Pt-Ni and Pt-M-Ni (M = Ru, Sn) Anode Catalysts for Low-Temperature Acidic Direct Alcohol Fuel Cells: A Review
Abstract
:1. Introduction
2. Structural Characteristics of Pt-Ni Catalysts
3. Methanol Oxidation on Pt-Ni Catalysts
4. Ethanol Oxidation on Pt-Ni Catalysts
5. Stability of Pt-Ni Catalysts in Fuel Cell Environment
5.1. General Overview
5.2. Acid Immersion
5.3. Repetitive Potential Cycling (RPC)
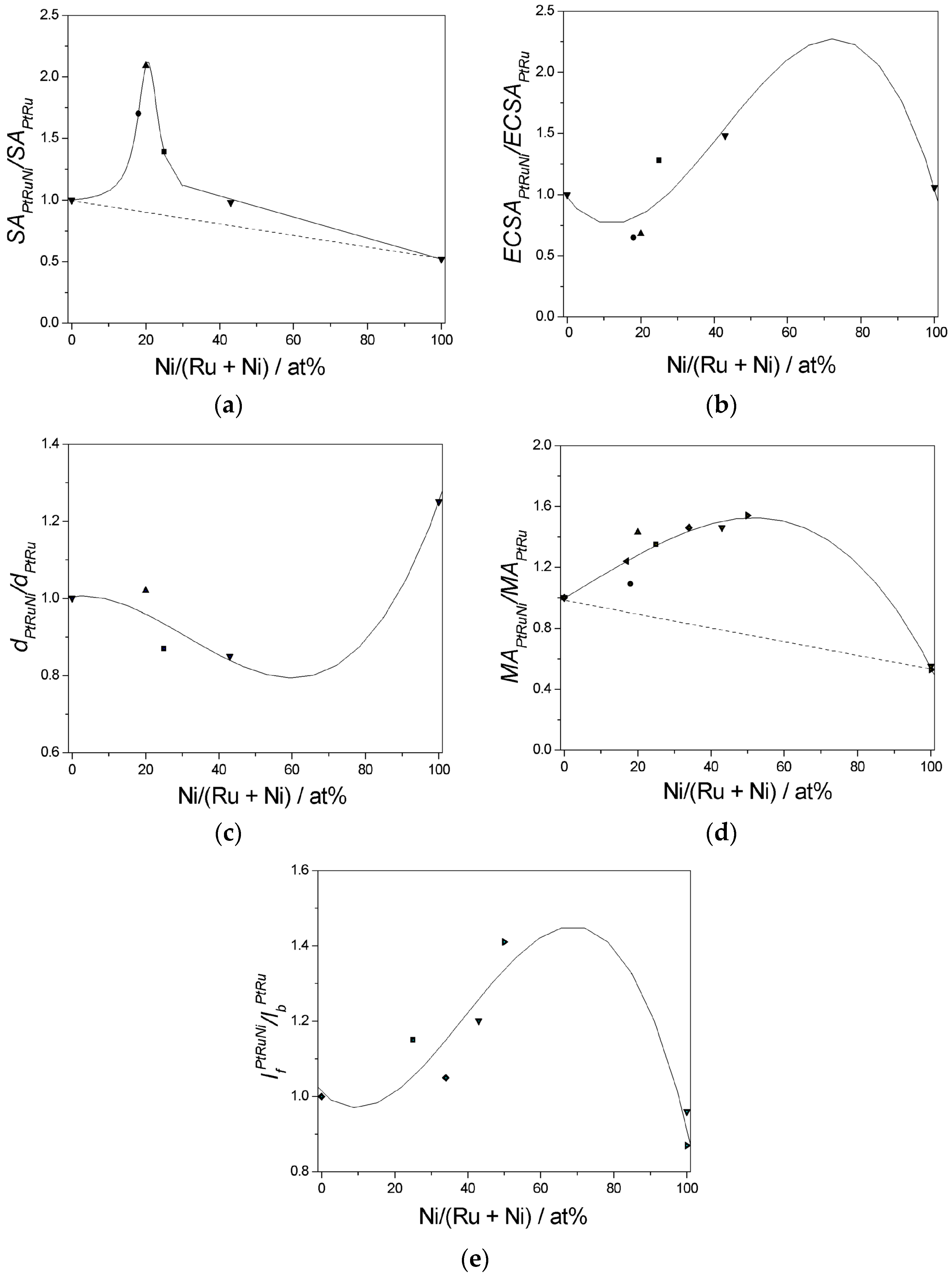
6. Methanol Oxidation on Ternary Pt-Ru-Ni Catalysts
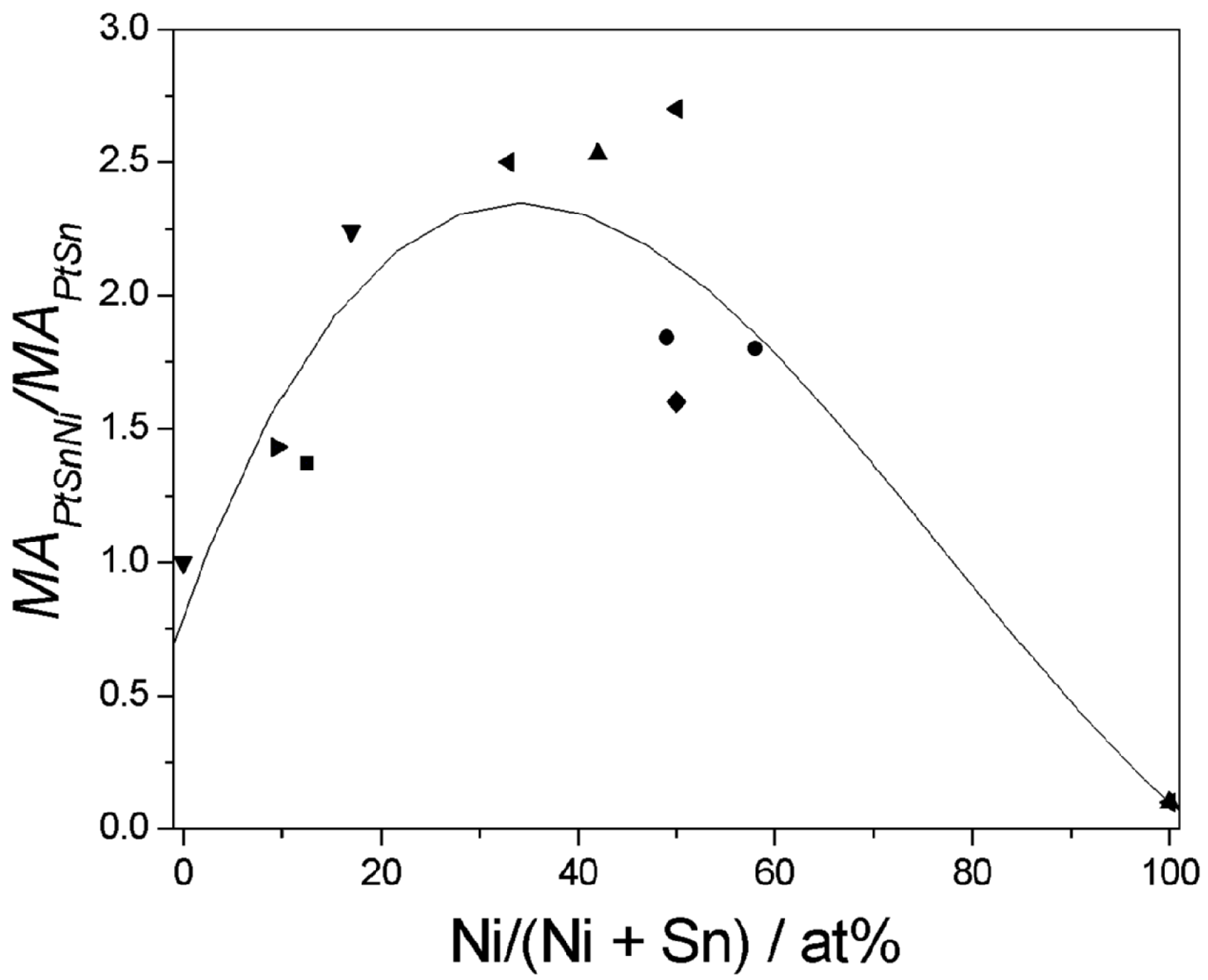
7. Ethanol Oxidation on Ternary Pt-Sn-Ni and Pt-Ru-Ni Catalysts
8. Conclusions
Conflicts of Interest
References
- Aricò, A.S.; Srinivasan, S.; Antonucci, V. DMFCs: From fundamental aspects to technology development. Fuel Cells 2001, 1, 133–161. [Google Scholar] [CrossRef]
- Manthiram, A.; Zhao, X.; Li, W. Developments in membranes, catalysts and membrane electrode assemblies for direct methanol fuel cells (DMFCs). Funct. Mater. Sustain. Energy Appl. 2012, 11, 312–369. [Google Scholar]
- Antolini, E. Catalysts for direct ethanol fuel cells. J. Power Sources 2007, 170, 1–12. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Markovic, N.M.; Gasteiger, H.A.; Ross, P.N.; Jiang, X.; Villegas, I.; Weaver, M.J. Electro-oxidation mechanisms of methanol and formic acid on Pt-Ru alloy surfaces. Electrochim. Acta 1995, 40, 91–98. [Google Scholar] [CrossRef]
- Gojkovic, S.L.; Vidakovic, T.R.; Durovic, D.R. Kinetic study of methanol oxidation on carbon-supported PtRu electrocatalyst. Electrochim. Acta 2003, 48, 3607–3614. [Google Scholar] [CrossRef]
- Iwasita, T. Electrocatalysis of methanol oxidation. Electrochim. Acta 2002, 47, 3663–3674. [Google Scholar] [CrossRef]
- Christensen, P.A.; Hamnett, A.; Troughton, G.L. The role of morphology in the methanol electro-oxidation reaction. J. Electroanal. Chem. 1993, 362, 207–218. [Google Scholar] [CrossRef]
- Antolini, E. Platinum alloys as anode catalysts for direct methanol fuel cells. In Electrocatalysis of Direct Methanol Fuel Cells: From Fundamentals to Applications; Liu, H., Zhang, J., Eds.; Wiley-VCH: Weinheim, Germany, 2009; pp. 227–255. [Google Scholar]
- Antolini, E. The problem of Ru dissolution from Pt–Ru catalysts during fuel cell operation: Analysis and solutions. J. Solid State Electrochem. 2011, 15, 455–472. [Google Scholar] [CrossRef]
- Zignani, S.C.; Baglio, V.; Linares, J.J.; Monforte, G.; Gonzalez, E.R.; Aricò, A.S. Endurance study of a solid polymer electrolyte direct ethanol fuel cell based on a Pt–Sn anode catalyst. Int. J. Hydrogen Energy 2013, 38, 11576–11582. [Google Scholar] [CrossRef]
- Park, K.-W.; Choi, J.-H.; Kwon, B.-K.; Lee, S.-A.; Sung, Y.-E.; Ha, H.-Y.; Hong, S.-A.; Kim, H.; Wieckowski, A. Chemical and electronic effects of Ni in Pt/Ni and Pt/Ru/Ni alloy nanoparticles in methanol electrooxidation. J. Phys. Chem. B 2002, 106, 1869–1877. [Google Scholar] [CrossRef]
- Lu, X.-G.; Sundman, B. Thermodynamic assessments of the Ni-Pt and Al-Ni-Pt systems. Calphad 2009, 33, 450–456. [Google Scholar] [CrossRef]
- Staunton, J.; Weinberger, P.; Gyorffy, B.L. On the electronic structure of paramagnetic NicPt1-c alloys: A relativistic calculation. J. Phys. F Met. Phys. 1983, 13, 779–786. [Google Scholar] [CrossRef]
- De Temmerman, L.; Creemers, C.; Van Hove, H.; Neyens, A.; Bertolini, J.C.; Massardier, J. Experimental determination of equilibrium surface segregation in Pt-Ni single crystal alloys. Surf. Sci. 1986, 178, 888–896. [Google Scholar] [CrossRef]
- Wang, G.; Van Hove, M.A.; Ross, P.N.; Baskes, M.I. Quantitative prediction of surface segregation in bimetallic Pt–M alloy nanoparticles (M = Ni, Re, Mo). Prog. Surf. Sci. 2005, 79, 28–45. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Schmidt, T.J.; Ross, P.N.; Markovic, N.M. Surface composition effect in electrocatalysis: Kinetics of oxygen reduction on well-defined Pt3Ni and Pt3Co alloy surfaces. J. Phys. Chem. B 2002, 106, 11970–11979. [Google Scholar] [CrossRef]
- Park, K.W.; Choi, J.H.; Sung, Y.E. Structural, chemical, and electronic properties of Pt/Ni thin film electrodes for methanol electrooxidation. J. Phys. Chem. B 2003, 107, 5851–5856. [Google Scholar] [CrossRef]
- Page, T.; Johnson, R.; Hormes, J.; Noding, S.; Rambabu, B. A study of methanol electro-oxidation reactions in carbon membrane electrodes and structural properties of Pt alloy electro-catalysts by EXAFS. J. Electroanal. Chem. 2000, 485, 34–41. [Google Scholar] [CrossRef]
- Shen, Y.; Xiao, K.; Xi, J.; Qiu, X. Comparison study of few-layered graphene supported platinum and platinum alloys for methanol and ethanol electro-oxidation. J. Power Sources 2015, 278, 235–244. [Google Scholar] [CrossRef]
- Zhou, Y.-Y.; Liu, C.-H.; Liu, J.; Cai, X.-L.; Lu, Y.; Zhang, H.; Sun, X.-H.; Wang, S.-D. Self-decoration of PtNi alloy nanoparticles on multiwalled carbon nanotubes for highly efficient methanol electro-oxidation. Nano-Micro Lett. 2016, 8, 371–380. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Rangarajan, J. Electro-catalytic activity of nano-sized Pt-Ni bimetallic alloy particles supported on carbon for methanol electro-oxidation. Int. J. Sci. Eng. Res. 2014, 5, 1544–1551. [Google Scholar]
- Amin, R.S.; Abdel Hameed, R.M.; El-Khatib, K.M.; Youssef, M.E.; Elzatahry, A.A. Pt–NiO/C anode electrocatalysts for direct methanol fuel cells. Electrochim. Acta 2012, 59, 499–508. [Google Scholar] [CrossRef]
- Nagashree, K.L.; Raviraj, N.H.; Ahmed, M.F. Carbon paste electrodes modified by Pt and Pt–Ni microparticles dispersed in polyindole film for electrocatalytic oxidation of methanol. Electrochim. Acta 2010, 55, 2629–2635. [Google Scholar] [CrossRef]
- Zhao, Y.; Yifeng, E.; Fan, L.; Qiu, Y.; Yang, S. A new route for the electrodeposition of platinum–nickel alloy nanoparticles on multi-walled carbon nanotubes. Electrochim. Acta 2007, 52, 5873–5878. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, L.; Ren, J.; Hong, B. Electrodeposition of Pt–Ru and Pt–Ru–Ni nanoclusters on multi-walled carbon nanotubes for direct methanol fuel cell. Int. J. Hydrogen Energy 2014, 39, 4544–4557. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Lin, J.-Y. Fabrication of bimetallic Pt–M (M = Fe, Co, and Ni) nanoparticle/carbon nanotube electrocatalysts for direct methanol fuel cells. J. Power Sources 2009, 188, 347–352. [Google Scholar] [CrossRef]
- Antolini, A.; Salgado, J.R.C.; Gonzalez, E.R. Oxygen reduction on a Pt70Ni30/C electrocatalyst prepared by the borohydride method in H2SO4/CH3OH solutions. J. Power Sources 2006, 155, 161–166. [Google Scholar] [CrossRef]
- Yang, H.; Coutanceau, C.; Léger, J.-M.; Alonso-Vante, N.; Lamy, C. Methanol tolerant oxygen reduction on carbon-supported Pt–Ni alloy nanoparticles. J. Electroanal. Chem. 2005, 576, 305–313. [Google Scholar] [CrossRef]
- Drillet, J.-F.; Ee, A.; Friedemann, J.; Kötz, R.; Schnyder, B.; Schmidt, V.M. Oxygen reduction at Pt and Pt70Ni30 in H2SO4/CH3OH solution. Electrochim. Acta 2002, 47, 1983–1988. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.R.C.; Gonzalez, E.R. The methanol oxidation reaction on platinum alloys with the first row transition metals: The case of Pt-Co and -Ni alloy electrocatalysts for DMFCs: A short review. Appl. Catal. B Environ. 2006, 63, 137–149. [Google Scholar] [CrossRef]
- Manoharan, R.; Goodenough, J.B. Methanol oxidation in acid on ordered NiTi. J. Mater. Chem. 1992, 2, 875–887. [Google Scholar] [CrossRef]
- Habibi, B.; Dadashpour, E. Carbon-ceramic supported bimetallic PteNi nanoparticles as an electrocatalyst for electrooxidation of methanol and ethanol in acidic media. Int. J. Hydrogen Energy 2013, 38, 5425–5434. [Google Scholar] [CrossRef]
- Guo, W.; Tian, W.Q.; Lian, X.; Liu, F.; Zhou, M.; Xiao, P.; Zhang, Y. A comparison of the dominant pathways for the methanol dehydrogenation to CO on Pt7 and Pt7−xNix (x = 1, 2, 3) bimetallic clusters: A DFT study. Comput. Theor. Chem. 2014, 1032, 73–83. [Google Scholar] [CrossRef]
- Xu, C.; Hou, J.; Pang, X.; Li, X.; Zhu, M.; Tang, B. Nanoporous PtCo and PtNi alloy ribbons for methanol electrooxidation. Int. J. Hydrogen Energy 2012, 37, 10489–10498. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Norskov, J.K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Zhang, D.-F.; Guo, L. Phase-segregated Pt-Ni chain-like nanohybrids with high electrocatalytic activity towards methanol oxidation reaction. Nanoscale 2014, 6, 4635–4641. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, Y.; Tao, H.; Jian, G.; Wang, X.; Fan, Y.; Zhu, J.; Hu, Z. Highly dispersed Pt-Ni nanoparticles on nitrogen-doped carbon nanotubes for application in direct methanol fuel cells. J. Nanosci. Nanotechnol. 2010, 10, 3895–3900. [Google Scholar] [CrossRef] [PubMed]
- Nassr, A.B.A.A.; Sinev, I.; Grünert, W.; Bron, M. PtNi supported on oxygen functionalized carbon nanotubes: In depth structural characterization and activity for methanol electrooxidation. Appl. Catal. B Environ. 2013, 142–143, 849–860. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, X.; Zheng, Y.; Shen, J.; Yuan, J.; Wang, A.; Niu, L.; Huang, S. Size-controllable synthesis of ultrafine PtNi nanoparticles uniformly deposited on reduced graphene oxide as advanced anode catalysts for methanol oxidation. Int. J. Hydrogen Energy 2016, 41, 9303–9311. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, J.; Dong, L.; Pan, H.; Yuan, Y.; Wang, Y. Graphitized nanodiamond supporting PtNi alloy as stable anodic andcathodic electrocatalysts for direct methanol fuel cell. Electrochim. Acta 2013, 113, 583–590. [Google Scholar] [CrossRef]
- Luo, B.; Xu, S.; Yan, X.; Xue, Q. PtNi alloy nanoparticles supported on polyelectrolyte functionalized graphene as effective electrocatalysts for methanol oxidation. J. Electrochem. Soc. 2013, 160, F262–F268. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, P.; Yin, Y.; Zhang, H.; Cai, C. Effects of structure, composition, and carbon support properties on the electrocatalytic activity of Pt-Ni-graphene nanocatalysts for the methanol oxidation. Appl. Catal. B Environ. 2012, 111–112, 208–217. [Google Scholar] [CrossRef]
- Zhou, X.-W.; Zhang, R.-H.; Zhou, Z.-Y.; Sun, S.-G. Preparation of PtNi hollow nanospheres for the electrocatalytic oxidation of methanol. J. Power Sources 2011, 196, 5844–5848. [Google Scholar] [CrossRef]
- Yang, H.; Vogel, W.; Lamy, C.; Alonso-Vante, N. Structure and electrocatalytic activity of carbon-supported Pt-Ni alloy nanoparticles toward the oxygen reduction reaction. J. Phys. Chem. B 2004, 108, 11024–11034. [Google Scholar] [CrossRef]
- Comignani, V.; Sieben, J.M.; Brigante, M.E.; Duarte, M.M. Carbon supported Pt-NiO nanoparticles for ethanol electro-oxidation in acid media. J. Power Sources 2015, 278, 119–127. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.; Lu, J.; Nan, C.; Li, Y. Synthesis of Pt-Ni-graphene via in situ reduction and its enhanced catalyst activity for the methanol oxidation. Chem. Commun. 2013, 49, 7486–7488. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E.; Salgado, J.R.C.; dos Santos, A.M.; Gonzalez, E.R. Carbon-supported Pt-Ni alloys prepared by the borohydride method as electrocatalysts for DMFCs. Electrochem Solid-State Lett. 2005, 8, A226–A230. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Lei, Z.; Zhang, Z.; Wang, R. The performance of carbon-supported platinum-decorated nickel electrocatalyst for ethanol oxidation. Chin. J. Catal. 2011, 32, 1519–1524. [Google Scholar] [CrossRef]
- Beyhan, S.; Léger, J.-M.; Kadırgan, F. Pronounced synergetic effect of the nano-sized PtSnNi/C catalyst for ethanol oxidation in direct ethanol fuel cell. Appl. Catal. B Environ. 2013, 130–131, 305–313. [Google Scholar] [CrossRef]
- Cantillo, N.M.; Solla-Gullon, J.; Herrero, E.; Sanchez, C. Ethanol electrooxidation on PtSnNi/C nanoparticles prepared in water-in-oil microemulsion. ECS Trans. 2011, 41, 1307–1316. [Google Scholar]
- Ponmani, K.; Kiruthika, S.; Muthukumaran, B. Investigation of nanometals (Ni and Sn) in platinum-based ternary electrocatalysts for ethanol electro-oxidation in membraneless fuel cells. J. Electrochem. Sci. Technol. 2015, 6, 95–105. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K. Electrochemical dissolution of surface alloys in acids: Thermodynamic trends from first-principles calculations. Electrochim. Acta 2007, 52, 5829–5836. [Google Scholar] [CrossRef]
- Bonakdarpour, A.; Wenzel, J.; Stevens, D.A.; Sheng, S.; Monchesky, T.L.; Lobel, R.; Atanasoski, R.T.; Schmoeckel, A.K.; Vernstrom, G.D.; Debe, M.K.; et al. Studies of transition metal dissolution from combinatorially sputtered, nanostructured Pt1−xMx (M = Fe, Ni; 0 < x < 1) electrocatalysts for PEM fuel cells. J. Electrochem. Soc. 2005, 152, A61–A72. [Google Scholar]
- Hoshi, Y.; Yoshida, T.; Nishikata, A.; Tsuru, T. Dissolution of Pt–M (M: Cu, Co, Ni, Fe) binary alloys in sulfuric acid solution. Electrochim. Acta 2011, 56, 5302–5309. [Google Scholar] [CrossRef]
- Colón-Mercado, H.R.; Kim, H.; Popov, B.N. Durability study of Pt3Ni1 catalysts as cathode in PEM fuel cells. Electrochem. Commun. 2004, 6, 795–799. [Google Scholar] [CrossRef]
- Colón-Mercado, H.R.; Popov, B.N. Stability of platinum based alloy cathode catalysts in PEM fuel cells. J. Power Sources 2006, 155, 253–263. [Google Scholar] [CrossRef]
- Zignani, S.C.; Antolini, E.; Gonzalez, E.R. Stability of Pt-Ni/C (1:1) and Pt/C electrocatalysts as cathode materials for polymer electrolyte fuel cells: Effect of ageing tests. J. Power Sources 2009, 191, 344–350. [Google Scholar] [CrossRef]
- Borup, R.L.; Davey, J.R.; Garzon, F.H.; Wood, D.L.; Inbody, M.A. PEM fuel cell electrocatalyst durability measurements. J. Power Sources 2006, 163, 76–81. [Google Scholar] [CrossRef]
- Watanabe, M.; Tsurumi, K.; Mizukami, T.; Nakamura, T.; Stonehart, P. Activity and stability of ordered and disordered Co-Pt alloys for phosphoric acid fuel cells. J. Electrochem. Soc. 1994, 141, 2659–2668. [Google Scholar] [CrossRef]
- Zou, L.; Li, J.; Yuan, T.; Zhou, Y.; Li, X.; Yang, H. Structural transformation of carbon-supported PtCr nanoparticles from a disordered to an ordered phase as a durable oxygen reduction electrocatalyst. Nanoscale 2014, 6, 10686–10692. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. Platinum-based ternary catalysts for low temperature fuel cells: Part I. Preparation methods and structural characteristics. Appl. Catal. B Environ. 2007, 74, 324–336. [Google Scholar] [CrossRef]
- Huang, T.; Liu, J.; Li, R.; Cai, W.; Yu, A. A novel route for preparation of PtRuMe (Me = Fe, Co, Ni) and their catalytic performance for methanol electrooxidation. Electrochem. Commun. 2009, 11, 643–646. [Google Scholar] [CrossRef]
- Jeon, M.K.; Lee, K.R.; Daimon, H.; Nakahara, A.; Woo, S.I. Pt45Ru45M10/C (M = Fe, Co, and Ni) catalysts for methanol electro-oxidation. Catal. Today 2008, 132, 123–126. [Google Scholar] [CrossRef]
- Kang, D.K.; Noh, C.S.; Kim, N.H.; Cho, S.-H.; Sohn, J.M.; Kim, T.J.; Park, Y.-K. Effect of transition metals (Ni, Sn and Mo) in Pt5Ru4M alloy ternary electrocatalyst on methanol electro-oxidation. J. Ind. Eng. Chem. 2010, 16, 385–389. [Google Scholar] [CrossRef]
- Papaderakis, A.; Pliatsikas, N.; Prochaska, C.; Papazisi, K.M.; Balomenou, S.P.; Tsiplakides, D.; Patsalas, P.; Sotiropoulos, S. Ternary Pt-Ru-Ni catalytic layers for methanol electrooxidation prepared by electrodeposition and galvanic replacement. Front. Chem. 2014, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, J.; Huang, Q.; Li, X.; Zou, Z.; Yang, H. Methanol oxidation on carbon-supported Pt-Ru-Ni ternary nanoparticle electrocatalysts. J. Power Sources 2008, 175, 159–165. [Google Scholar] [CrossRef]
- Wang, Z.B.; Yin, G.P.; Shi, P.F.; Sun, Y.C. Novel Pt-Ru-Ni/C catalysts for methanol electro-oxidation in acid medium. Electrochem. Solid-State Lett. 2006, 9, A13–A15. [Google Scholar] [CrossRef]
- Martínez-Huerta, M.V.; Rojas, S.; Gomez de la Fuente, J.L.; Terreros, P.; Peña, M.A.; Fierro, J.L.G. Effect of Ni addition over PtRu/C based electrocatalysts for fuel cell applications. Appl. Catal. B Environ. 2006, 69, 75–84. [Google Scholar] [CrossRef]
- Ye, F.; Chen, S.; Dong, X.; Lin, W. Carbon nanotubes supported Pt-Ru-Ni as methanol electro-oxidation catalyst for direct methanol fuel cells. J. Nat. Gas Chem. 2007, 16, 162–166. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Liu, Z.; Hu, W.; Zhao, C.; Shi, D. An advanced electrocatalyst with exceptional eletrocatalytic activity via ultrafine Pt-based trimetallic nanoparticles on pristine grapheme. Carbon 2015, 87, 116–127. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, P.K.; Jiang, S.P. Enhanced activity and stability of core–shell structured PtRuNix electrocatalysts for direct methanol fuel cells. Int. J. Hydrogen Energy 2016, 41, 1935–1943. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Effect of synthesis method and structural characteristics of Pt-Sn fuel cell catalysts on the electro-oxidation of CH3OH and CH3CH2OH in acid medium. Catal. Today 2011, 160, 28–38. [Google Scholar] [CrossRef]
- Beyhan, S.; Coutanceau, C.; Léger, J.-M.; Napporn, T.W.; Kadırgan, F. Promising anode candidates for direct ethanol fuel cell: Carbon supported PtSn-based trimetallic catalysts prepared by Bönnemann method. Int. J. Hydrogen Energy 2013, 38, 6830–6841. [Google Scholar] [CrossRef]
- Parreira, L.S.; da Silva, J.C.M.; D’Villa-Silva, M.; Simões, F.C.; Garcia, S.; Gaubeur, I.; Cordeiro, M.A.L.; Leite, E.R.; dos Santos, M.C. PtSnNi/C nanoparticle electrocatalysts for the ethanol oxidation reaction: Ni stability study. Electrochim. Acta 2013, 96, 243–252. [Google Scholar] [CrossRef]
- Spinacé, E.V.; Linardi, M.; Oliveira Neto, A. Co-catalytic effect of nickel in the electro-oxidation of ethanol on binary Pt-Sn electrocatalysts. Electrochem. Commun. 2005, 7, 365–369. [Google Scholar] [CrossRef]
- Ribadeneira, E.; Hoyos, B.A. Evaluation of Pt-Ru-Ni and Pt-Sn-Ni catalysts as anodes in direct ethanol fuel cells. J. Power Sources 2008, 180, 238–242. [Google Scholar] [CrossRef]
- Bonesi, A.; Garaventa, G.; Triaca, W.E.; Castro Luna, A.M. Synthesis and characterization of new electrocatalysts for ethanol oxidation. Int. J. Hydrogen Energy 2008, 33, 3499–3501. [Google Scholar] [CrossRef]
- Bonesi, A.R.; Moreno, M.S.; Triaca, W.E.; Castro Luna, A.M. Modified catalytic materials for ethanol oxidation. Int. J. Hydrogen Energy 2010, 35, 5999–6004. [Google Scholar] [CrossRef]
- Almeida, T.S.; Van Wassen, A.R.; VanDover, R.B.; de Andrade, A.R.; Abruña, H.D. Combinatorial PtSnM (M = Fe, Ni, Ru and Pd) nanoparticle catalyst library toward ethanol electrooxidation. J. Power Sources 2015, 284, 623–630. [Google Scholar] [CrossRef]
- Beyhan, S.; Léger, J.-M.; Kadırgan, F. Understanding the influence of Ni, Co, Rh and Pd addition to PtSn/C catalyst for the oxidation of ethanol by in situ Fourier transform infrared spectroscopy. Appl. Catal. B Environ. 2014, 144, 66–74. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Yin, G.-P.; Zhang, J.; Sun, Y.-C.; Shi, P.-F. Investigation of ethanol electrooxidation on a Pt–Ru–Ni/C catalyst for a direct ethanol fuel cell. J. Power Sources 2006, 160, 37–43. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Zuo, P.-J.; Wang, G.-J.; Du, C.-Y.; Yin, G.-P. Effect of Ni on PtRu/C catalyst performance for ethanol electrooxidation in acidic medium. J. Phys. Chem. C 2008, 112, 6582–6587. [Google Scholar] [CrossRef]
- Antolini, E.; Perez, J. The renaissance of unsupported nanostructured catalysts for low-temperature fuel cells: From the size to the shape of metal nanostructures. J. Mater. Sci. 2011, 46, 4435–4457. [Google Scholar] [CrossRef]
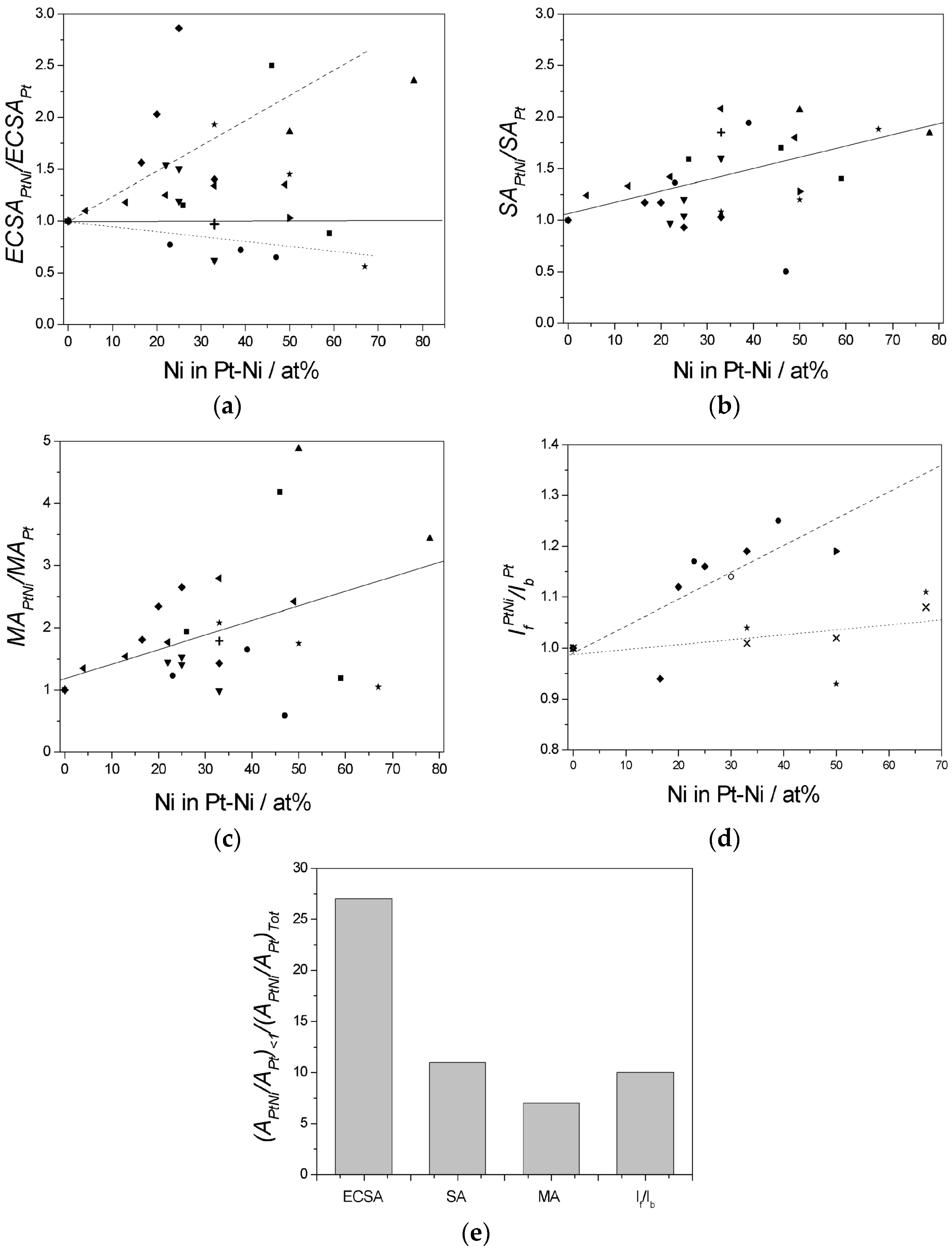
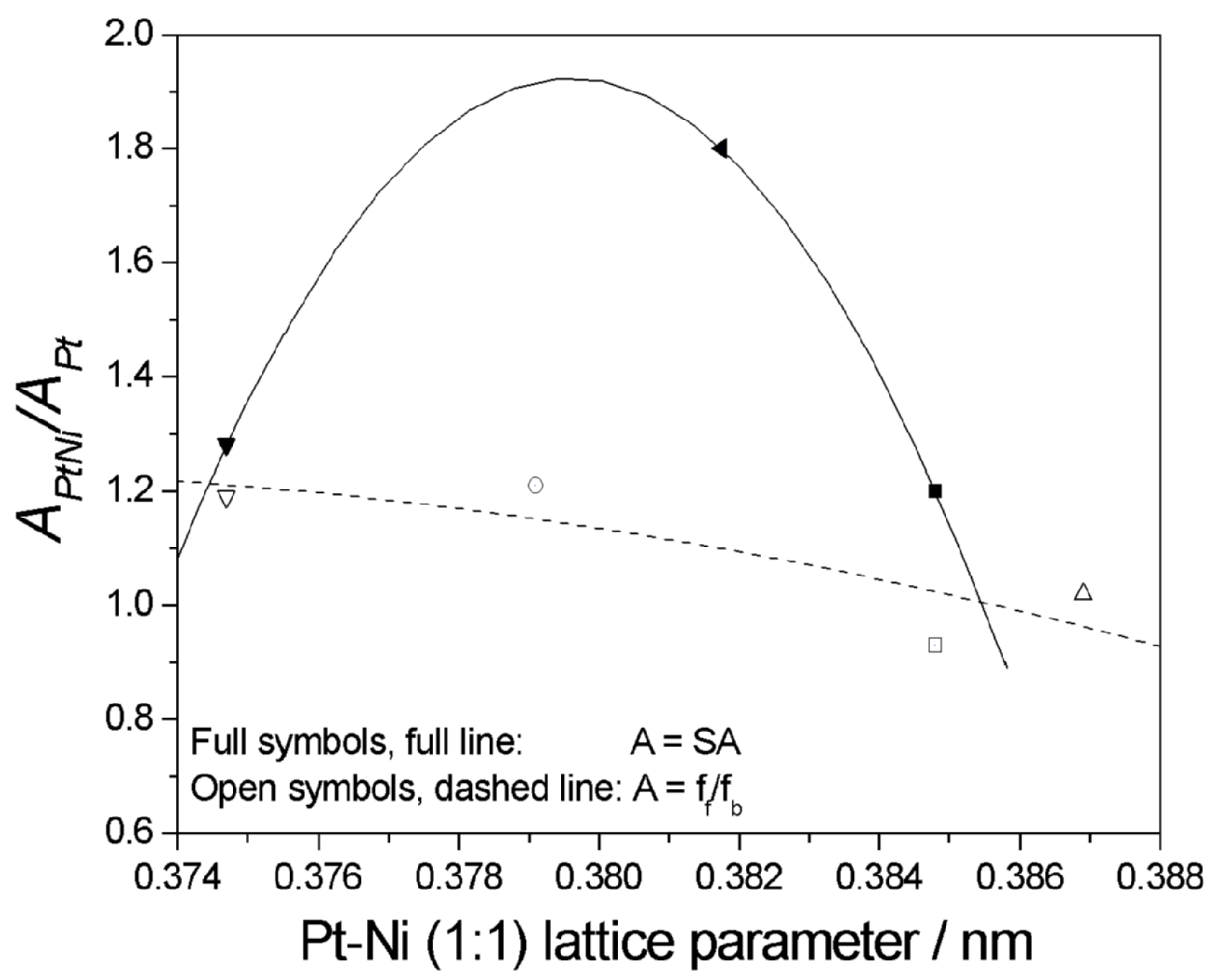
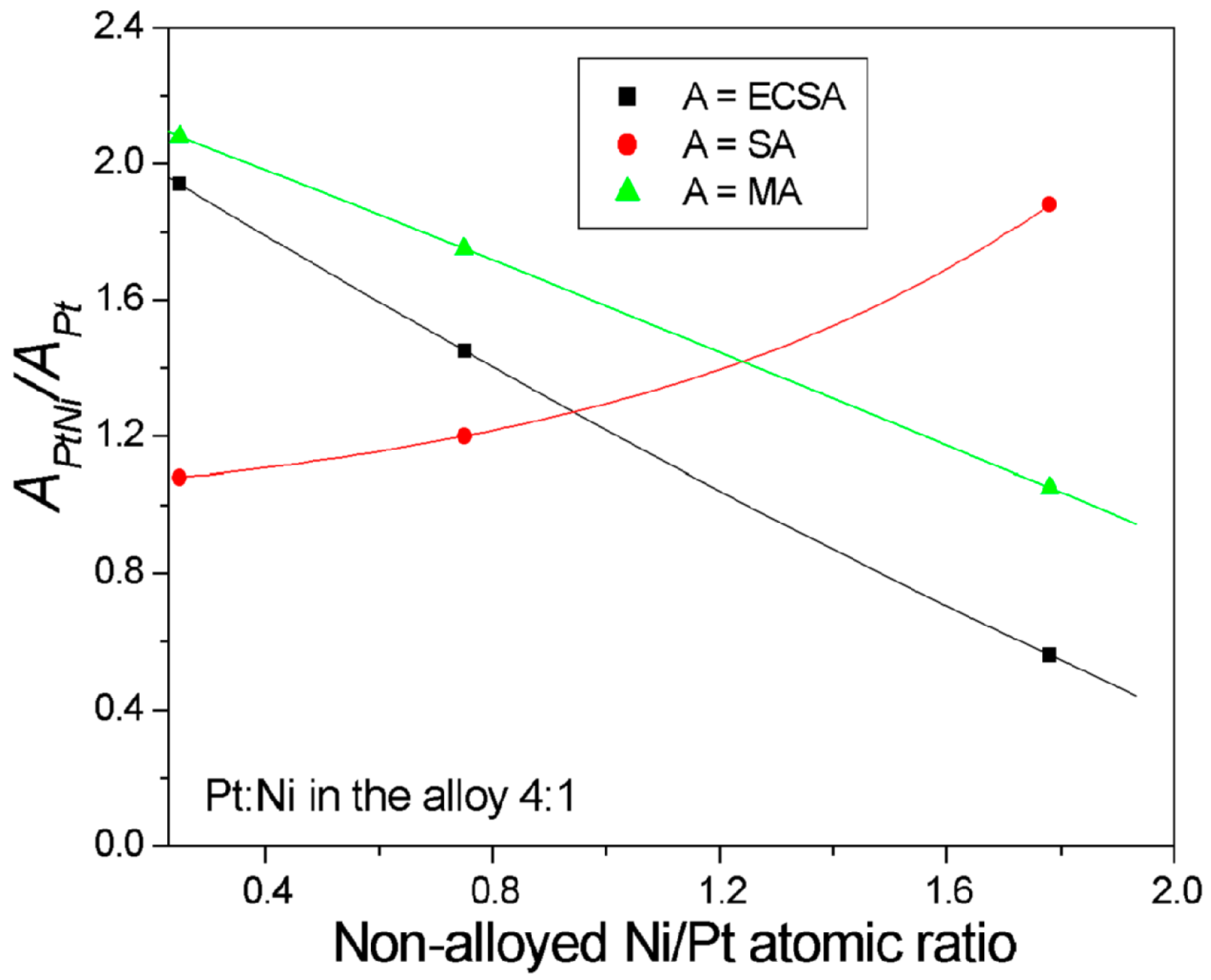
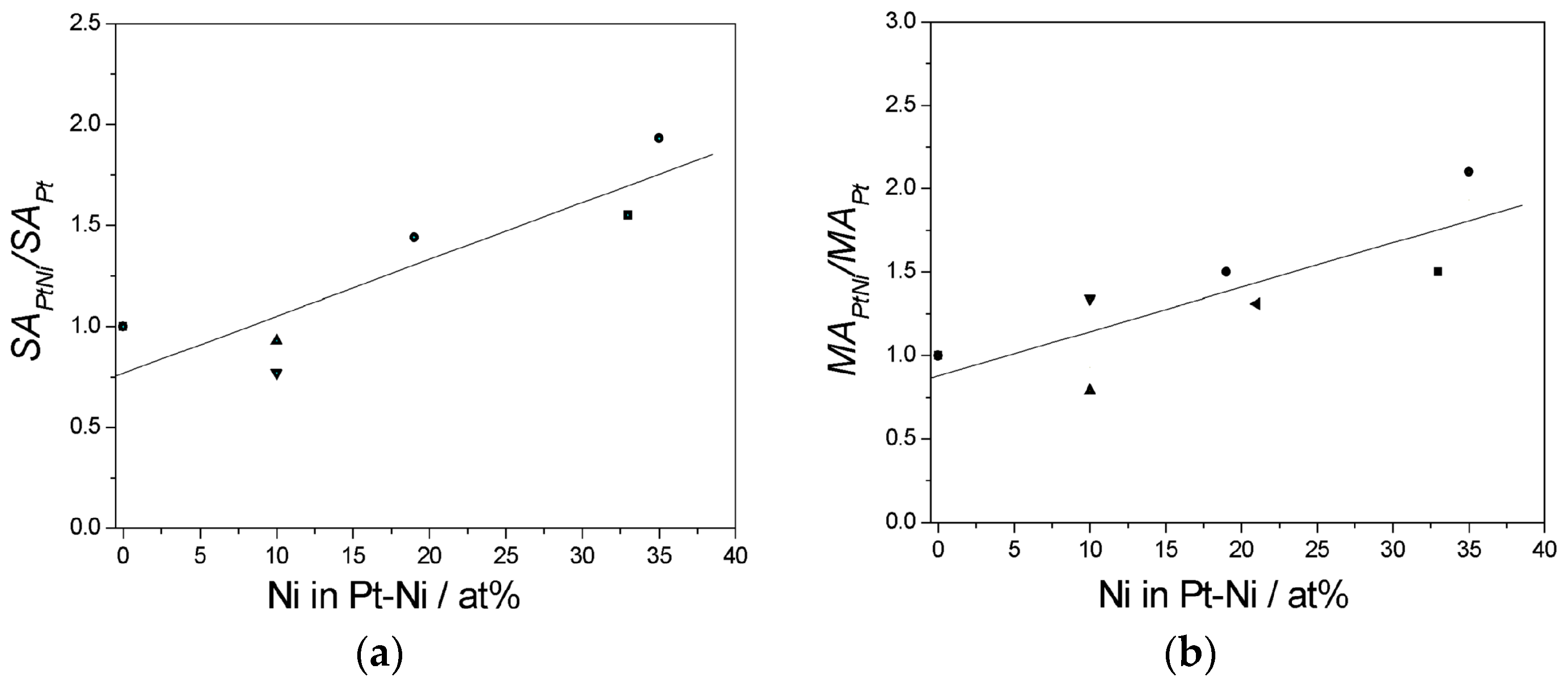

| Pt:Ni Atomic Ratio | Characteristics/Synthesis Method | ΔVoPtNi−VoPt (mV) | ΔVoPtNi−VoPtRu (mV) | Reference |
|---|---|---|---|---|
| 3:1 | NaBH4 reduction method at room T. Unsupported nanoparticles | −30 | +55 | [18] |
| 1:1 | −60 | +25 | ||
| 1:1 | Carbon supported Pt-Ni | −15 (25 °C) | +70 (25 °C) | [19] |
| −10 (50 °C) | +55 (50 °C) | |||
| −40 (75 °C) | +30 (75 °C) | |||
| 2:1 | Few-layered graphene (FLG) supported Pt-Ni. Polyol assisted reduction method | −100 | 0 | [20] |
| 1:1 | Sself-decorated PtNi alloy nanoparticles, on MWCNT in [BMIm][BF4] ionic liquid | −60 | [21] | |
| 0.3:1 | −90 | |||
| 24:1 | Adsorption of nickel laurate on Pt/C followed by reduction at 900 °C in H2/N2 atmosphere | −13 | [22] | |
| 6.7:1 | −25 | |||
| 3:1 | −25 | |||
| 2:1 | −50 | |||
| 1:1 | −37 | |||
| 0.9:1 | NiO loaded on Vulcan XC-72R carbon black. Pt was further chemically reduced by sodium borohydride on that NiO/C using the impregnation and the microwave methods. Partially alloyed Pt-Ni | −35 | [23] | |
| 1.2:1 | −92 | |||
| 2:1 | Pt-Ni modified polyindole (Pin) films | −100 | [24] | |
| 1.1 | −100 | |||
| 1:1 | Electrodeposition of Pt-Ni on MWCNT | −50 | [25] | |
| 3:1 | Synthesis of PtNi/CNT by chemical oxidation of CNTs, two-step refluxing and subsequent hydrogen reduction | +20 | [27] | |
| 2.3:1 | Carbon supported Pt-Ni | +65 | [28] | |
| 2:1 | Carbon supported Pt-Ni | +100 | [29] | |
| 1.5:1 | +100 | |||
| 1:1 | +100 | |||
| 2.3:1 | Pt and Pt0.7Ni0.3 disc electrodes with a diameter of 12 and 2 mm in thickness | 0 | [30] |
© 2017 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antolini, E. Pt-Ni and Pt-M-Ni (M = Ru, Sn) Anode Catalysts for Low-Temperature Acidic Direct Alcohol Fuel Cells: A Review. Energies 2017, 10, 42. https://doi.org/10.3390/en10010042
Antolini E. Pt-Ni and Pt-M-Ni (M = Ru, Sn) Anode Catalysts for Low-Temperature Acidic Direct Alcohol Fuel Cells: A Review. Energies. 2017; 10(1):42. https://doi.org/10.3390/en10010042
Chicago/Turabian StyleAntolini, Ermete. 2017. "Pt-Ni and Pt-M-Ni (M = Ru, Sn) Anode Catalysts for Low-Temperature Acidic Direct Alcohol Fuel Cells: A Review" Energies 10, no. 1: 42. https://doi.org/10.3390/en10010042






