Experimental Study of Sulfonate Gemini Surfactants as Thickeners for Clean Fracturing Fluids
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
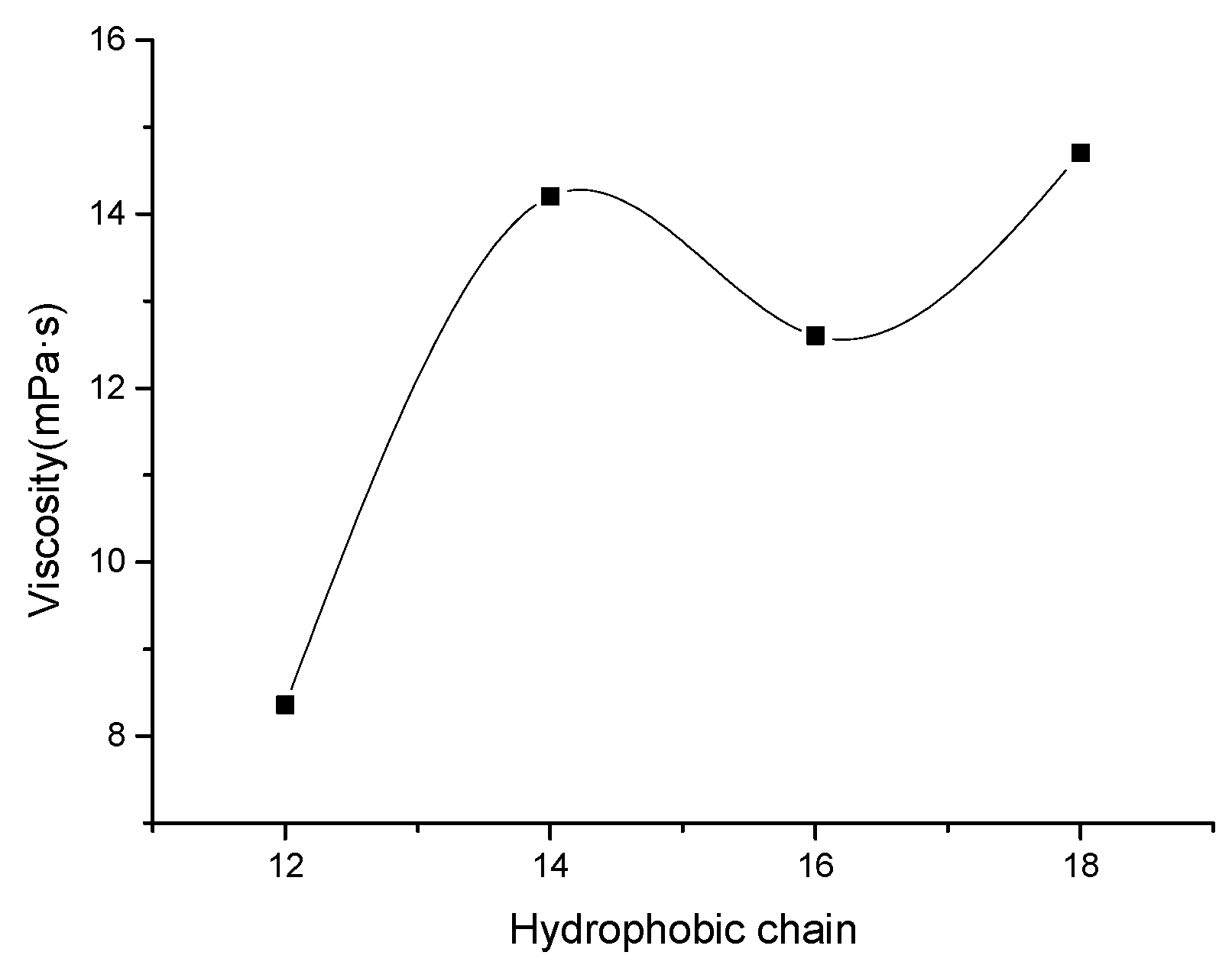
3.1. Effect of Hydrophobic Chain on the Viscosity of Sulfonate Gemini Surfactant Solution
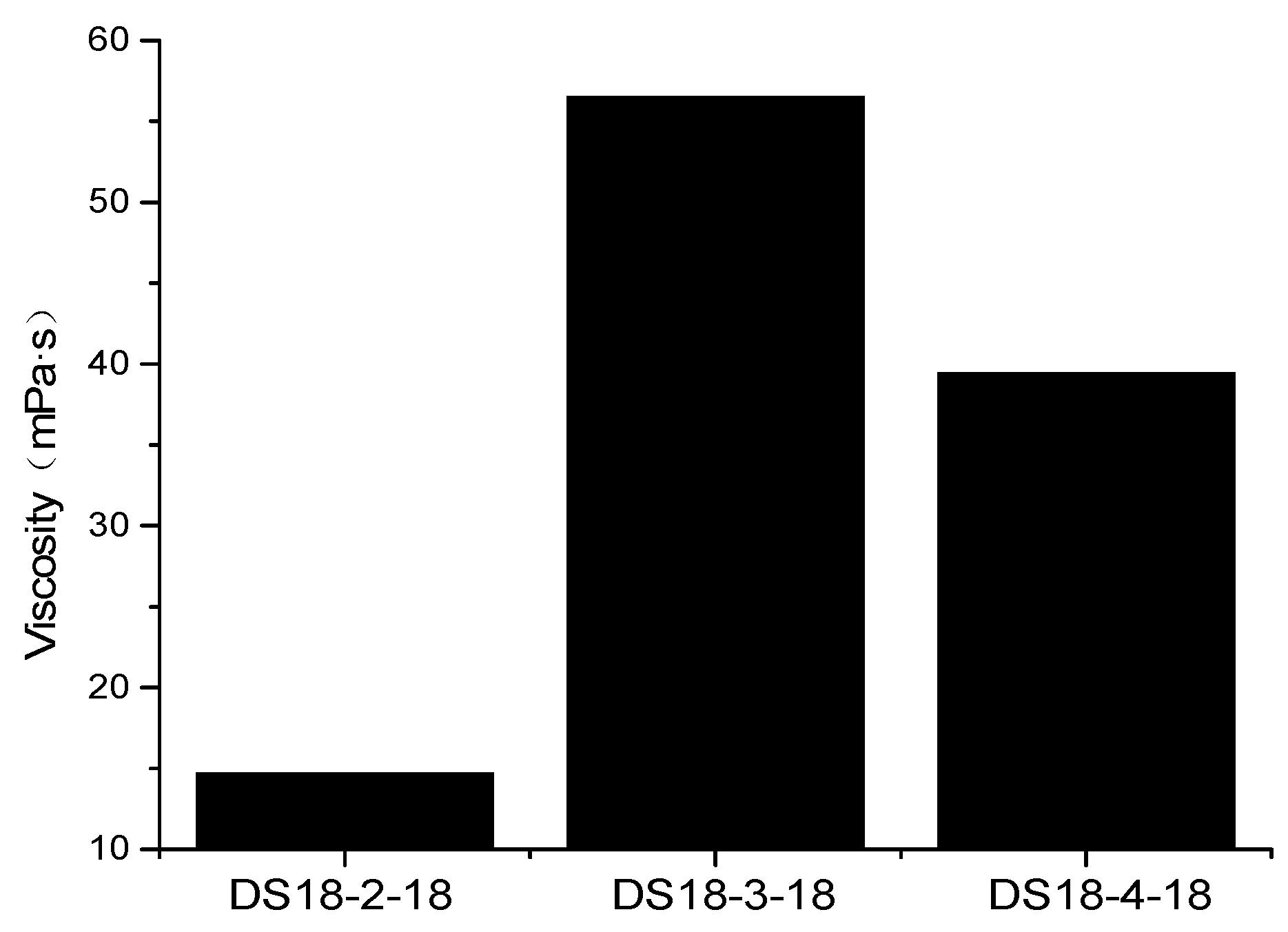
3.2. Effect of Spacer Group on the Viscosity of Sulfonate Gemini Surfactant Solution
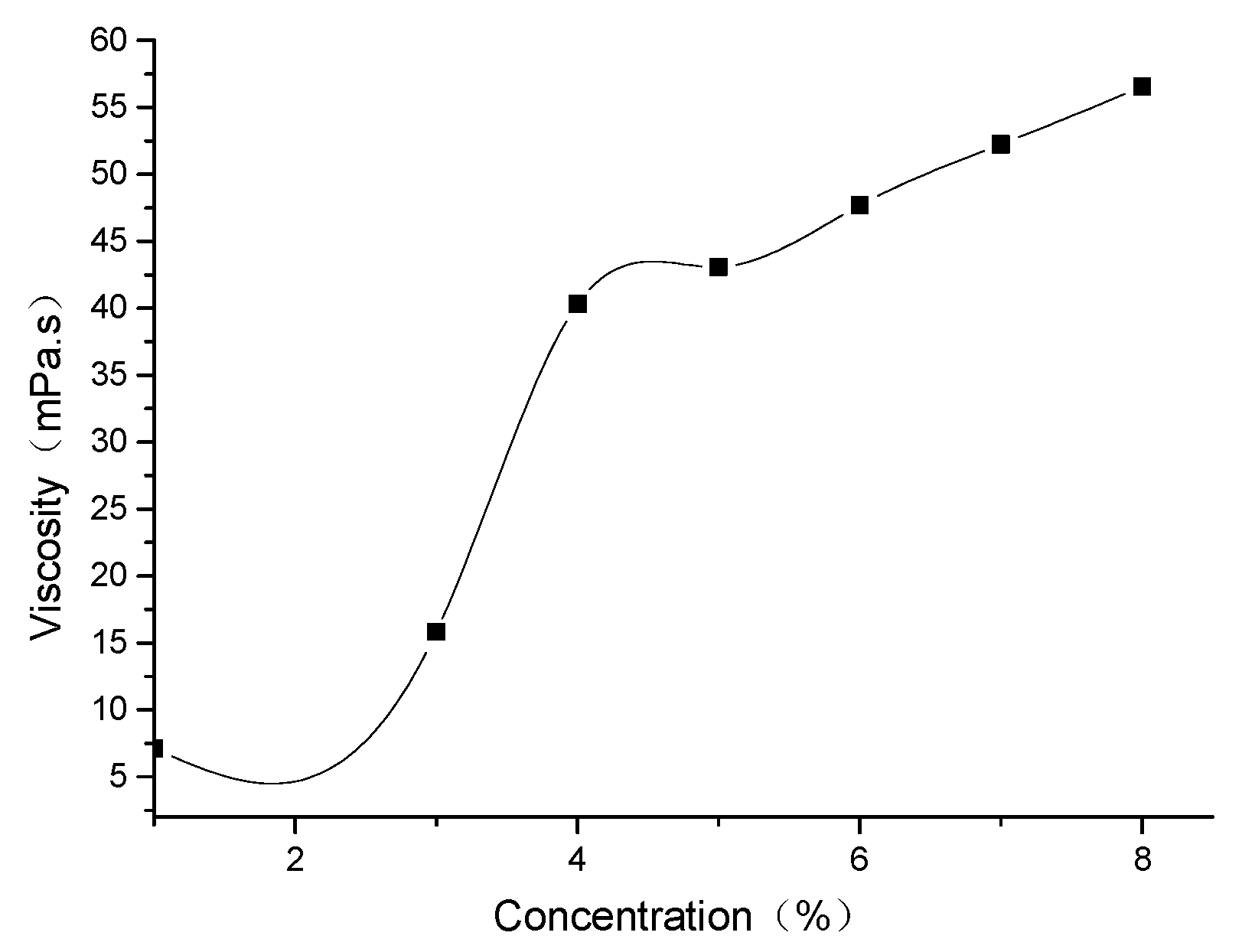
3.3. Effect of Concentration on the Viscosity of DS18-3-18 Solution
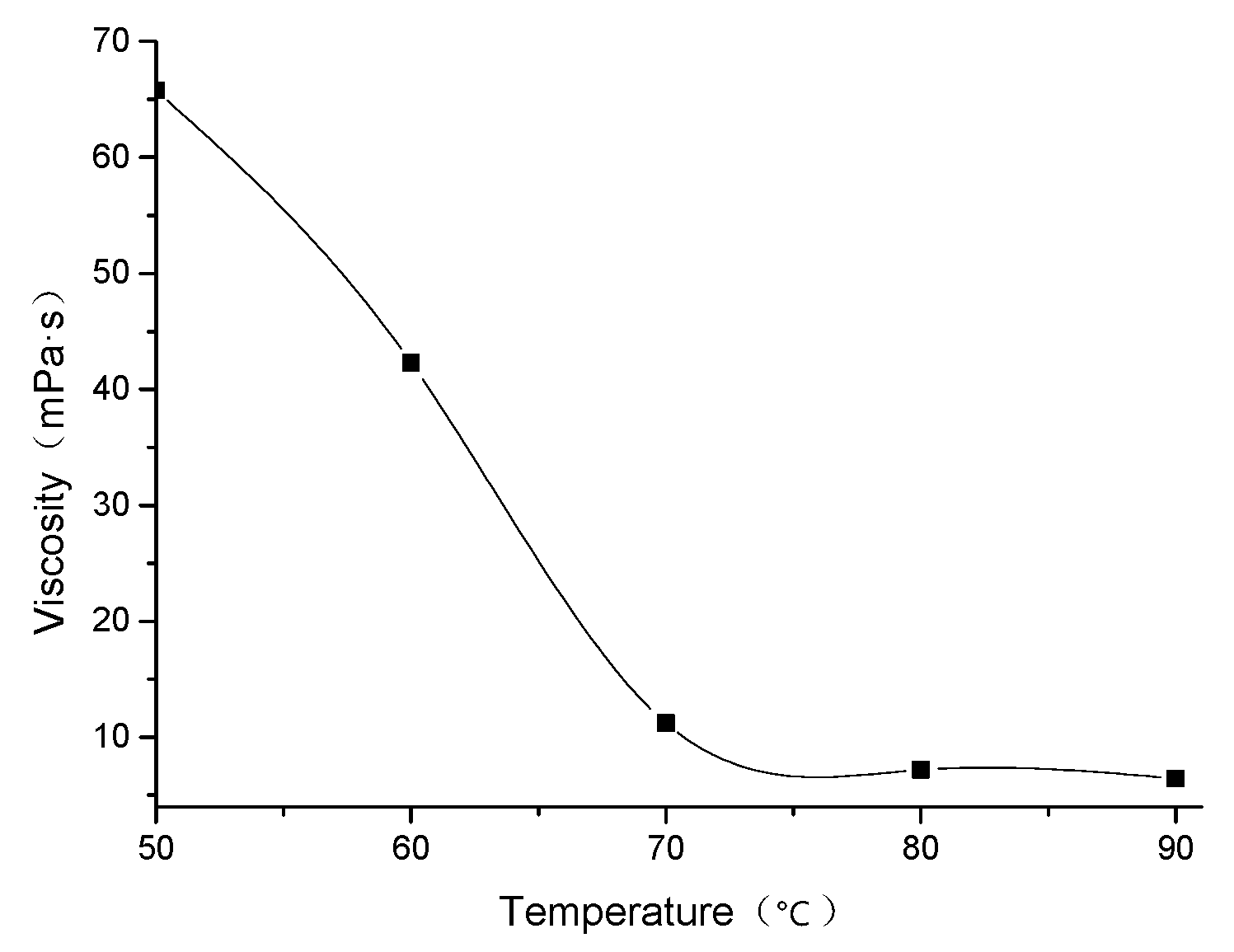
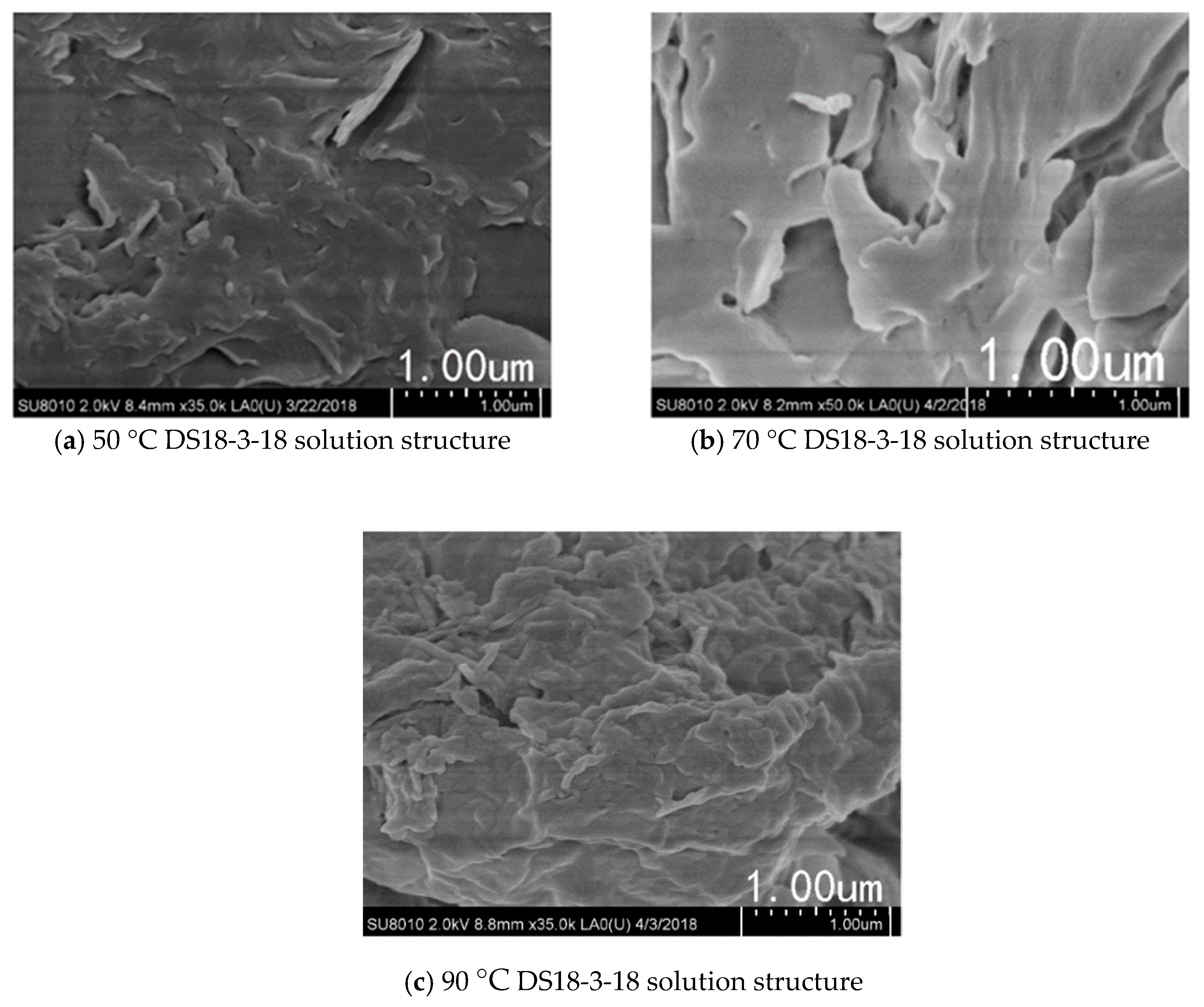
3.4. Effect of Temperature on Viscosity of DS18-3-18 Solution
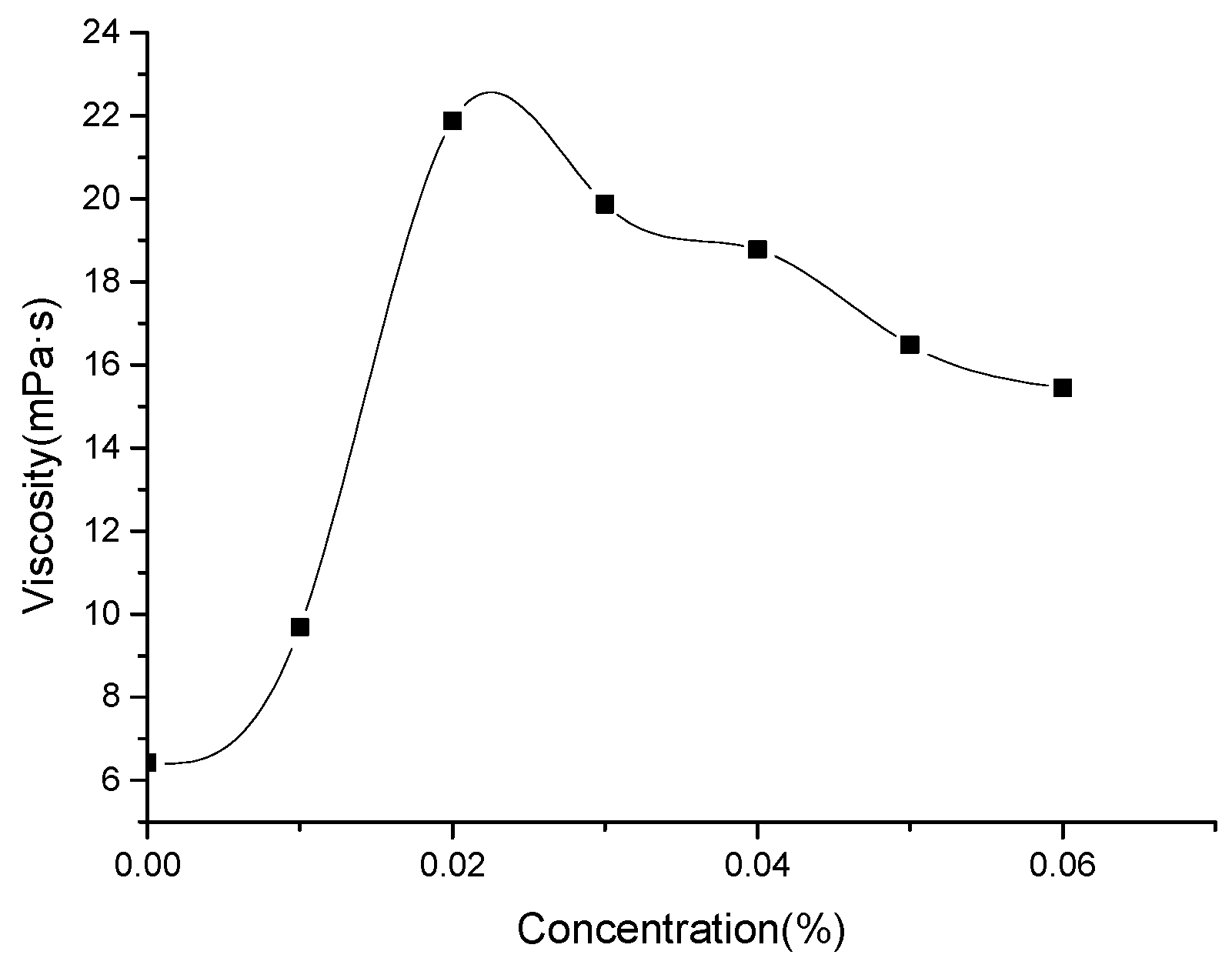
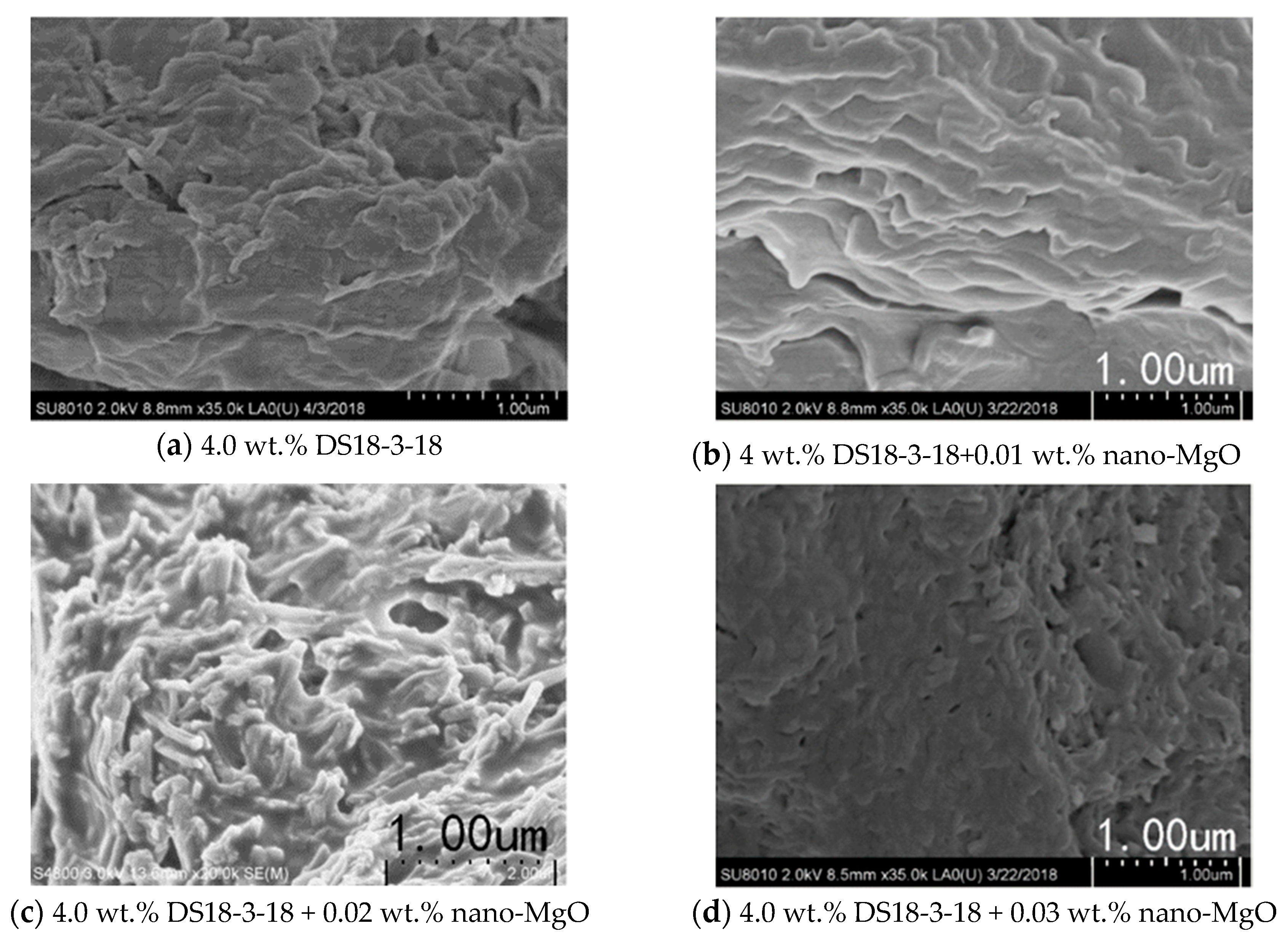
3.5. Effect of Nano-MgO on the Viscosity of 4.0 wt.% DS18-3-18
4. Conclusions
- (1)
- The viscosity of the DSm-s-m solution showed a fluctuating upward trend with increasing the length of hydrophobic chain at s = 2. Meanwhile, the viscosity of the DS18-s-18 solution increased firstly and decreased later with increasing spacer group length. Moreover, DS18-3-18 showed prominent viscosity behavior.
- (2)
- The viscosity of the DS18-3-18 solution increased as the increase of surfactant concentration, and the tendency of the increase became slow when the solution concentration exceeded 4.0 wt.%.
- (3)
- 4.0 wt.% DS18-3-18 solution had better temperature-resistance, and the viscosity of the solution kept above 6 mPa·s at 90 °C and 170 s−1, which satisfied the experimental requirements for a clean fracturing fluid thickener.
- (4)
- The addition of nano-MgO improved the temperature-resistance of 4.0 wt.% DS18-3-18 solution. The viscosity of the solution increased firstly and decreased later with increasing nano-MgO concentration. The viscosity of the solution reached its maximum of 21.87 mPa·s at 90 °C when 0.02 wt.% nano-MgO was added, which achieved the required viscosity of the clean fracturing fluid.
- (5)
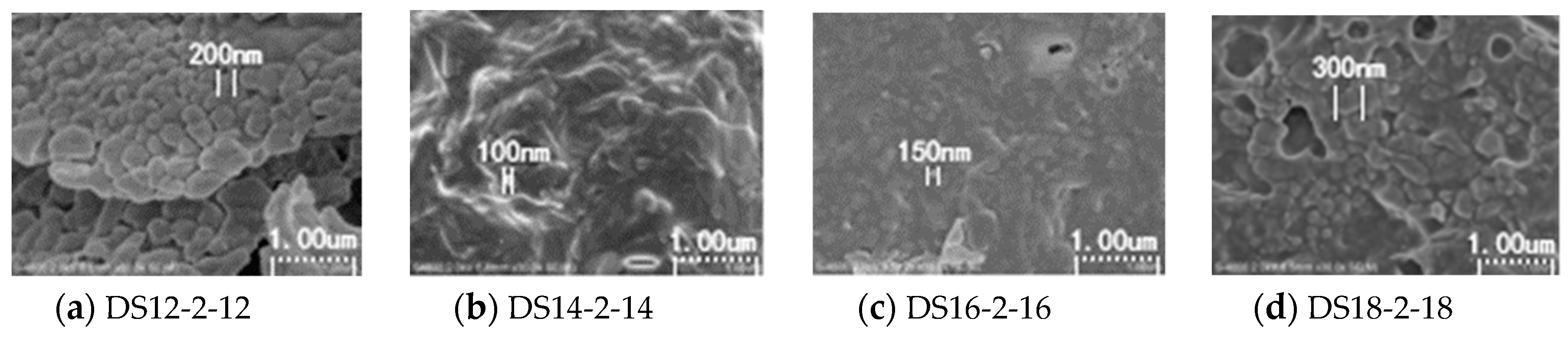
- DS18-3-18 self-assembled intodense layered micelles, and the micelles entangled with each other to form a network structure, contributing to the better temperature-resistance and higher viscosity. Nano-MgO further enhanced the temperature-resistance of 4.0 wt.% DS18-3-18 solution by changing the micellar morphology.
- (6)
- The limitation of this experimental study was that the temperature resistance of the sulfonate Gemini surfactant can only reach 90 °C, and the cost was slightly higher.
- (7)
- Compared with other studies, DS18-3-18 had a temperature resistance of 90 °C, and the viscosity after adding nano-MgO could reach up to 21.87 mPa·s, which meet the viscosity requirements of a clean fracturing fluid and has good application prospects.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Kou, Z.; Wang, H.; Zhao, Y.; Dejam, M.; Guo, J.; Du, J. Performance analysis for a model of a multi-wing hydraulically fractured vertical well in a coalbed methane gas reservoir. J. Pet. Sci. Eng. 2018, 166, 104–120. [Google Scholar] [CrossRef]
- Dejam, M.; Hassanzadeh, H.; Chen, Z. Semi-analytical solution for pressure transient analysis of a hydraulically fractured vertical well in a bounded dual-porosity reservoir. J. Hydrol. 2018, 565, 289–301. [Google Scholar] [CrossRef]
- Seright, R.S. How Much Polymer Should Be Injected During a Polymer Flood? In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Saboorian-Jooybari, H.; Dejam, M.; Chen, Z. Heavy oil polymer flooding from laboratory core floods to pilot tests and field applications: Half-century studies. J. Pet. Sci. Eng. 2016, 142, 85–100. [Google Scholar] [CrossRef]
- Rui, Z.; Wang, X.; Zhang, Z.; Lu, J.; Chen, G.; Zhou, X.; Patil, S. A realistic and integrated model for evaluating oil sands development with Steam Assisted Gravity Drainage technology in Canada. Appl. Energy 2018, 213, 76–91. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, C.; Zhao, M.; Feng, H.; Gao, B.; Li, M. Research and Application Progress of Cleaning Fracturing Fluid. Oilfield Chem. 2015, 32, 141–145. [Google Scholar]
- Yan, Z.; Dai, C.; Zhao, M.; Sun, Y. Rheological characterizations and molecular dynamics simulations of self-assembly in an anionic/cationic surfactant mixture. Soft Matter 2016, 12, 6058–6066. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, F. Wettability alteration of silica induced by surfactant adsorption. Oilfield Chem. 1993, 10, 195–200. [Google Scholar]
- Zhang, J.; Zhang, M.; Zhang, S.; Bai, B.; Gao, B. Development and Field Pilot Test of a Novel Viscoelastic Anionic-Surfactant Fracturing Fluid. In Proceedings of the Society of Petroleum Engineers Western North American Regional Meeting—In Collaboration with the Joint Meetings of the Pacific Section AAPG and Cordlleran Section GSA, Anaheim, CA, USA, 27–29 May 2010. [Google Scholar]
- Lai, L.; Mei, P.; Wu, X.; Hou, C.; Zheng, Y.; Liu, Y. Micellization of anionic gemini surfactants and their interaction with polyacrylamide. Colloid Polym. Sci. 2014, 292, 2821–2830. [Google Scholar] [CrossRef]
- Pei, X.; Zhao, J.; Ye, Y.; You, Y.; Wei, X. Wormlike micelles and gels reinforced by hydrogen bonding in aqueous cationic gemini surfactant systems. Soft Matter 2011, 7, 2953–2960. [Google Scholar] [CrossRef]
- Zana, R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: A review. Adv. Colloid Interface Sci. 2002, 97, 205–253. [Google Scholar] [CrossRef]
- Li, G.; Zhao, L.; Wang, P.; Ning, X.; Qin, P.; Xue, M. Synthesis and Surface Activity of Cationic Gemini Surfactant, N,N′-bis(Octadecyl/tetradecyl dimethyl)-1,2-dichloro-1,4-benzene Dimethyl Ammonium Salt. Fine Chem. 2016, 33, 519–523. [Google Scholar]
- Tang, S.; Cui, Y.; Xiong, X.; Lei, X. Viscosity and Influencing Factors of Anionic Gemini Surfactant (GA-16) Solution. J. Oil Gas Technol. 2014, 36, 146–149. (In Chinese) [Google Scholar]
- Akbas, H.; Elemenli, A.; Boz, M. Aggregation and Thermodynamic Properties of Some Cationic Gemini Surfactants. J. Surfactants Deterg. 2012, 15, 33–40. [Google Scholar] [CrossRef]
- Zhu, H.; Niu, H.; Lou, P.; Ding, H.; Zhang, H. Synthesis and Performance of Gemini Surfactant—Containing Acidic Clean Fracturing Fluid. Adv. Fine Petrochem. 2011, 12, 5–8. [Google Scholar]
- Yang, J.; Guan, B.; Lu, Y. Viscoelastic Evaluation of Gemini Surfactant Gel for Hydraulic Fracturing. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Noordwijk, The Netherlands, 5–7 June 2013. [Google Scholar]
- Yu, H.; Shen, Y.; Yang, X.; Liu, G.; Zhang, L. Interfacial activity and rheological behavior of N,N′-bis (hexadecyldimethyl)-1,2-dibro-mide-ethanediyl ammonium salt. Acta Pet. Sin. 2014, 30, 542–547. [Google Scholar]
- Liu, Y.; Guo, L.; Bi, K.; Ren, W.; Liu, L.; Li, Y.; Han, L. Synthesis of Cationic Gemini Surfactant and Its Application in Water Blocking. Fine Spec. Chem. 2011, 19, 8–10. (In Chinese) [Google Scholar]
- Tang, S.; Liu, Z.; Liu, S.; Hu, X.; Wu, R. Research on Rheological Behavior of Anion Gemini Surfactant Solution. Adv. Mater. Res. 2011, 146–147, 536–541. [Google Scholar] [CrossRef]
- Pi, Y.; Zhang, L.; Liu, Z.; Gao, D.; Tang, S. Research on Rheological Properties of Sulfuric Acid Ester Salt Gemini Surfactant Solution. J. Oil Gas Technol. 2011, 33, 135–138. [Google Scholar]
- Du, X.; Li, L.; Lu, Y.; Yang, Z. Unusual viscosity behavior of a kind of anionic gemini surfactant. Colloid Surf. A 2007, 308, 147–149. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, C.; Tian, L.; Zou, T. Temperature-resistance clean fracturing fluid with carboxylate gemini surfactant: A case study of tight sandstone gas reservoirs in the Tarim Basin. Nat. Gas Ind. 2016, 36, 45–51. [Google Scholar]
- Mao, J.; Yang, X.; Chen, Y.; Zhang, Z.; Zhang, C.; Yang, B.; Zhao, J. Viscosity reduction mechanism in high temperature of a Gemini viscoelastic surfactant (VES) fracturing fluid and effect of counter-ion salt (KCl) on its heat resistance. J. Pet. Sci Eng. 2018, 164, 189–195. [Google Scholar] [CrossRef]
- Zana, R.; Talmon, Y. Dependence of aggregate morphology on structure of dimeric surfactants. Nature 1993, 362, 228–230. [Google Scholar] [CrossRef]
- Gao, D.; Yu, M. The Comparative Study of Influence Factors of Ionic Gemini Surfactant Solution’s Rheology. J. Chongqing Univ. Sci. Technol. (Nat. Sci. Ed.) 2012, 14, 101–104. [Google Scholar]
- Zhu, Q. Study on Synthesis, Properties and Applications in Enhanced Oil Recovery (EOR) of Novel Gemini Surfactant. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2009. [Google Scholar]
- Han, L.J.; Chen, H.; Luo, P.Y. Viscosity behavior of cationic gemini surfactants with long alkyl chains. Surf. Sci. 2004, 564, 141–148. [Google Scholar] [CrossRef]
- Chen, H.; Ye, Z.; Han, L.; Luo, P.; Zhang, L. Temperature-induced micelle transition of gemini surfactant in aqueous solution. Surf. Sci. 2007, 601, 2147–2151. [Google Scholar] [CrossRef]
- Yu, H. Preparation and Performance of Quaternary Ammonium Gemini Surfactant. Master’s Thesis, Shaanxi University of Science & Technology, Xi’an, China, 2010. [Google Scholar]
- Yang, J. Study on the Turbulent Flow and Heat Transfer Characteristics of Viscoelastic Fluid Based Nanofluid. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2013. [Google Scholar]
- Helgeson, M.E.; Hodgdon, T.K.; Kaler, E.W.; Wagner, N.J.; Vethamuthu, M.; Ananthapadmanabhan, K.P. Formation and Rheology of Viscoelastic “Double Networks” in Wormlike Micelle-Nanoparticle Mixtures. Langmuir 2010, 26, 8049–8060. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Clean Fracturing Fluid | Guanidine Gum Fracturing Fluid |
|---|---|---|
| Molecular weight category | Molecular weight less than 500 | Molecular weight greater than 50,000 |
| With or without crosslinker | No | Yes |
| With or without a breaker | No | Yes |
| Sand carrying mechanism | Viscoelastic body carrying sand, viscosity greater than 30 mPa·s at 100 s−1 | Fracturing fluid carrying sand, viscosity greater than 100 mPa·s at 100 s−1 |
| Filtration loss | Low filtration | High filtration |
| Diversion capacity | More than 93% | More than 70% |
| Craftsmanship | Easy to make on site | Difficult to make on site |
| Spacer | Sulfonate Gemini Surfactant (DSm-s-m) |
|---|---|
| s = 2 | DS12-2-12, DS14-2-14, DS16-2-16, DS18-2-18 |
| s = 3 | DS18-3-18 |
| s = 4 | DS18-4-18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Zheng, Y.; Yang, W.; Wang, J.; Fan, Y.; Lu, J. Experimental Study of Sulfonate Gemini Surfactants as Thickeners for Clean Fracturing Fluids. Energies 2018, 11, 3182. https://doi.org/10.3390/en11113182
Tang S, Zheng Y, Yang W, Wang J, Fan Y, Lu J. Experimental Study of Sulfonate Gemini Surfactants as Thickeners for Clean Fracturing Fluids. Energies. 2018; 11(11):3182. https://doi.org/10.3390/en11113182
Chicago/Turabian StyleTang, Shanfa, Yahui Zheng, Weipeng Yang, Jiaxin Wang, Yingkai Fan, and Jun Lu. 2018. "Experimental Study of Sulfonate Gemini Surfactants as Thickeners for Clean Fracturing Fluids" Energies 11, no. 11: 3182. https://doi.org/10.3390/en11113182





