Degradation of Low Concentrated Perfluorinated Compounds (PFCs) from Water Samples Using Non-Thermal Atmospheric Plasma (NTAP)
Abstract
:1. Introduction
2. Materials and Methods
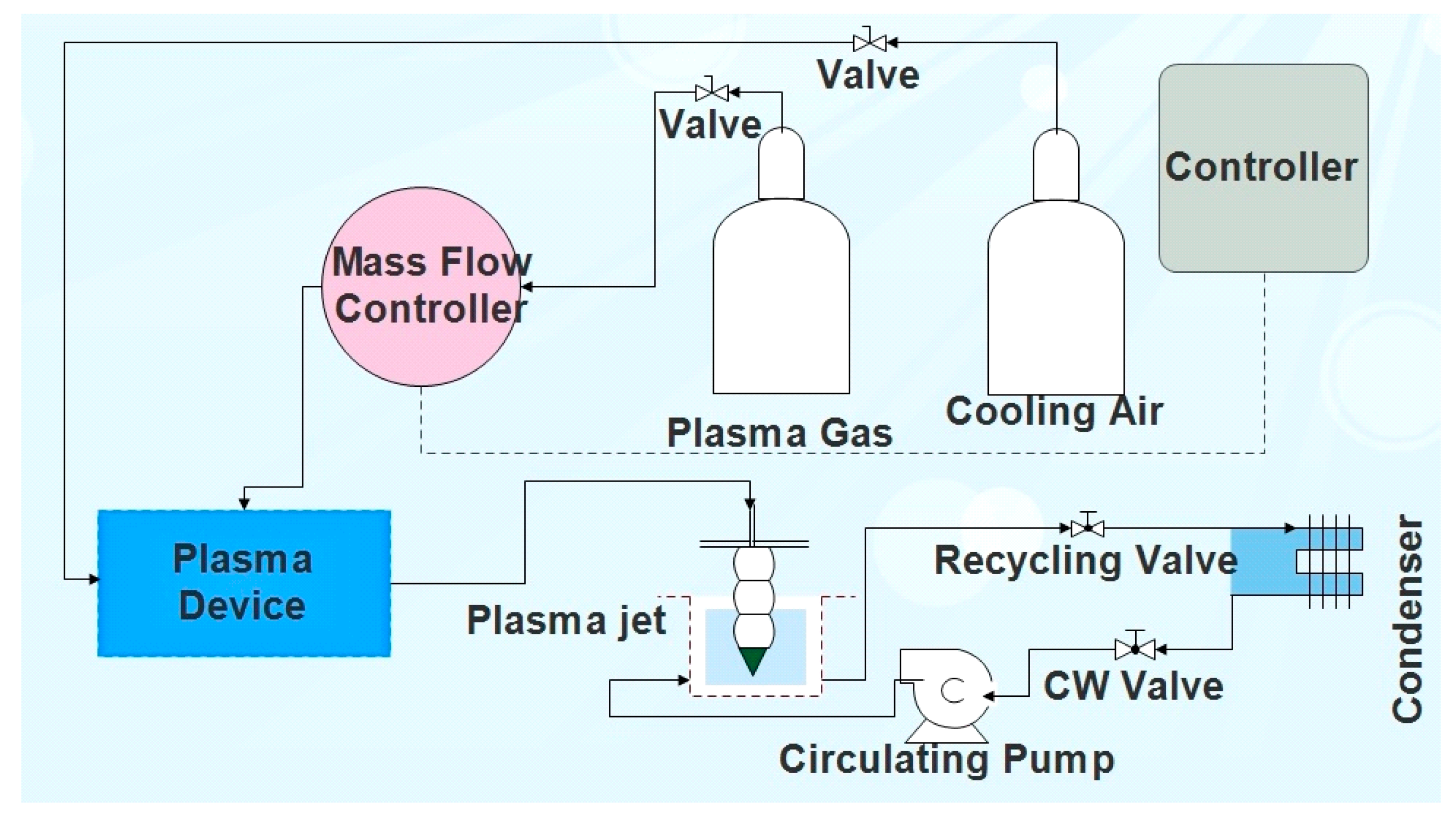
2.1. Experimental Set-up
2.2. Experimental Procedure
3. Results and Discussion
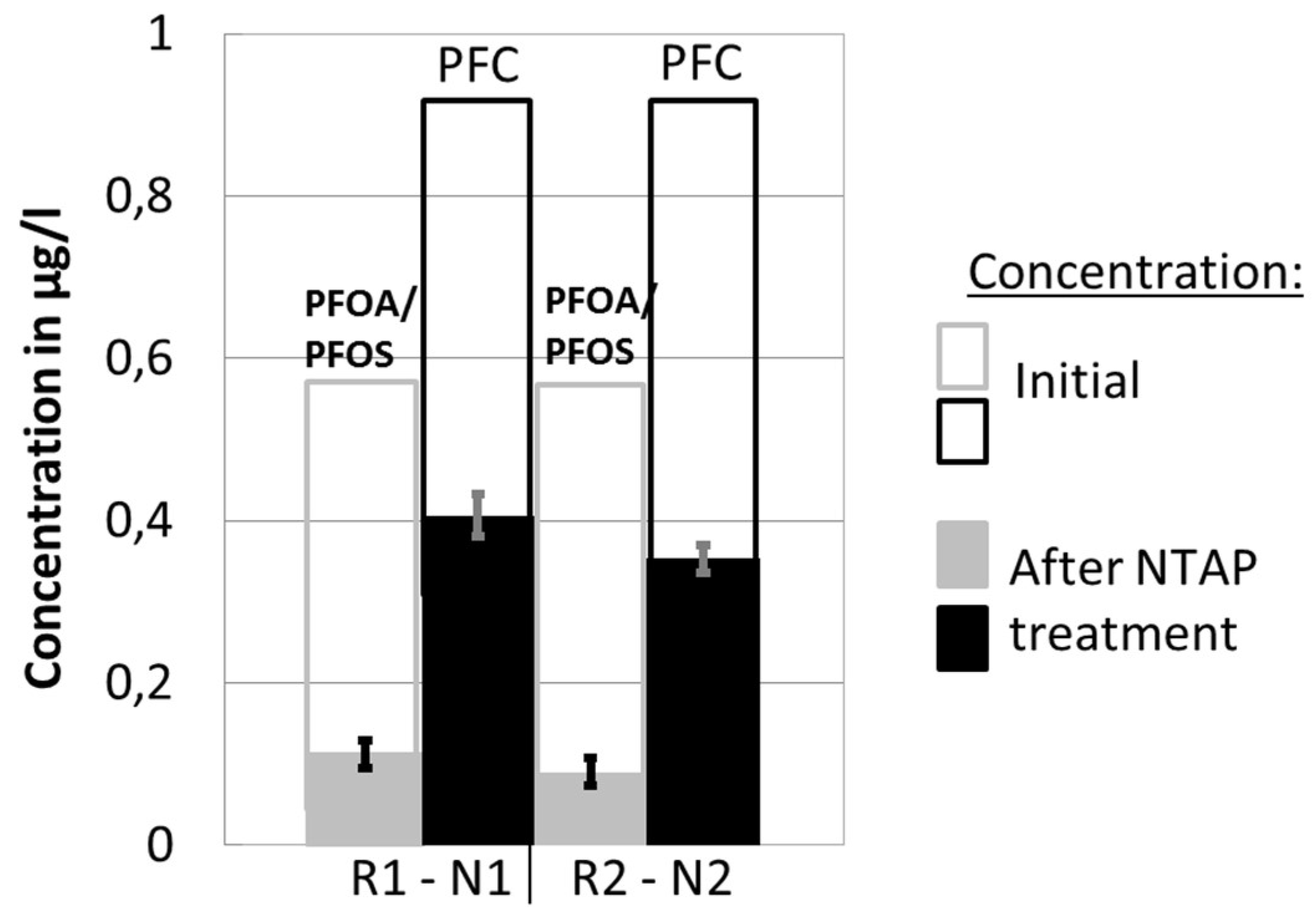
3.1. Repeatability
3.2. Perfluorinated Compound (PFC) Concentration and Influence of Non-Thermal Atmospheric Plasma (NTAP) Treatment
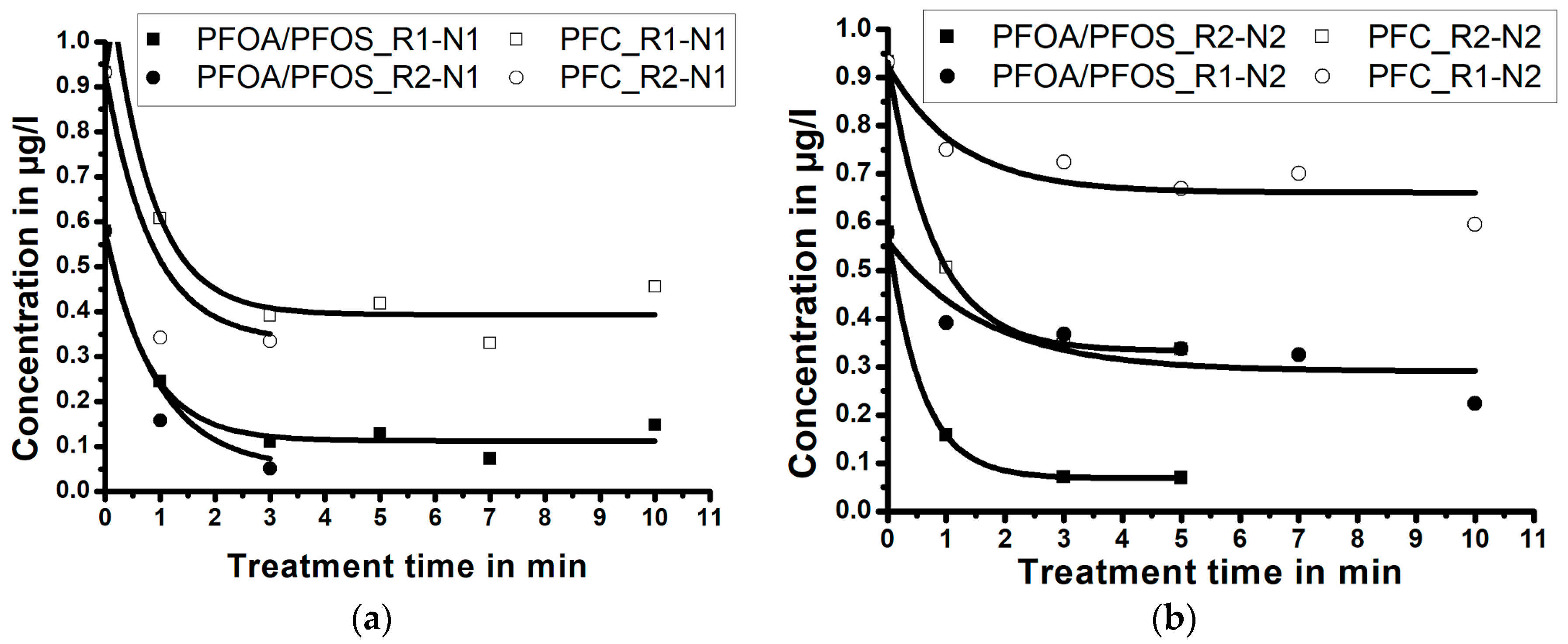
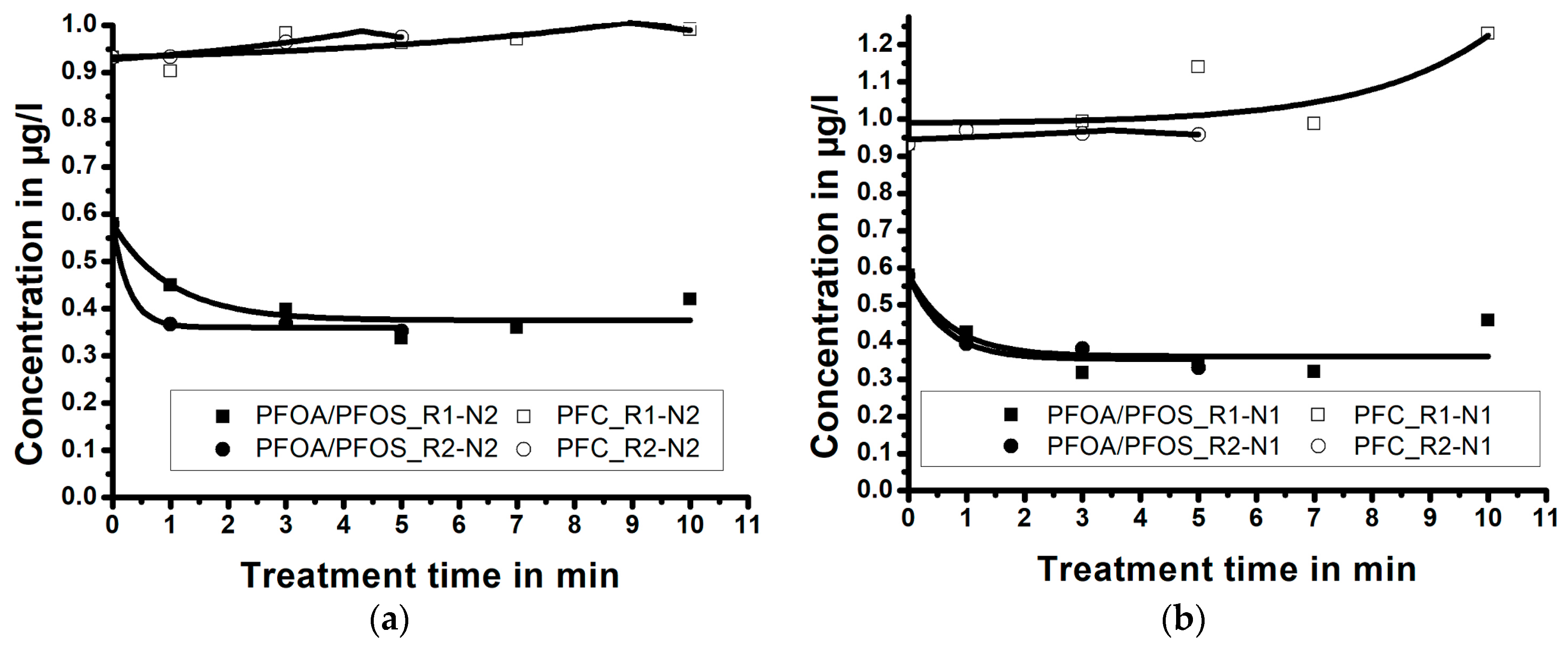
3.3. Influence of Treatment Time/Reactor Size/Nozzle Size
3.4. Influence of the pH-Value
3.5. Influence of the Plasma Gas
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Key, B.D.; Howell, R.D.; Criddle, C.S. Fluorinated organics in the biosphere. Environ. Sci. Technol. 1997, 31, 2445–2454. [Google Scholar] [CrossRef]
- Liou, J.S.-C.; Szostek, B.; DeRito, C.M.; Madsen, E.L. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 2010, 80, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Zareitalabad, P.; Siemens, J.; Hamer, M.; Amelung, W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater—A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J. Perfluorinated chemicals taint drinking water. Chem. Eng. News 2016, 94, 20–22. [Google Scholar]
- Parsons, J.R.; Saez, M.; Dolfing, J.; de Voogt, P. Biodegradation of perfluorinated compounds. Rev. Environ. Contam. Toxicol. 2008, 196, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Butt, C.M.; Berger, U.; Bossi, R.; Tomy, G.T. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci. Total Environ. 2010, 408, 2936–2965. [Google Scholar] [CrossRef] [PubMed]
- Kwadijk, C.J.; Korytár, P.; Koelmans, A.A. Distribution of Perfluorinated Compounds in Aquatic Systems in The Netherlands. Environ. Sci. Technol. 2010, 44, 3746–3751. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Polprasert, C.; Tanaka, S.; Lien, N.P.H.; Qiu, Y. New POPs in the water environment: Distribution, bioaccumulation and treatment of perfluorinated compounds: A review paper. J. Water Supp. Res. Technol. AQUA 2007, 56, 313–326. [Google Scholar] [CrossRef]
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2012, 116, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Sci. Total Environ. 2015, 524, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Lehmler, H.J. Synthesis of environmentally relevant fluorinated surfactants—A review. Chemosphere 2005, 58, 1471–1496. [Google Scholar] [CrossRef] [PubMed]
- Mhadhbi, L.; Rial, D.; Pérez, S.; Beiras, R. Ecological risk assessment of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in marine environment using Isochrysis galbana, Paracentrotus lividus, Siriella armata and Psetta maxima. J. Environ. Monit. 2012, 14, 1375–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutze, H.V.; Brekenfeld, J.; Naumov, S.; von Sonntag, C.; Schmidt, T.C. Degradation of perfluorinated compounds by sulfate radicals—New mechanistic aspects and economical considerations. Water Res. 2018, 129, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Ludwicki, J.K.; Goralczyk, K.; Strucinski, P.; Wojtyniak, B.; Rabczenko, D.; Loft, G.; Lindh, C.H.; Jonsson, B.A.G.; Lenters, V.; Heederik, D.; et al. Hazard quotient profiles used as a risk assessment tool for PFOS and PFOA serum levels in three distinctive European populations. Environ. Int. 2015, 74, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Liu, W.; Sato, I.; Nakayama, S.F.; Sasaki, K.; Saito, N.; Tsuda, S. PFOS and PFOA in environmental and tap water in China. Chemosphere 2009, 77, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Codling, G.; Vogt, A.; Jones, P.D.; Wang, T.; Wang, P.; Lu, Y.L.; Corcoran, M.; Bonina, S.; Li, A.; Sturchio, N.C.; et al. Historical trends of inorganic and organic fluorine in sediments of Lake Michigan. Chemosphere 2014, 114, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Buser, A.; Morf, L. Substance Flow Analysis for Switzerland: Perfluorinated Surfactants Perfluorooctanesulfonate (PFOS) and Perfluorooctanoic Acid (PFOA); Environmental Studies No. 0922; Federal Office for the Environment: Bern, Switzerland, 2009; p. 144. [Google Scholar]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011, 23, 38–52. [Google Scholar] [CrossRef]
- Bliss, J. Removal of PFOA from Water Using UV Treatment, Chemical Oxidation and Adsorption by Activated Carbon and Zeolites; Major Qualify Project, Worcester Polytechnic Institute: Worceste, MA, USA, 2012. [Google Scholar]
- Renner, R. Growing concern over perfluorinated chemicals. Environ. Sci. Technol. 2001, 35, 154A–160A. [Google Scholar] [CrossRef] [PubMed]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Li, Y.; Shang, E.; Xu, Z.; Liu, J. Electrochemical oxidation of perfluorinated compounds in water. Chemosphere 2016, 146, 526–538. [Google Scholar] [CrossRef] [PubMed]
- OECD. Results of Survey on Production and Use of PFOS, PFAS and PFOA, Related Substances and Products/Mixtures Containing These Substances; Health and Safety Publications Series on Risk Management No. 22; OECD Environment: Paris, France, 2006. [Google Scholar]
- US Environmental Protection Agency (US EPA): Basic Information about Per- and Polyfluoroalkyl Substances (PFASs). Available online: www.epa.gov (accessed on 1 April 2018).
- Steenland, K.; Fletcher, T.; Savitz, D.A. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ. Health Perspect. 2010, 118, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- US EPA, 2014: Emerging Contaminants—Perfluorooctane sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). Available online: https://nepis.epa.gov (accessed on 1 April 2018).
- Lu, Z.; Song, L.; Zhao, Z.; Ma, Y.; Wang, J.; Yang, H.; Ma, H.; Cai, M.; Codling, G.; Ebinghaus, R.; et al. Occurrence and trends in concentrations of perfluoroalkyl substances (PFASs) in surface waters of eastern China. Chemosphere 2015, 119, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.-H.; Cho, C.-R.; Lee, J.-S.; Soh, H.-Y.; Lee, B.-C.; Lee, J.-A.; Tatarozako, N.; Sasaki, K.; Saito, N.; Iwabuchi, K.; et al. Perfluorinated alkyl substances in water, sediment, plankton and fish from Korean rivers and lakes: A nationwide survey. Sci. Total Environ. 2014, 491, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Konwick, B.J.; Tomy, G.T.; Ismail, N.; Peterson, J.T.; Fauver, R.J.; Higginbotham, D.; Fisk, A.T. Concentrations and patterns of perfluoroalkyl acids in Georgia, USA surface waters near and distant to a major use source. Environ. Toxicol. Chem. 2008, 27, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Webster, G. Potential Human Health Effects of Perfluorinated Chemicals (PFCs). Available online: www.ncceh.ca/sites/default/files/Health_effects_PFCs_Oct_2010.pdf (accessed on 1 April 2018).
- Gorrochategui, E.; Perez-Albaladejo, E.; Casas, J.; Lacorte, S.; Porte, C. Perfluorinated chemicals: Differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol. Appl. Pharmacol. 2014, 277, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Liao, C.; Cai, Y.; Jiang, G. Perspectives on the inclusion of perfluorooctane sulfonate into the Stockholm convention on persistent organic pollutants. Environ. Sci. Technol. 2009, 43, 5171–5175. [Google Scholar] [CrossRef] [PubMed]
- COPs: Synergies among the Basel, Rotterdam and Stockholm Conventions. Available online: www.brsmeas.org (accessed on 1 April 2018).
- EPA Stewardship. Available online: www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program (accessed on 1 April 2018).
- Commonwealth Environmental Management Guidance on Perfluorooctane Sulfonic Acid (PFOS) and Perfluorooctanoic Acid (PFOA). October 2016. Available online: www.environment.gov.au/system/files/pages/dfb876c5-581e-48b7-868c-242fe69dad68/files/draft-environmental-mgt-guidance-pfos-pfoa.pdf (accessed on 5 April 2018).
- EU Directive 2013/39/EU. The European Parliament and the Council of the European Union, 2013. Available online: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:EN:PDF (accessed on 5 April 2018).
- Network for Industrially Co-Ordinated Sustainable Land Management in Europe (NICOLE). PFASs Summary. Emerging Contaminants Working Group. Available online: www.nicole.org/uploadedfiles/2017%20PFAS%20Summary,%20Report%20NICOLE%20Working%20Group%20PFAS.pdf (accessed on 5 April 2018).
- Moermond, C.T.A.; Verbruggen, E.M.J.; Smit, C.E. Environmental Risk Limits for PFOS: A Proposal for Water Quality Standards in Accordance with the Water Framework Directive. RIVM Report 601714013/2010. Available online: www.rivm.nl/bibliotheek/rapporten/601714013.pdf (accessed on 5 April 2018).
- Perfluorinated Compounds. Treatment and Removal. American Water Works Association (AWWA). Available online: www.awwa.org/Portals/0/files/resources/water%20knowledge/rc%20healtheffects/AWWAPFCFactSheetTreatmentandRemoval.pdf (accessed on 5 April 2018).
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Symsaris, E.C.; Andersen, H.R.; Antoniou, M.G.; Nikolaos, T.S.; Athanasios, S.S. Removal of perfluorinated compounds from water with activated carbon and advanced oxidation processes (AOPs). In Micropol & Ecohazard, Proceedings of the 8th IWA Specialist Conference on Assessment and Control of Micropollutants/Hazardous Substances in Water, Zurich, Switzerland, 16–20 June 2013; IWA Publishing: London, UK, 2013. [Google Scholar]
- Lutze, H.; Panglisch, S.; Bergmann, A.; Schmidt, T. Treatment Options for the Removal and Degradation of Polyfluorinated Chemicals. In Polyfluorinated Chemicals and Transformation Products; Springer: Berlin/Heidelberg, Germany, 2011; Volume 17, pp. 103–125. [Google Scholar] [CrossRef]
- Yasuoka, K.; Sasaki, K.; Hayashi, R.; Kosugi, A.; Takeuchi, N. Degradation of Perfluoro Compounds and —Recovery in Water Using Discharge Plasmas Generated within Gas Bubbles. Int. J. Plasma Environ. Sci. Technol. 2010, 4, 113–117. [Google Scholar]
- Zhang, T.; Pan, G.; Zhou, Q. Temperature effect on photolysis decomposing of perfluorooctanoic acid. J. Environ. Sci. 2016, 42, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ. Sci. Technol. 2005, 39, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in landfill groundwater: Environmental matrix effects. Environ. Sci. Technol. 2008, 42, 8057–8063. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.; Hoffmann, M.R. Sonochemical degradation of perfluorinated surfactants: Power and multiple frequency effects. Sep. Purif. Technol. 2015, 156, 1019–1027. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Kudo, T.; Sankoda, K. Combined sonochemical and short-wavelength UV degradation of hydrophobic perfluorinated compounds. Ultrason. Sonochem. 2017, 39, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.H.; Wang, B.B.; Yu, H.S.; Wang, L.L.; Yuan, S.H.; Chen, J. Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation. J. Hazard. Mater. 2010, 179, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.; Burgess, D.R.; Babushok, V. On the incinerability of highly fluorinated organic compounds. Combust. Sci. Technol. 1998, 139, 385–402. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Eng. 2009, 3, 129–151. [Google Scholar] [CrossRef]
- Niu, J.; Lin, H.; Gong, C.; Sun, X. Theoretical and experimental insights into the electrochemical mineralization mechanism of perfluorooctanoic acid. Environ. Sci. Technol. 2013, 47, 14341–14349. [Google Scholar] [CrossRef] [PubMed]
- Chang, J. Thermal Plasma Solid Waste and Water Treatments: A Critical Review What Is Plasmas Solid Liquid Gas. Int. J. Plasma Environ. Sci. Technol. 2009, 3, 67–84. [Google Scholar]
- Cheng, H.; Chen, S. Non-thermal plasma technology for degradation of organic compounds in wastewater control: A critical review. J. Environ. 2007, 17, 427–433. [Google Scholar]
- Foster, J.; Sommers, B.S.; Gucker, S.N.; Blankson, I.M.; Adamovsky, G. Prespectives on the Interaction of Plasmas With Liquid Water for Water Purification. IEEE Trans. Plasma Sci. 2012, 40, 1311–1323. [Google Scholar] [CrossRef]
- Choi, S.; Hong, S.H.; Lee, H.S.; Watanabe, T. A comparative study of air and nitrogen thermal plasmas for PFCs decomposition. Chem. Eng. J. 2012, 185, 193–200. [Google Scholar] [CrossRef]
- Holzer, F.; Kopinke, F.D.; Roland, U. Influence of ferroelectric materials and catalysts on the performance of Non-Thermal Plasma (NTP) for the removal of air pollutants. Plasma Chem. Plasma Process. 2005, 25, 595–611. [Google Scholar] [CrossRef]
- Hao, H.L.; Zhang, X.W.; Lei, L.C. Degradation characteristics of toxic contaminant with modified activated carbons in aqueous pulsed discharge plasma process. Carbon 2009, 47, 153–161. [Google Scholar] [CrossRef]
- Hayashi, R.; Obo, H.; Takeuchi, N.; Yasuoka, K. Decomposition of Perfluorinated Compounds in Water by DC Plasma within Oxygen Bubbles. Electr. Eng. Jpn. 2015, 190, 767–772. [Google Scholar] [CrossRef]
- Martinsen, K. Polyfluorinated compounds at fire training facilities. In Proceedings of the Section for Waste Treatment and Contaminated Ground Common Forum Meeting, Bilbao, Spain, 23–24 October 2012. [Google Scholar]
- Seow, J. Firefighting Foams with Perfluororchemicals—Environmental Review; Department of Environment and Conservation Western Australia, Hemming Information Services: Sandgate, Australia, 2013. [Google Scholar]
- Frank-Kamenezki, D.A. Plasma—Der Vierte Aggregatzustand; Progress Verlag: Moskow, Russia, 1963. [Google Scholar]
- Piel, A. Plasma Physics; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Bogaerts, A.; Neyts, E.; Gijbels, R.; van der Mullen, J. Gas discharge plasmas and their applications. Spectrochim. Acta B 2002, 57, 609–658. [Google Scholar] [CrossRef]
- Kempe, V.; Jovicic, V.; Almeida Ribeiro, M.; Delgado, A. Untersuchungen zur Vergasung von Biomasse mit nicht-thermischem Stickstoff Plasma. In Proceedings of the Deutscher Flammentag Vrebrennung und Feuerung, Duisburg, Germany, 3–5 September 2013; Volume 2161, pp. 353–363. [Google Scholar]
- Al-Amayreh, M.; Al-Salaymeh, A.; Kovicic, V.; Delgado, A. Gasification of Jordanian oil shale using a nitrogen non-thermal plasma. In Proceedings of the 31st Oil shale symposium, Colorado School of Mines, Golden, CO, USA, 17–19 October 2011. [Google Scholar]
- Jovicic, V.; Jung, I.; Zbogar-Rasic, A.; Khan, M.J.; Delgado, A. Cleaning of the surfaces in the food industry using cold plasma. In Experimentelle Strömungsmesstechnik, Proceedings of the GALA Conference, Karlsruhe, Germany, 5–7 September 2017; German Association for Laser Anemometry: Karlsruhe, Germany, 2017. [Google Scholar]
- Jovicic, V.; Zbogar-Rasic, A.; Groß, F.; Jung, I.; Seok, J.; Kim, Y.; Delgado, A. Overview of experimental evaluation of innovative non-thermal plasma treatment of biotic and abiotic matter. In Proceedings of the International Nonthermal Processing Workshop, Athens, Greece, 12–13 November 2015. [Google Scholar]
- Company Diener Electronic GmbH und Co.KG (Ebhausen, Germany). Available online: www.plasma.com/plasmatechnik/ (accessed on 1 April 2018).
- Company Bronkhorst High-Tech, B.V. (AK Ruurlo, The Netherlands). Available online: www.bronkhorst.com/ (accessed on 1 April 2018).
- Thomas, M.; Mittal, K.L. (Eds.) Atmospheric Pressure Plasma Treatment of Polymers: Relevance to Adhesion; Scrivener Publishing LLC: Beverly, MA, USA, 2013. [Google Scholar]
- Company R&H Umwelt (Nuremberg, Germany). Available online: www.rh-umwelt.de/de/ (accessed on 1 April 2018).
- Uhm, H.S. Generation of various radicals in nitrogen plasma and their behavior in media. Phys. Plasmas 2015, 22, 123506. [Google Scholar] [CrossRef]

| Reactor | Nozzle | Initial PFC Concentration | Final PFC Concentration | Test Time | PFC Conc. Reduction |
|---|---|---|---|---|---|
| R1 | N1 | 0.932 μg/L | 0.391 μg/L | 3 min. | 58.0% |
| N2 | 0.669 μg/L | 5 min. | 28.2% | ||
| R2 | N1 | 0.336 μg/L | 3 min. | 63.9% | |
| N2 | 0.346 μg/L | 5 min. | 63.0% |
| R1-N1 | R2-N1 | R1-N2 | R2-N2 | |
|---|---|---|---|---|
| Initial concentration of PFC (μg/L) | 0.443 | 0.303 | 0.513 | 0.277 |
| End concentration of PFC (μg/L) | 0.380 | 0.173 | 0.418 | 0.265 |
| PFC conc. reduction rate (%) | 14 | 43 | 19 | 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovicic, V.; Khan, M.J.; Zbogar-Rasic, A.; Fedorova, N.; Poser, A.; Swoboda, P.; Delgado, A. Degradation of Low Concentrated Perfluorinated Compounds (PFCs) from Water Samples Using Non-Thermal Atmospheric Plasma (NTAP). Energies 2018, 11, 1290. https://doi.org/10.3390/en11051290
Jovicic V, Khan MJ, Zbogar-Rasic A, Fedorova N, Poser A, Swoboda P, Delgado A. Degradation of Low Concentrated Perfluorinated Compounds (PFCs) from Water Samples Using Non-Thermal Atmospheric Plasma (NTAP). Energies. 2018; 11(5):1290. https://doi.org/10.3390/en11051290
Chicago/Turabian StyleJovicic, Vojislav, Muhammad Jehanzaib Khan, Ana Zbogar-Rasic, Nataliia Fedorova, Alexander Poser, Peter Swoboda, and Antonio Delgado. 2018. "Degradation of Low Concentrated Perfluorinated Compounds (PFCs) from Water Samples Using Non-Thermal Atmospheric Plasma (NTAP)" Energies 11, no. 5: 1290. https://doi.org/10.3390/en11051290







