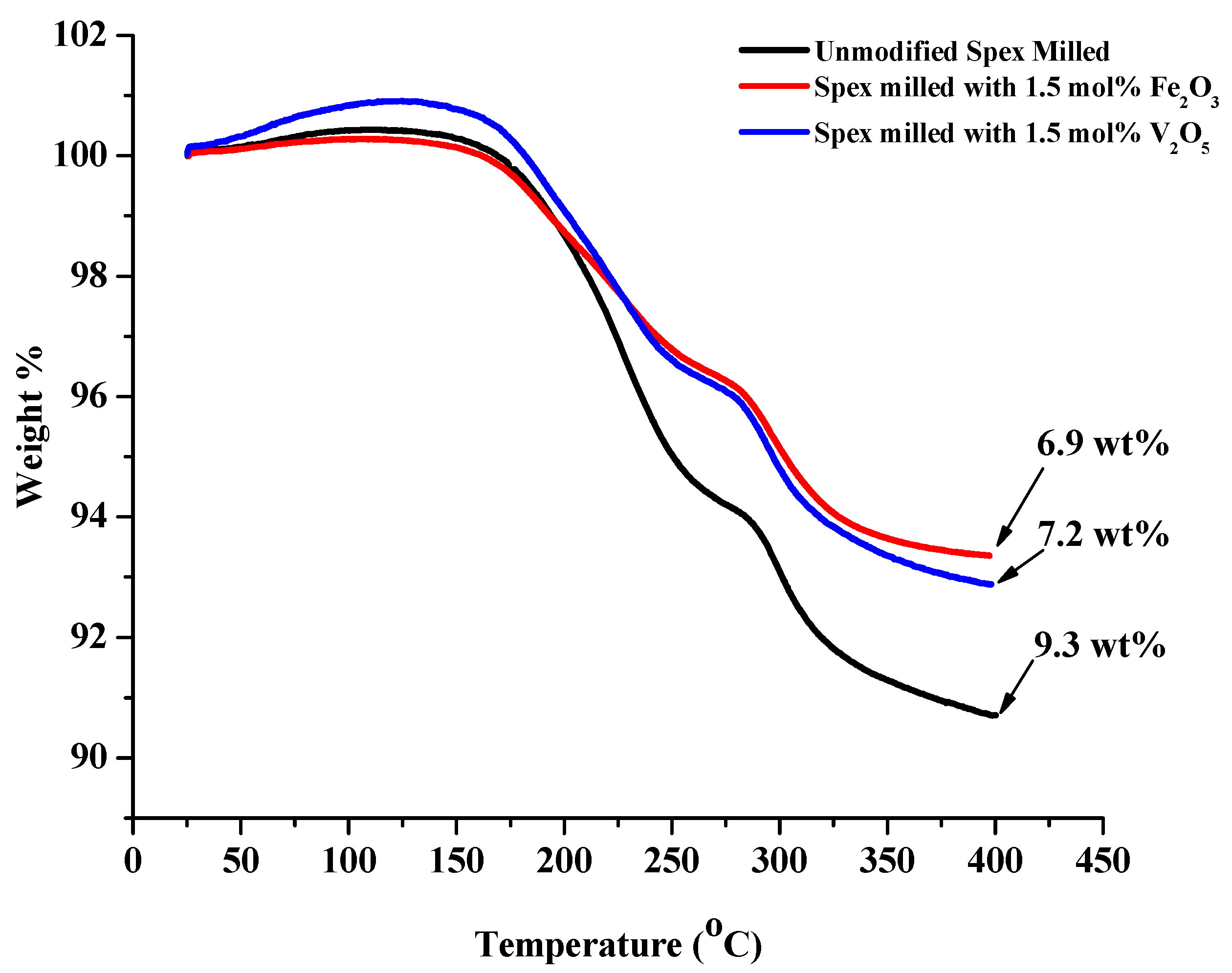
| Compositional Modification | Theoretical H2 Weight % | Total Weight % Released | H1 | H2 | Peak Ammonia Release Temperature |
|---|---|---|---|---|---|
| No Modification | 8.2 | 9.3 | 240 | 345 | 285 °C |
| 1.5 mol% Fe2O3 | 7.45 | 6.9 | 225 | 330 | 270 °C |
| 1.5 mol% V2O5 | 7.36 | 7.2 | 220 | 325 | 270 °C |
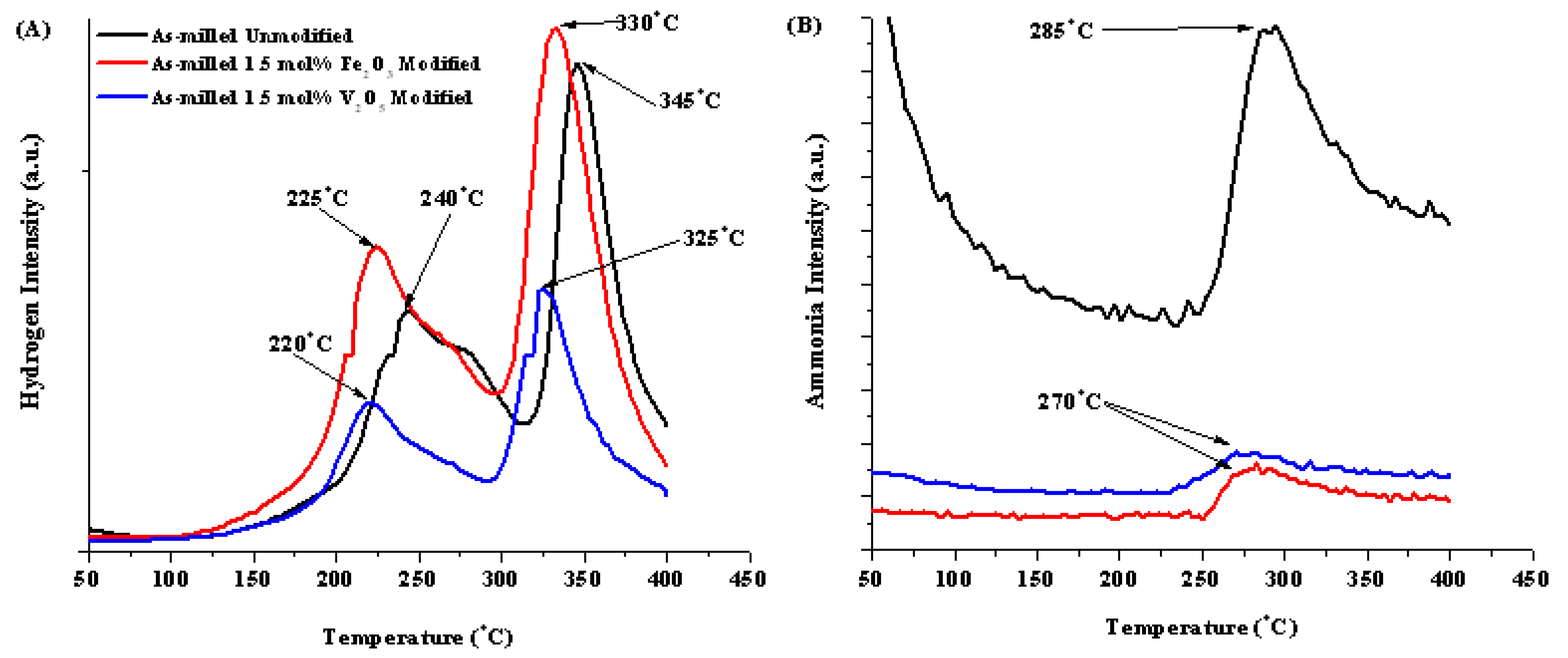
2.2.3. Isothermal Hydrogenation/Dehydrogenation of Modified 1:1 LiNH2:MgH2 System

2.2.4. Phase Identification after Cycling

3. Experimental Section
4. Conclusions
Acknowledgements
References
- Pinkerton, F.E. Decomposition kinetics of lithium amide for hydrogen storage materials. J. Alloys Compd. 2005, 400, 76–82. [Google Scholar] [CrossRef]
- Matsumoto, M.; Haga, T.; Kawai, Y.; Kojima, Y. Hydrogen desorption reactions of Li–N–H hydrogen storage system: Estimation of activation free energy. J. Alloys Compd. 2007, 439, 358–362. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.R.; Ozoliņš, V.; Wolverton, C. First-Principles Determination of Multicomponent Hydride Phase Diagrams: Application to the Li–Mg–N–H System. Adv. Mater. 2007, 19, 3233–3239. [Google Scholar] [CrossRef]
- Luo, W.; Sickafoose, S. Thermodynamic and structural characterization of the Mg–Li–N–H hydrogen storage system. J. Alloys Compd. 2006, 407, 274–281. [Google Scholar] [CrossRef]
- Araujo, C.M.; Scheicher, R.H.; Ahuja, R. Thermodynamic analysis of hydrogen sorption reactions in Li–Mg–N–H systems. Appl. Phys. Lett. 2008, 92, 021907–021903. [Google Scholar] [CrossRef]
- Luo, W. LiNH2–MgH2: A viable hydrogen storage system. J. Alloys Compd. 2004, 381, 284–287. [Google Scholar] [CrossRef]
- Leng, H.Y.; Ichikawa, T.; Hino, S.; Hanada, N.; Isobe, S.; Fujii, H. New Metal−N−H System Composed of Mg(NH2)2 and LiH for Hydrogen Storage. J. Phys. Chem. B 2004, 108, 8763–8765. [Google Scholar] [CrossRef]
- Luo, W.; Rönnebro, E. Towards a viable hydrogen storage system for transportation application. J. Alloys Compd. 2005, 404–406, 392–395. [Google Scholar]
- Xiong, Z.; Wu, G.; Hu, J.; Chen, P. Ternary Imides for Hydrogen Storage. Adv. Mater. 2004, 16, 1522–1525. [Google Scholar] [CrossRef]
- Hu, J.; Fichtner, M. Formation and Stability of Ternary Imides in the Li–Mg–N–H Hydrogen Storage System. Chem. Mater. 2009, 21, 3485–3490. [Google Scholar] [CrossRef]
- Janot, R.; Eymery, J.-B.; Tarascon, J.-M. Investigation of the processes for reversible hydrogen storage in the Li–Mg–N–H system. J. Power Sources 2007, 164, 496–502. [Google Scholar] [CrossRef]
- Weidner, E.; Dolci, F.; Hu, J.; Lohstroh, W.; Hansen, T.; Bull, D.J.; Fichtner, M. Hydrogenation Reaction Pathway in Li2Mg(NH)2. J. Phys. Chem. C 2009, 113, 15772–15777. [Google Scholar] [CrossRef]
- Dolci, F.; Weidner, E.; Hoelzel, M.; Hansen, T.; Moretto, P.; Pistidda, C.; Brunelli, M.; Fichtner, M.; Lohstroh, W. In-situ neutron diffraction study of magnesium amide/lithium hydride stoichiometric mixtures with lithium hydride excess. Int. J. Hydrog. Energy 2010, 35, 5448–5453. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C. Activation of hydrogen storage materials in the Li–Mg–N–H system: Effect on storage properties. J. Alloys Compd. 2007, 430, 334–338. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Yang, L.; Wu, G.; Luo, W. Mechanistic Investigations on the Heterogeneous Solid-State Reaction of Magnesium Amides and Lithium Hydrides. J. Phys. Chem. B 2006, 110, 14221–14225. [Google Scholar] [CrossRef] [PubMed]
- Leng, H.; Ichikawa, T.; Hino, S.; Nakagawa, T.; Fujii, H. Mechanism of Hydrogenation Reaction in the Li–Mg–N–H System. J. Phys. Chem. B 2005, 109, 10744–10748. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Noritake, T.; Nakamori, Y.; Towata, S.; Orimo, S. Dehydriding and rehydriding properties of Mg(NH2)2-LiH systems. J. Alloys Compd. 2007, 446–447, 328–331. [Google Scholar]
- Aoki, M.; Noritake, T.; Kitahara, G.; Nakamori, Y.; Towata, S.; Orimo, S. Dehydriding reaction of Mg(NH2)2–LiH system under hydrogen pressure. J. Alloys Compd. 2007, 428, 307–311. [Google Scholar] [CrossRef]
- Chu, L.; Yongfeng, L.; Kun, L.; Bo, L.; Mingxia, G.; Hongge, P.; Qidong, W. Reaction Pathways Determined by Mechanical Milling Process for Dehydrogenation/Hydrogenation of the LiNH2–MgH2. Chem. A Eur. J. 2010, 16, 693–702. [Google Scholar] [CrossRef]
- Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Noritake, T.; Towata, S.; Orimo, S. Hydrogen storage properties of Li–Mg–N–H systems. J. Alloys Compd. 2005, 404–406, 396–398. [Google Scholar]
- Alapati, S.V.; Johnson, J.K.; Sholl, D.S. Identification of Destabilized Metal Hydrides for Hydrogen Storage Using First Principles Calculations. J. Phys. Chem. B 2006, 110, 8769–8776. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Fang, Z.Z.; Choi, Y.J.; Sohn, H.Y. Potential of Binary Lithium Magnesium Nitride for Hydrogen Storage Applications. J. Phys. Chem. C 2007, 111, 12129–12134. [Google Scholar] [CrossRef]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y. Effect of milling intensity on the formation of LiMgN from the dehydrogenation of LiNH2-MgH2 (1:1) mixture. J. Power Sources 2010, 195, 1992–1997. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, K.; Gao, M.; Wang, J.; Pan, H.; Wang, Q. Hydrogen Storage in a LiNH2–MgH2 (1:1) System. Chem. Mater. 2008, 20, 3521–3527. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.; Luo, K.; Li, B.; Gao, M.; Pan, H.; Wang, Q. Reaction Pathways Determined by Mechanical Milling Process for Dehydrogenation/Hydrogenation of the LiNH2/MgH2 System. Chem. A Eur. J. 2010, 16, 693–702. [Google Scholar] [CrossRef]
- Osborn, W.; Markmaitree, T.; Shaw, L.L. Evaluation of the hydrogen storage behavior of a LiNH2 + MgH2 system with 1:1 ratio. J. Power Sources 2007, 172, 376–378. [Google Scholar] [CrossRef]
- Michel, K.J.; Akbarzadeh, A.R.; Ozolins, V. First-Principles Study of the Li–Mg–N–H System: Compound Structures and Hydrogen-Storage Properties. J. Phys. Chem. C 2009, 113, 14551–14558. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, K.; Luo, K.; Gao, M.; Pan, H.; Wang, Q. Size-Dependent Kinetic Enhancement in Hydrogen Absorption and Desorption of the Li–Mg–N–H System. J. Am. Chem. Soc. 2009, 131, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Price, C.; Gray, J.; Lascola, R.; Anton, D. The Affects of Halide Modifiers on the Sorption Kinetics of 1:1 LiNH2:MgH2. Int. J. Hydrog. Energy 2010, in press. [Google Scholar]
- Bald, C.P.; Hereijgers, B.P.C.; Bitter, J.H.; de Jong, K.P. Sodium Alanate Nanoparticles-Linking Size to Hydrogen Storage Properties. J. Am. Chem. Soc. 2008, 130, 6761–6765. [Google Scholar] [CrossRef] [PubMed]
- Varin, R.A.; Jang, M.; Polanski, M. The effects of ball milling and molar ratio of LiH on the hydrogen storage properties of nanocrystalline lithium amide and lithium hydride (LiNH2 + LiH) system. J. Alloys Compd. 2010, 491, 658–667. [Google Scholar] [CrossRef]
- Markmaitree, T.; Osborn, W.; Shaw, L.L. Comparative studies of reaction rates of NH3 with MgH2 and LiH. J. Power Sources 2008, 180, 535–538. [Google Scholar] [CrossRef]
- Markmaitree, T.; Osborn, W.; Shaw, L.L. Comparisons between MgH2- and LiH-containing systems for hydrogen storage applications. Int. J. Hydrog. Energy 2008, 33, 3915–3924. [Google Scholar] [CrossRef]
- Luo, W.; Stewart, K. Characterization of NH3 formation in desorption of Li–Mg–N–H storage system. J. Alloys Compd. 2007, 440, 357–361. [Google Scholar] [CrossRef]
- DOE Targets for Onboard Hydrogen Storage Systems for Light-Duty Vehicles. Available online: http://www1.eere.energy.gov/hydrogenandfuelcells/storage/pdfs/targets_onboard_hydro_storage.pdf (accessed on 1 December 2010).
- Hu, Y.H.; Ruckenstein, E. Ultrafast Reaction between LiH and NH3 during H2 Storage in Li3N. J. Phys. Chem. A 2003, 107, 9737–9739. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scripta Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Huang, Z.G.; Guo, Z.P.; Calka, A.; Wexler, D.; Lukey, C.; Liu, H.K. Effects of iron oxide (Fe2O3, Fe3O4) on hydrogen storage properties of Mg-based composites. J. Alloys Compd. 2006, 422, 299–304. [Google Scholar] [CrossRef]
- Dehouche, Z.; Klassen, T.; Oelerich, W.; Goyette, J.; Bose, T.K.; Schulz, R. Cycling and thermal stability of nanostructured MgH2–Cr2O3 composite for hydrogen storage. J. Alloys Compd. 2002, 347, 319–323. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anton, D.L.; Price, C.J.; Gray, J. Affects of Mechanical Milling and Metal Oxide Additives on Sorption Kinetics of 1:1 LiNH2/MgH2 Mixture. Energies 2011, 4, 826-844. https://doi.org/10.3390/en4050826
Anton DL, Price CJ, Gray J. Affects of Mechanical Milling and Metal Oxide Additives on Sorption Kinetics of 1:1 LiNH2/MgH2 Mixture. Energies. 2011; 4(5):826-844. https://doi.org/10.3390/en4050826
Chicago/Turabian StyleAnton, Donald L., Christine J. Price, and Joshua Gray. 2011. "Affects of Mechanical Milling and Metal Oxide Additives on Sorption Kinetics of 1:1 LiNH2/MgH2 Mixture" Energies 4, no. 5: 826-844. https://doi.org/10.3390/en4050826




