Facile Route for Fabrication of Ferrimagnetic Mn3O4 Spinel Material for Supercapacitors with Enhanced Capacitance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of Mn3O4 and Electrode Fabrication
2.3. Characterization Techniques
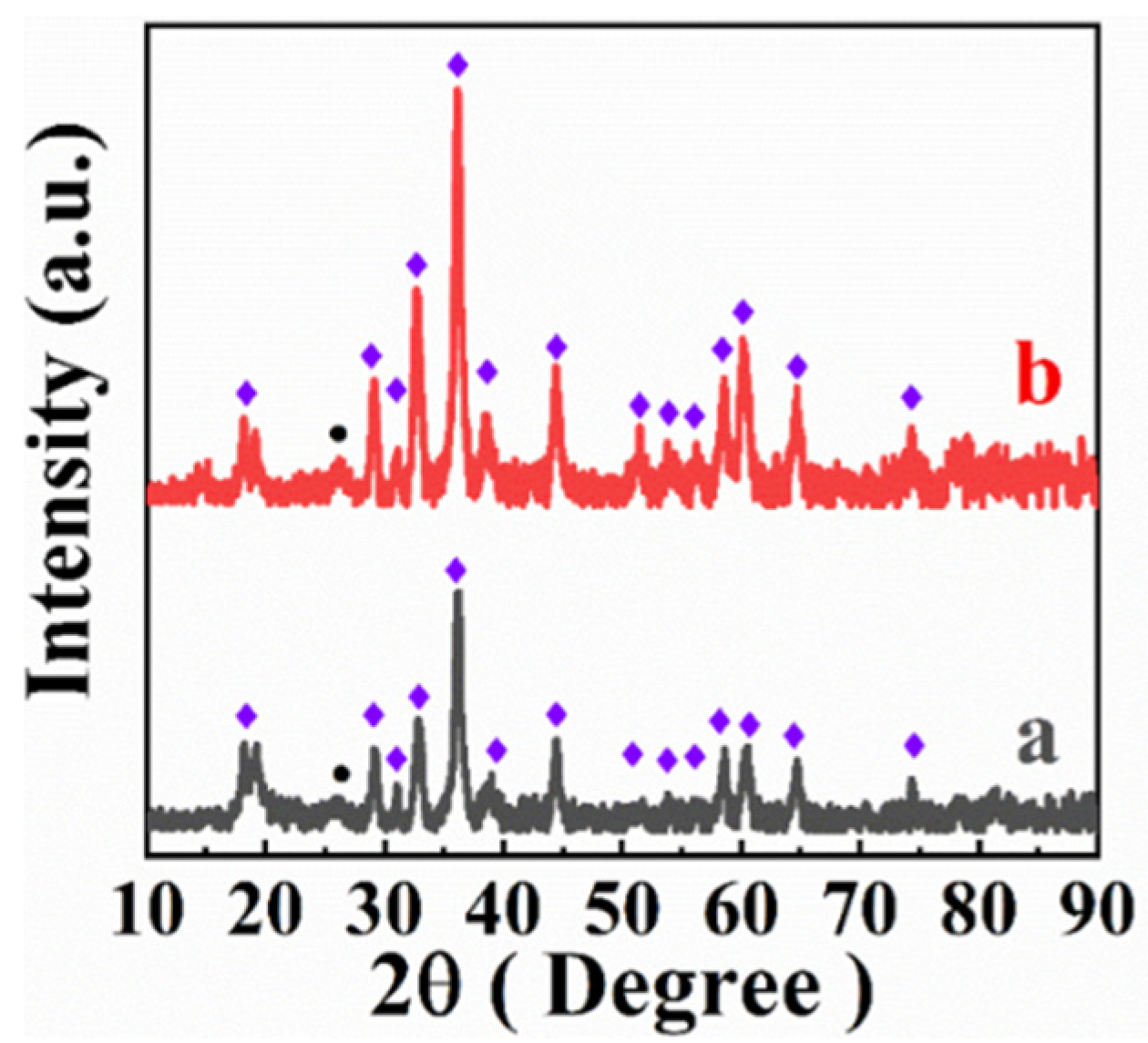
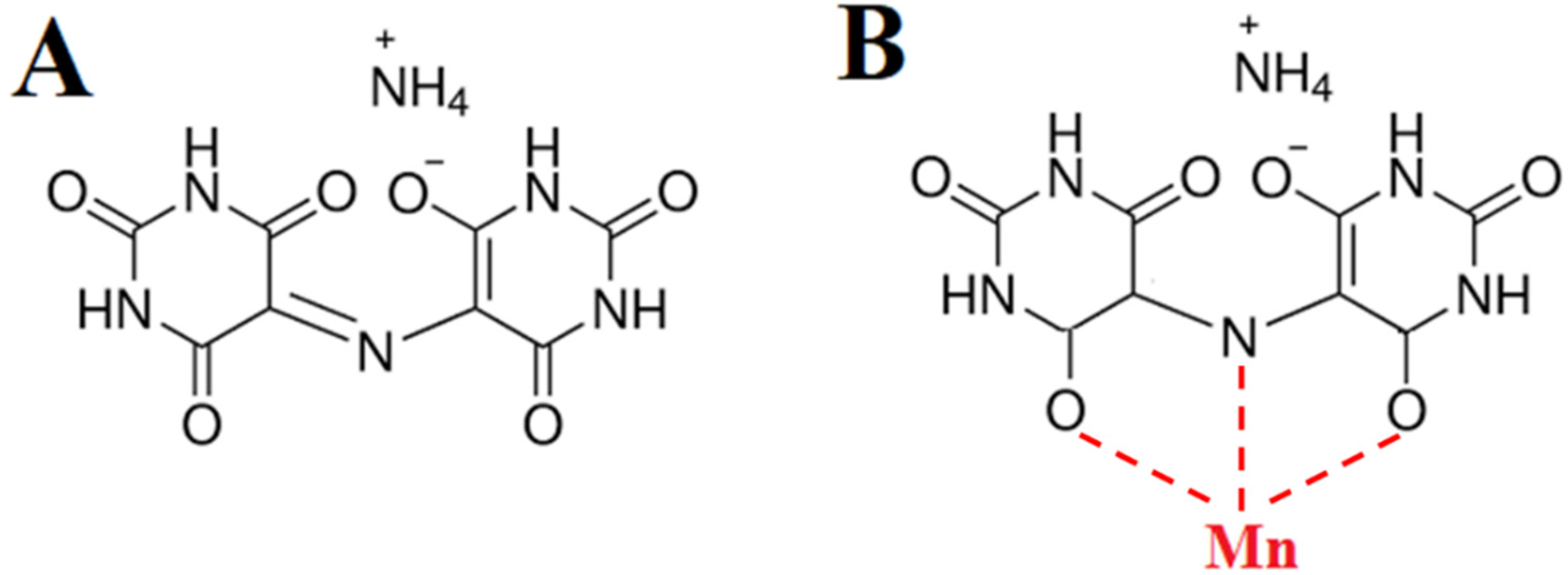
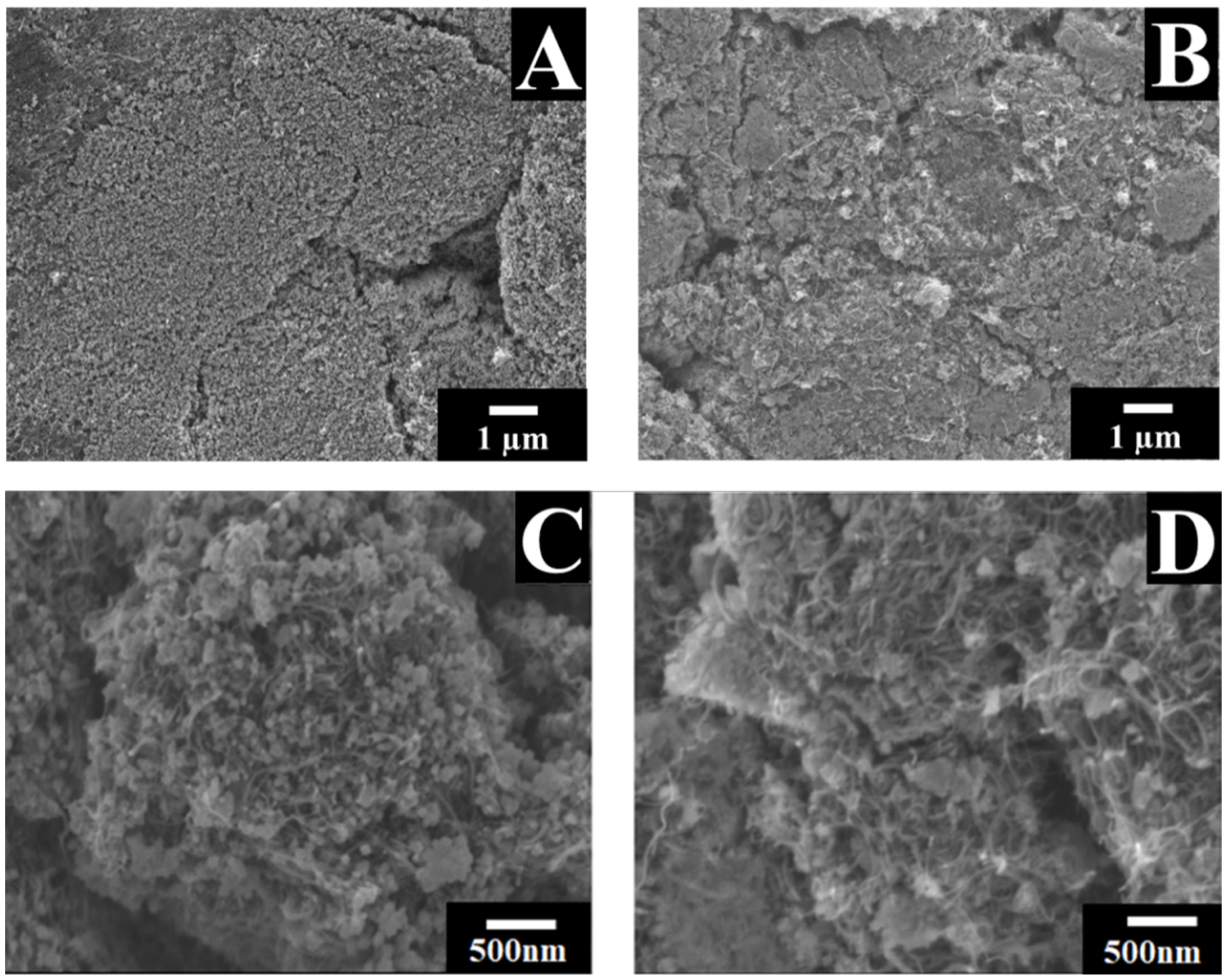
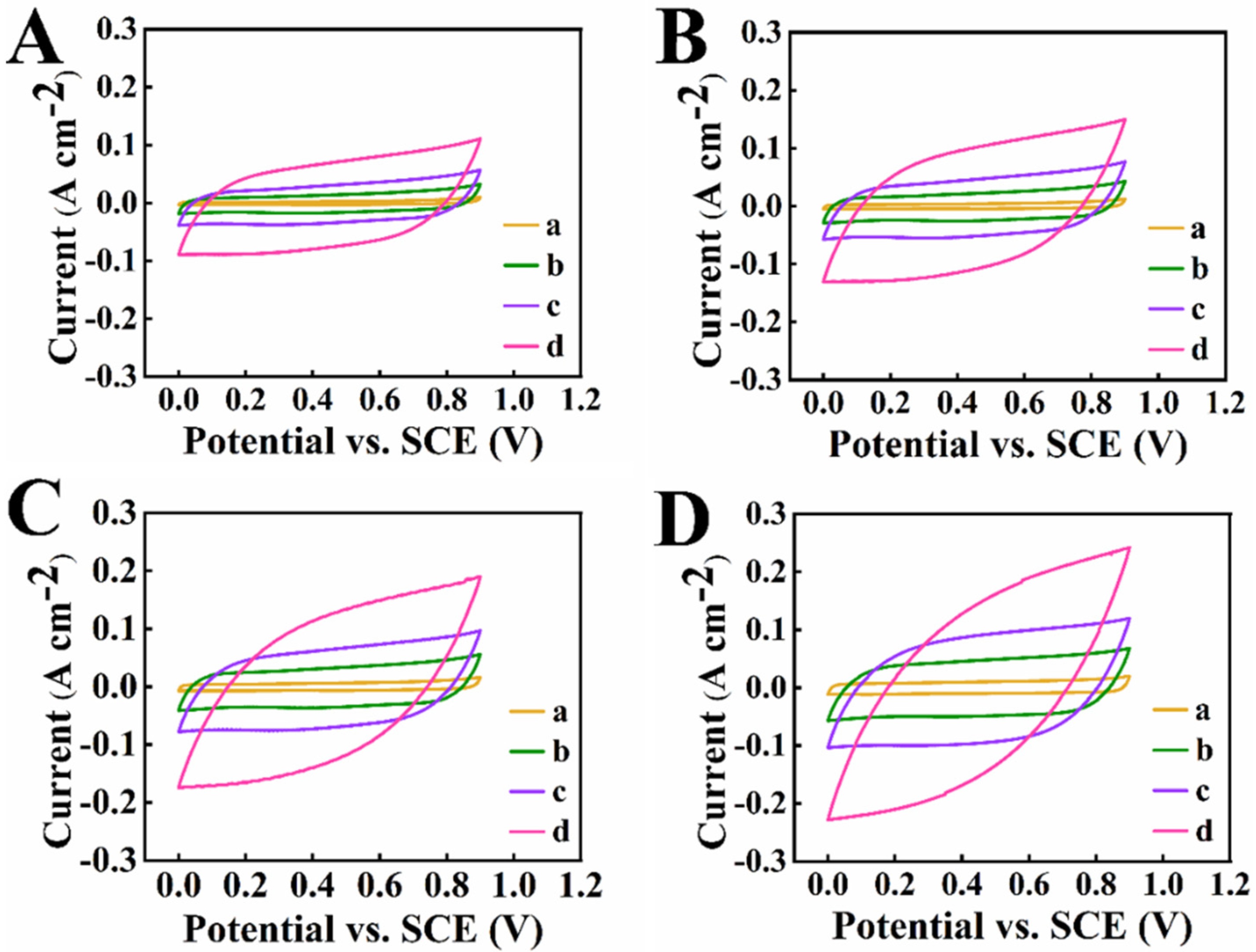
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, C.; Li, B.; Lin, X.; Liu, H.; Xu, Y.; Mao, J.; Duan, C.; Li, T.; Ni, Y. The recent progress on three-dimensional porous graphene-based hybrid structure for supercapacitor. Compos. Part B Eng. 2019, 165, 10–46. [Google Scholar] [CrossRef]
- Liu, C.; Hou, Y.; Li, Y.; Xiao, H. Heteroatom-doped porous carbon microspheres derived from ionic liquid-lignin solution for high performance Supercapacitors. J. Colloid Interface Sci. 2022, 614, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bai, Y.; Chen, H.; Wu, J.; Li, C.M.; Su, X.; Zhang, L. Oxygen-defect-rich 3D porous cobalt-gallium layered double hydroxide for high-performance supercapacitor application. J. Colloid Interface Sci. 2022, 608, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Cheng, Y.; Wang, R.; Sun, J. Recent advances in 3D porous MXenes: Structures, properties and applications. J. Phys. D Appl. Phys. 2021, 55, 093001. [Google Scholar] [CrossRef]
- Wang, T.; He, X.; Gong, W.; Kou, Z.; Yao, Y.; Fulbright, S.; Reardon, K.F.; Fan, M. Three-dimensional, heteroatom-enriched, porous carbon nanofiber flexible paper for free-standing supercapacitor electrode materials derived from microalgae oil. Fuel Process. Technol. 2022, 225, 107055. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The Development of Pseudocapacitor Electrodes and Devices with High Active Mass Loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Cui, W.; Qiu, Z.; Hu, Z.; Zhang, Z. 2D porous layered NiFe2O4 by a facile hydrothermal method for asymmetric supercapacitor. Ionics 2021, 27, 1347–1355. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Bi, J.; Chen, Y.; Hao, X.; Sun, X.; Zhang, J. Morphology-controllable preparation of NiFe2O4 as high performance electrode material for supercapacitor. Electrochim. Acta 2019, 296, 181–189. [Google Scholar] [CrossRef]
- Nawwar, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. Pseudocapacitive behavior of ferrimagnetic NiFe2O4-carbon nanotube electrodes prepared with a multifunctional dispersing agent. Open Ceram. 2021, 6, 100127. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, E.; He, W.; Deng, X.; Huang, J.; Ding, M.; Wei, X.; Liu, X.; Xu, X. NiCo2O4-based supercapacitor nanomaterials. Nanomaterials 2017, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Meena, S.; Anantharaju, K.S.; Malini, S.; Dey, A.; Renuka, L.; Prashantha, S.C.; Vidya, Y.S. Impact of temperature-induced oxygen vacancies in polyhedron MnFe2O4 nanoparticles: As excellent electrochemical sensor, supercapacitor and active photocatalyst. Ceram. Int. 2021, 47, 14723–14740. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahmed, J.; Alhabarah, A.N.; Ahamad, T.; Ahmad, T. Nitrogen-Doped Cobalt Ferrite/Carbon Nanocomposites for Supercapacitor Applications. ChemElectroChem 2017, 4, 2952–2958. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Chen, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. High areal capacitance of Fe3O4-decorated carbon nanotubes for supercapacitor electrodes. Carbon Energy 2019, 1, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, V.V.; Nagaswarupa, H.P.; Raghavendra, N. Development of Co-doped MnFe2O4 nanoparticles for electrochemical supercapacitors. Ceram. Int. 2021, 47, 10268–10273. [Google Scholar] [CrossRef]
- Angelopoulou, P.; Kassavetis, S.; Papavasiliou, J.; Karfaridis, D.; Słowik, G.; Patsalas, P.; Avgouropoulos, G. Enhanced Performance of LiAl0.1Mn1.9O4 Cathode for Li-Ion Battery via TiN Coating. Energies 2021, 14, 825. [Google Scholar] [CrossRef]
- Abdel-Ghany, A.; Hashem, A.M.; Mauger, A.; Julien, C.M. Lithium-Rich Cobalt-Free Manganese-Based Layered Cathode Materials for Li-Ion Batteries: Suppressing the Voltage Fading. Energies 2020, 13, 3487. [Google Scholar] [CrossRef]
- Vu, N.H.; Dao, V.-D.; Tran Huu, H.; Im, W.B. Effect of Synthesis Temperature on Structure and Electrochemical Performance of Spinel-Layered Li1.33MnTiO4+z in Li-Ion Batteries. Energies 2020, 13, 2962. [Google Scholar] [CrossRef]
- Venevtsev, Y.N.; Gagulin, V.V.; Zhitomirsky, I.D. Material science aspects of seignette-magnetism problem. Ferroelectrics 1987, 73, 221–248. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. Fe3O4 spinel-Mn3O4 spinel supercapacitor prepared using Celestine blue as a dispersant, capping agent and charge transfer mediator. Ceram. Int. 2020, 46, 18851–18858. [Google Scholar] [CrossRef]
- Guan, D.; Gao, Z.; Yang, W.; Wang, J.; Yuan, Y.; Wang, B.; Zhang, M.; Liu, L. Hydrothermal synthesis of carbon nanotube/cubic Fe3O4 nanocomposite for enhanced performance supercapacitor electrode material. Mater. Sci. Eng. B 2013, 178, 736–743. [Google Scholar] [CrossRef]
- Nipan, G.; Smirnova, M.; Kornilov, D.Y.; Kop’eva, M.; Nikiforova, G.; Gubin, S. Transformation of Solid Solution with Spinel-Type Structure Within the Range LiMn2−x(Ni0.33Co0.33Fe0.33)xO4 (0 ≤ x ≤ 2). J. Phase Equilibria Diffus. 2020, 41, 819–826. [Google Scholar] [CrossRef]
- Joo, H.; Lee, J.; Yoon, J. Short Review: Timeline of the Electrochemical Lithium Recovery System Using the Spinel LiMn2O4 as a Positive Electrode. Energies 2020, 13, 6235. [Google Scholar] [CrossRef]
- Abbas, S.M.; Hashem, A.M.; Abdel-Ghany, A.E.; Ismail, E.H.; Kotlár, M.; Winter, M.; Li, J.; Julien, C.M. Ag-Modified LiMn2O4 Cathode for Lithium-Ion Batteries: Coating Functionalization. Energies 2020, 13, 5194. [Google Scholar] [CrossRef]
- Cao, K.; Jia, Y.; Wang, S.; Huang, K.-J.; Liu, H. Mn3O4 nanoparticles anchored on carbon nanotubes as anode material with enhanced lithium storage. J. Alloy. Compd. 2021, 854, 157179. [Google Scholar] [CrossRef]
- Dai, Y.; Men, Y.; Wang, J.; Liu, S.; Li, S.; Li, Y.; Wang, K.; Li, Z. Tailoring the morphology and crystal facet of Mn3O4 for highly efficient catalytic combustion of ethanol. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127216. [Google Scholar] [CrossRef]
- Rizal, M.Y.; Saleh, R.; Taufik, A.; Yin, S. Photocatalytic decomposition of methylene blue by persulfate-assisted Ag/Mn3O4 and Ag/Mn3O4/graphene composites and the inhibition effect of inorganic ions. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100408. [Google Scholar] [CrossRef]
- Patil, T.S.; Gangawane, S.A.; Takale, M.V. A Review on Mn3O4 and Its Composite Nanomaterials of Diverse Morphologies as an Electrode Material in Supercapacitors. Int. J. Sci. Res. Sci. Technol. 2021, 8, 520–526. [Google Scholar]
- Sayyed, S.G.; Shaikh, A.V.; Dubal, D.P.; Pathan, H.M. Paving the Way towards Mn3O4 Based Energy Storage Systems. ES Energy Environ. 2021, 14, 3–21. [Google Scholar] [CrossRef]
- Rorabeck, K.; Zhitomirsky, I. Salting-out aided dispersive extraction of Mn3O4 nanoparticles and carbon nanotubes for application in supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126451. [Google Scholar] [CrossRef]
- Poon, R.; Zhitomirsky, I. High areal capacitance of Mn3O4-carbon nanotube electrodes. Mater. Lett. 2018, 215, 4–7. [Google Scholar] [CrossRef]
- Nagarajan, N.; Cheong, M.; Zhitomirsky, I. Electrochemical capacitance of MnOx films. Mater. Chem. Phys. 2007, 103, 47–53. [Google Scholar] [CrossRef]
- Milne, J.; Zhitomirsky, I. Application of octanohydroxamic acid for liquid-liquid extraction of manganese oxides and fabrication of supercapacitor electrodes. J. Colloid Interface Sci. 2018, 515, 50–57. [Google Scholar] [CrossRef]
- Poon, R.; Liang, W.; Zhitomirsky, I. Mn3O4 and (ZnFe) OOH Composites for Supercapacitors with High Active Mass. Metall. Mater. Trans. A 2020, 51, 855–862. [Google Scholar] [CrossRef]
- Ata, M.S.; Milne, J.; Zhitomirsky, I. Fabrication of Mn3O4—carbon nanotube composites with high areal capacitance using cationic and anionic dispersants. J. Colloid Interface Sci. 2018, 512, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.M.; Warnan, J. Molecular Dye-Sensitized Photocatalysis with Metal-Organic Framework and Metal Oxide Colloids for Fuel Production. Energies 2021, 14, 4260. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; Liu, H.; Wei, Q.; Gong, D.; Mo, L.; Tao, H.; Cui, S.; Wang, L. One-Step Synthesis of Highly Dispersed and Stable Ni Nanoparticles Confined by CeO2 on SiO2 for Dry Reforming of Methane. Energies 2020, 13, 5956. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Gal-Or, L. Formation of hollow fibers by electrophoretic deposition. Mater. Lett. 1999, 38, 10–17. [Google Scholar] [CrossRef]
- Lee, S.H.; Woo, S.P.; Kakati, N.; Kim, D.-J.; Yoon, Y.S. A Comprehensive Review of Nanomaterials Developed Using Electrophoresis Process for High-Efficiency Energy Conversion and Storage Systems. Energies 2018, 11, 3122. [Google Scholar] [CrossRef] [Green Version]
- Ata, M.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. RSC Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Martin, R.L.; White, A.H.; Willis, A.C. Structural studies in metal–purpurate complexes. Part 1. Crystal structures of potassium purpurate trihydrate and ammonium purpurate monohydrate (murexide). J. Chem. Soc. Dalton Trans. 1977, 8, 1336–1342. [Google Scholar] [CrossRef]
- Masoud, M.; Kassem, T.; Shaker, M.; Ali, A. Studies on transition metal murexide complexes. J. Therm. Anal. Calorim. 2006, 84, 549–555. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Qiu, Y.; Zhou, Z.; Zhong, W.; Li, X. Multiwalled carbon nanotubes as a solid-phase extraction adsorbent for the determination of three barbiturates in pork by ion trap gas chromatography–tandem mass spectrometry (GC/MS/MS) following microwave assisted derivatization. Anal. Chim. Acta 2007, 586, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Wallar, C.; Poon, R.; Zhitomirsky, I. High Areal Capacitance of V2O3-Carbon Nanotube Electrodes. J. Electrochem. Soc. 2017, 164, A3620–A3627. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True performance metrics in electrochemical energy storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, K.; Zhitomirsky, I. Fabrication of Polypyrrole-Coated Carbon Nanotubes Using Oxidant–Surfactant Nanocrystals for Supercapacitor Electrodes with High Mass Loading and Enhanced Performance. ACS Appl. Mater. Interfaces 2013, 5, 13161–13170. [Google Scholar] [CrossRef]
- Rorabeck, K.; Zhitomirsky, I. Dispersant Molecules with Functional Catechol Groups for Supercapacitor Fabrication. Molecules 2021, 26, 1709. [Google Scholar] [CrossRef]
- Shi, K.; Ren, M.; Zhitomirsky, I. Activated Carbon-Coated Carbon Nanotubes for Energy Storage in Supercapacitors and Capacitive Water Purification. ACS Sustain. Chem. Eng. 2014, 2, 1289–1298. [Google Scholar] [CrossRef]
- Poon, R.; Zhao, X.; Ata, M.S.; Clifford, A.; Zhitomirsky, I. Phase transfer of oxide particles for application in thin films and supercapacitors. Ceram. Int. 2017, 43, 8314–8320. [Google Scholar] [CrossRef]
- Milne, J.; Silva, R.M.; Zhitomirsky, I. Surface modification and dispersion of ceramic particles using liquid-liquid extraction method for application in supercapacitor electrodes. J. Eur. Ceram. Soc. 2019, 39, 3450–3455. [Google Scholar] [CrossRef]
- Poon, R.; Zhitomirsky, I. Application of Cyrene as a solvent and dispersing agent for fabrication of Mn3O4-carbon nanotube supercapacitor electrodes. Colloid Interface Sci. Commun. 2020, 34, 100226. [Google Scholar] [CrossRef]
- Rorabeck, K.; Zhitomirsky, I. Application of Octanohydroxamic Acid for Salting out Liquid–Liquid Extraction of Materials for Energy Storage in Supercapacitors. Molecules 2021, 26, 296. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhitomirsky, I. Aqueous electrostatic dispersion and heterocoagulation of multiwalled carbon nanotubes and manganese dioxide for the fabrication of supercapacitor electrodes and devices. RSC Adv. 2014, 4, 45481–45489. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, K.; Zhitomirsky, I. New colloidal route for electrostatic assembly of oxide nanoparticle–carbon nanotube composites. Colloids Surf. A Physicochem. Eng. Asp. 2014, 446, 15–22. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhitomirsky, I. Surface modification of MnO2 and carbon nanotubes using organic dyes for nanotechnology of electrochemical supercapacitors. J. Mater. Chem. A 2013, 1, 12519–12526. [Google Scholar] [CrossRef]
- Li, J.; Yang, Q.M.; Zhitomirsky, I. Nickel foam-based manganese dioxide–carbon nanotube composite electrodes for electrochemical supercapacitors. J. Power Sources 2008, 185, 1569–1574. [Google Scholar] [CrossRef]

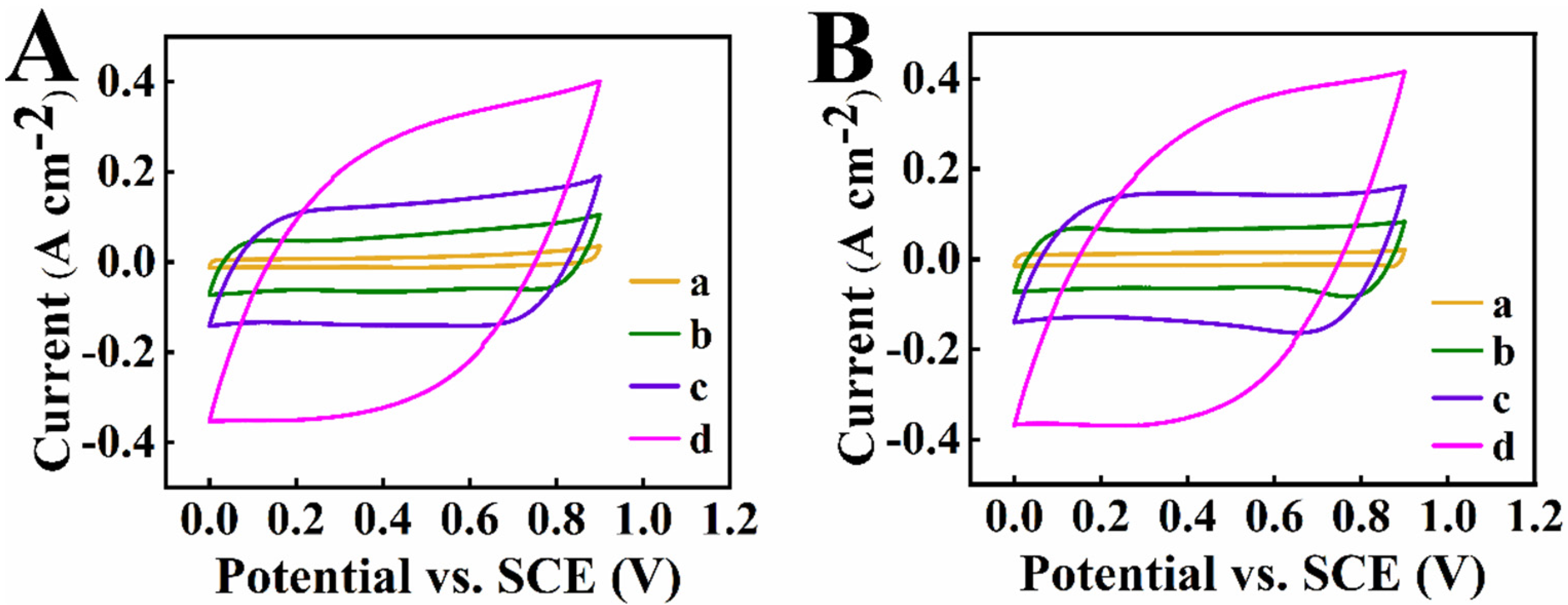

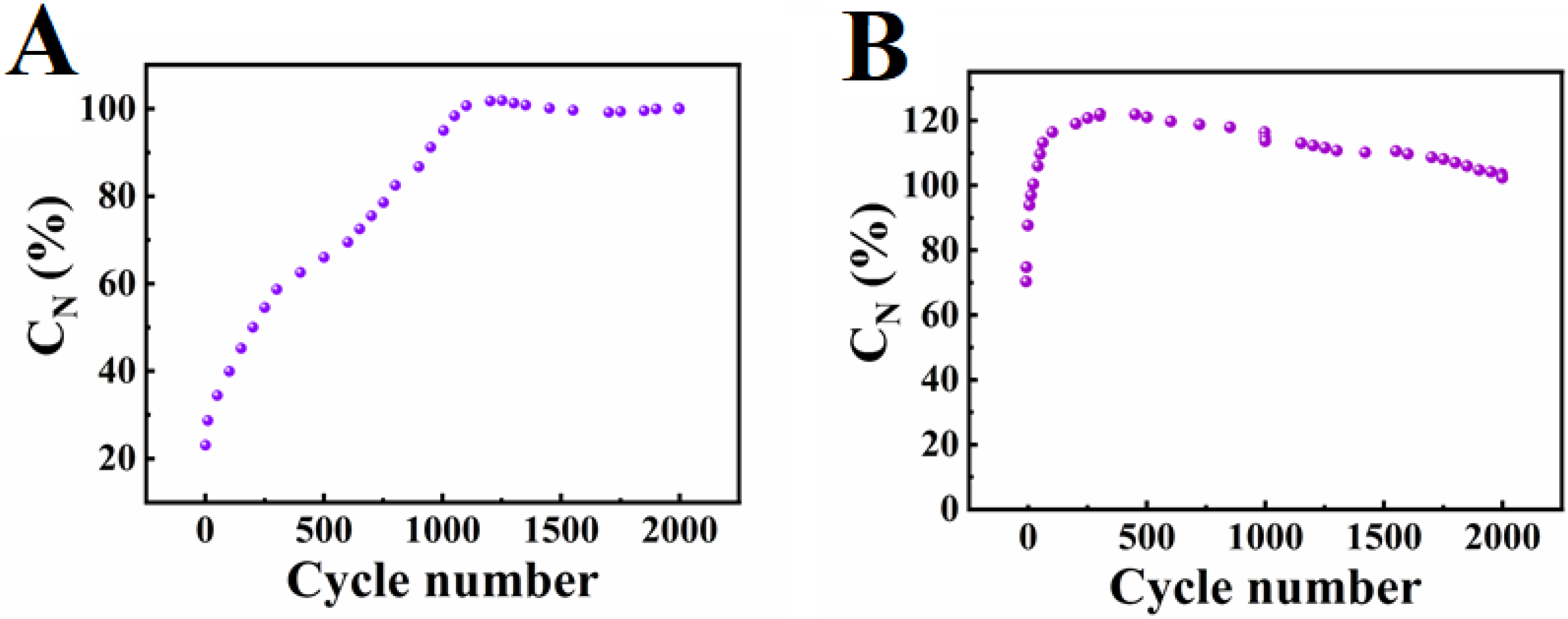
| Material | Active Mass (mg cm−2) | Areal Capacitance (F cm−2) | Reference |
|---|---|---|---|
| Mn3O4 | 28.4 | 2.8 | [35] |
| Mn3O4 | 30.4 | 2.63 | [49] |
| Mn3O4 | 33.0 | 4.2 | [33] |
| Mn3O4 | 35.0 | 3.5 | [31] |
| Mn3O4 | 36.0 | 3.1 | [50] |
| Mn3O4 | 36.0 | 3.79 | [51] |
| Mn3O4 | 40.1 | 4.3 | [52] |
| Mn3O4 | 40.0 | 6.67 | this work |
| MnO2 | 40.0 | 5.26 | [53] |
| MnO2 | 40.0 | 5.3 | [54] |
| MnO2 | 40.0 | 5.9 | [55] |
| MnO2 | 40.0 | 6.2 | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Nawwar, M.; Zhitomirsky, I. Facile Route for Fabrication of Ferrimagnetic Mn3O4 Spinel Material for Supercapacitors with Enhanced Capacitance. Energies 2022, 15, 1812. https://doi.org/10.3390/en15051812
Yang W, Nawwar M, Zhitomirsky I. Facile Route for Fabrication of Ferrimagnetic Mn3O4 Spinel Material for Supercapacitors with Enhanced Capacitance. Energies. 2022; 15(5):1812. https://doi.org/10.3390/en15051812
Chicago/Turabian StyleYang, Wenjuan, Mohamed Nawwar, and Igor Zhitomirsky. 2022. "Facile Route for Fabrication of Ferrimagnetic Mn3O4 Spinel Material for Supercapacitors with Enhanced Capacitance" Energies 15, no. 5: 1812. https://doi.org/10.3390/en15051812







