Esterification of Oleic Acid for Biodiesel Production Catalyzed by SnCl2: A Kinetic Investigation
Abstract
:1. Introduction
1.1. Homogeneous catalysts based on Brønsted acids for FFAs esterification reactions.
1.2. Environmental benefits of use of Lewis acid catalysts for Biodiesel production from FFA esterification reactions.
2. Experimental Section
2.1. Chemicals
2.2. General reaction procedure
2.2.1. The SnCl2 catalyst versus H2SO4
2.2.2. Esterification of soybean oil with high amounts of oleic acid
2.3. Kinetic measurements
2.4. Identification of the reaction products
3. Results and Discussion
3.1. General aspects

3.2. The SnCl2 catalyst versus H2SO4: A comparative study
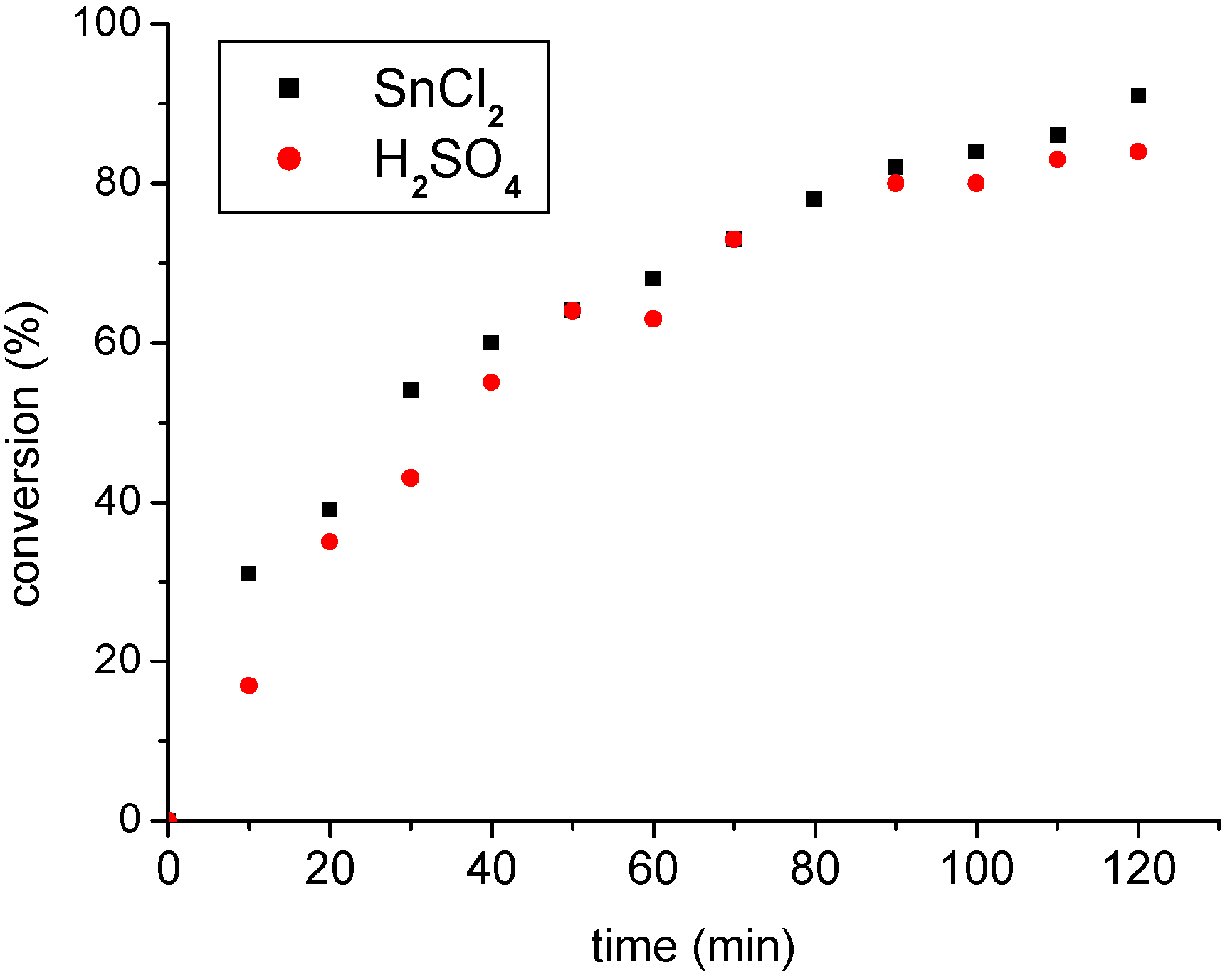
| Time (h) | SnCl2 | H2SO4 | ||
|---|---|---|---|---|
| Conversion (%) | Selectivity (%) | Conversion (%) | Selectivity (%) | |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 62 | 92 | 65 | 90 |
| 2 | 93 | 94 | 92 | 89 |
| 4 | 90 | 95 | 94 | 87 |
| 6 | 90 | 93 | 95 | 89 |
| 8 | 91 | 93 | 94 | 88 |
3.3. Ethanolysis of oleic acid catalyzed by SnCl2·2H2O: kinetic studies
3.3.1. The effect of oleic acid concentration

| Catalyst | Linear Equation | T1/2 (min) | R2 |
|---|---|---|---|
| H2SO4 | ln[oleic acid] = -0.00359 *t-2.4414 | 206 | 0.9850 |
| SnCl2 | ln[oleic acid] = -0.00278 *t-2.3155 | 249 | 0.9700 |
3.3.2. The effect of SnCl2·2H2O concentration
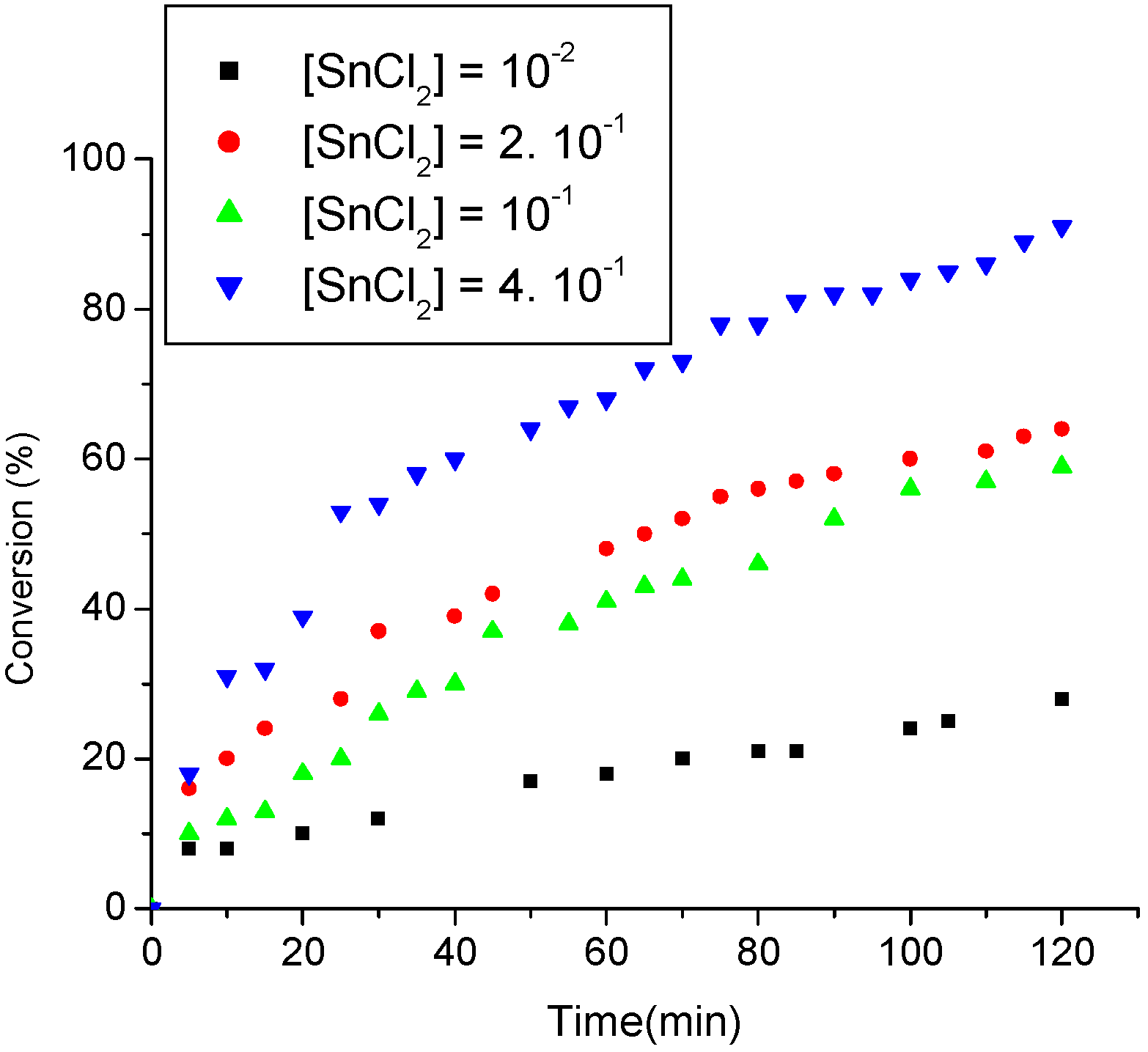
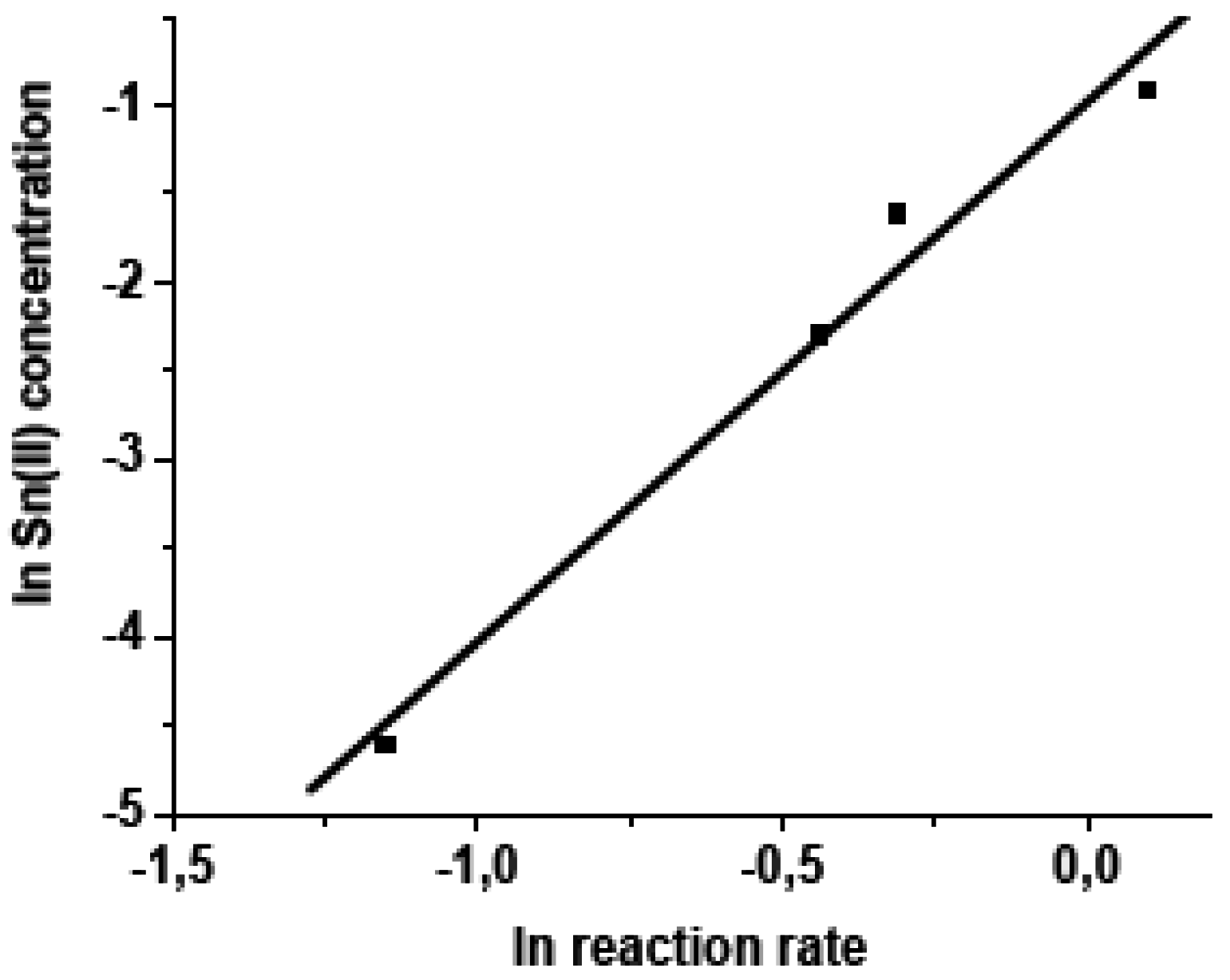
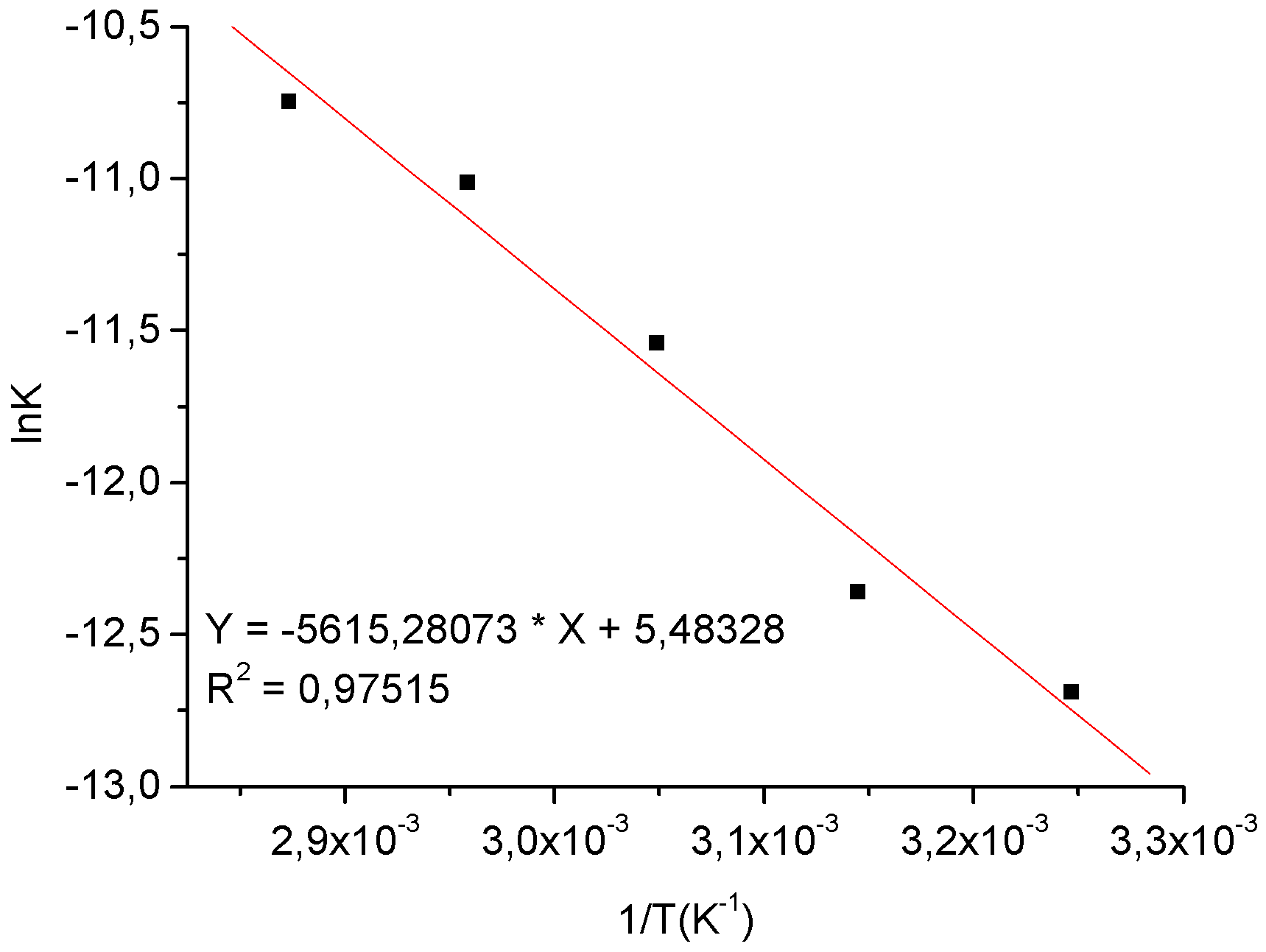
3.3.3. The effect of temperature on the ethanolysis of oleic acid SnCl2-catalyzed
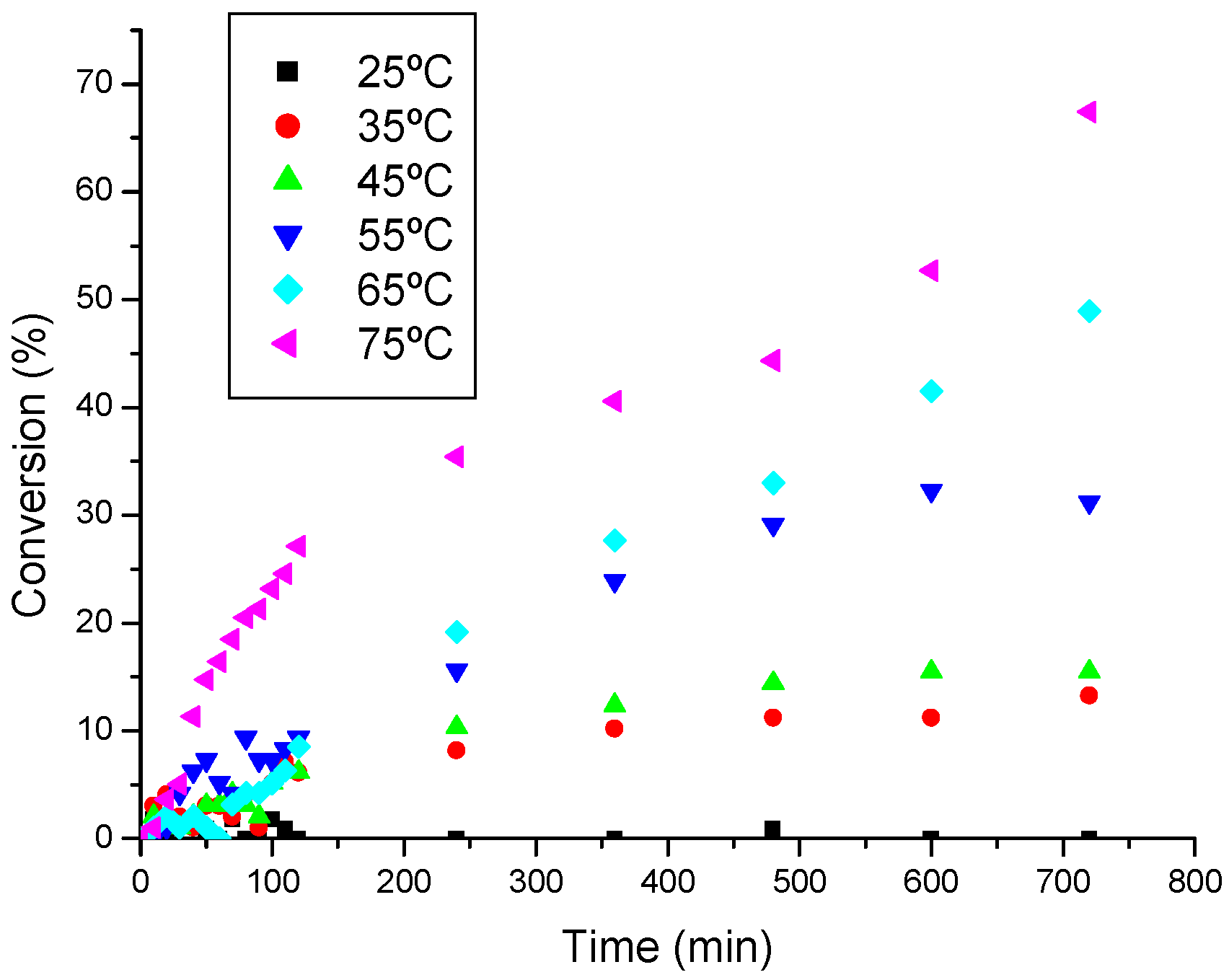
| Temperature (ºC) | ln([oleic acid]/[oleic acid]o) = -kt | Linearity Coeffcient R2 |
|---|---|---|
| 35 | –3.0892 x10-6.t | -0.9090 |
| 45 | –4.2939 x 10-6.t | -0.9547 |
| 55 | –9.7396 x 10-6.t | -0.9762 |
| 65 | –1.6522 x 10-5.t | -0.9938 |
| 75 | –2.1535 x 10-5.t | -0.9701 |
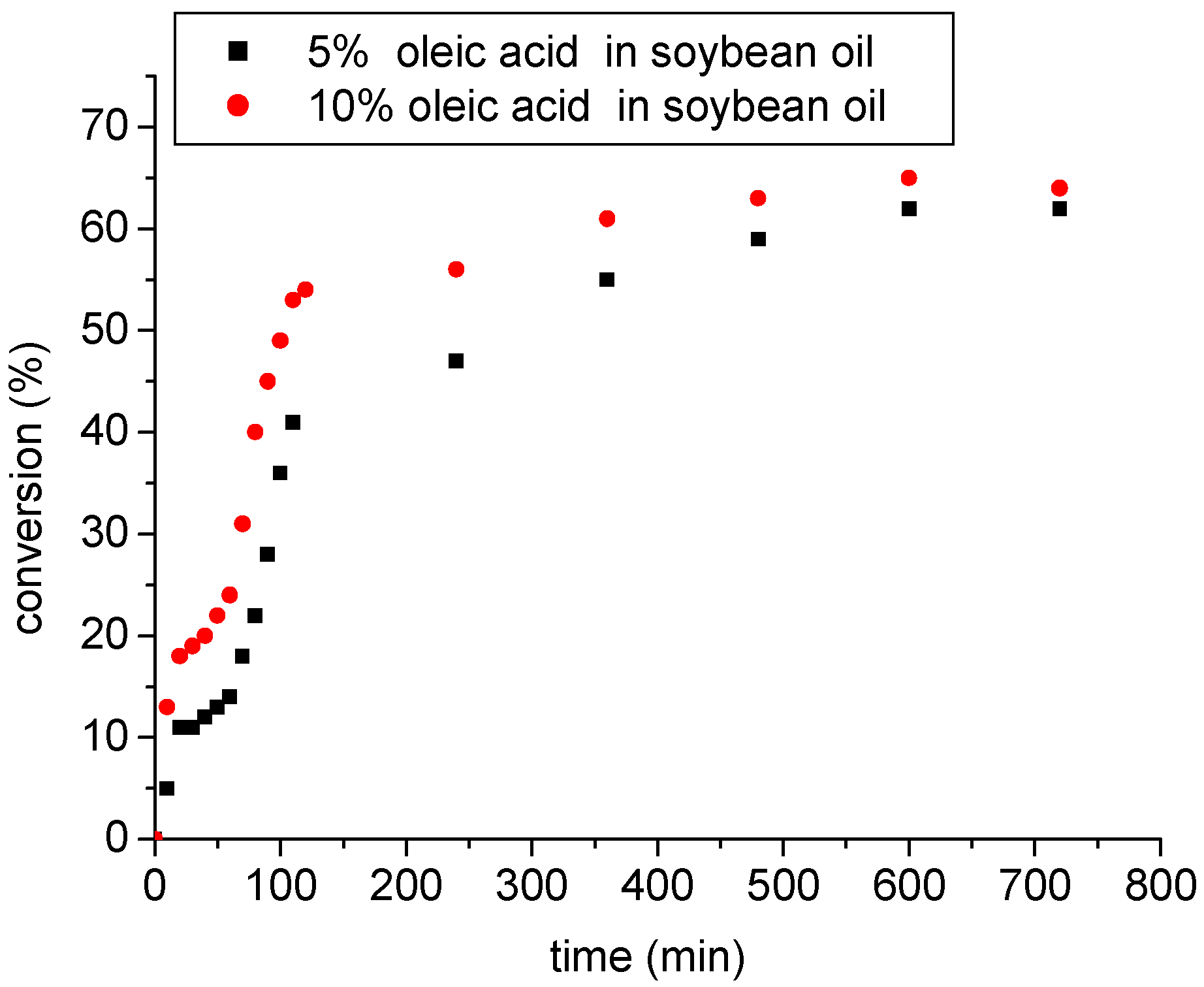
3.3.4. Esterification of soybean oil with high amounts of FFA catalyzed by SnCl2.
4. Conclusions
Acknowledgements
References
- Maa, F.; Hannab, M.A. Biodiesel Production-A Review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar]
- Zheng, S.; Kates, M.; Dubé, M.A.; Mclean, D.D. Acid-catalyzed production of biodiesel from waste frying oil. Biomass Bioener. 2006, 30, 267–272. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste Cooking Oil as an Alternate Feedstok for Biodiesel Prouction. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef]
- Kima, H.J.; Kang, B.S.; Kim, M.J.; Park, Y.M.; Kimb, D.K.; Lee, J.S.; Lee, K.Y. Transesterification of vegetable oil to biodiesel using heterogeneous base catalyst. Catal. Today 2004, 93–95, 315–320. [Google Scholar] [CrossRef]
- Haas, M.J.; Karen, M.S.; Marmer, W.N.; Foglia, T.A. In situ alkaline transesterification: an effective method for the production of fatty acid esters from vegetable oils. J. Am. Oil Chem. Soc. 2004, 81, 83–89. [Google Scholar] [CrossRef]
- Haas, M.J. Improving the economics of biodiesel production through the use of low value lipids as feedstocks: vegetable oil soapstock. Fuel Process Technol. 2005, 86, 1087–1096. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of Biodiesel via Acid Catalysis. Ind. Eng. Chem. Res. 2005, 44, 25353–25363. [Google Scholar] [CrossRef]
- Zullaikah, S.; Lai, C.C.; Vali, S.R.; Ju, Y.H. A Two-step acid-catalyzed process for the production of biodiesel from rice bran oil. Bioresour. Technol. 2005, 96, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcıa, H. Lewis acids: From conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem. Ver. 2003, 103, 4307–4365. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Heterogeneous esterification of oil with high amount of free fatty acids. Fuel 2007, 86, 906–910. [Google Scholar] [CrossRef]
- Timofeeva, M.N. Acid Catalysis by Heteropoly Acids. Appl. Catal. 2003, 256, 19–35. [Google Scholar]
- Reis, S.C.M.; Lachter, E.R.R.; Nascimento, R.S.V.; Rodrigues Jr., J.A.; Reid, M.G. Transesterification of Brazilian vegetable oils with methanol over ion-exchange resins. J. Am. Oil Chem. Soc. 2005, 82, 661–665. [Google Scholar] [CrossRef]
- Ferreira, D.A.C.; Meneghetti, M.R.; Meneghetti, S.M.P.; Wolf, C.R. Methanolysis of soybean oil in the presence of tin(IV) complexes. Appl. Catal. 2007, 317, 58–61. [Google Scholar] [CrossRef]
- Alizadeh, M.H.; Kermanil, T.; Tayebee, R. A method for the acetylation of alcohols catalyzed by heteropolyoxometallates. Monatsh Chem. 2007, 138, 165–170. [Google Scholar] [CrossRef]
- Narasimharao, K.; Brown, D.R.; Lee, A.F.; Newman, A.D.; Siril, P.F.; Tavener, S.J.; Wilson, K. Structure–activity relations in Cs-doped heteropolyacid catalysts for biodiesel production. J. Catal. 2007, 248, 226–234. [Google Scholar] [CrossRef]
- Einloft, S.; Magalhães, T.O.; Donato, A.; Dullius, J.; Ligabue, R. Biodiesel from Rice Bran Oil: Transesterification by Tin Compounds. Ener. Fuels 2008, 22, 671–675. [Google Scholar] [CrossRef]
- Saito, S.; Yamamoto, H. Designer Lewis acid catalysts—bulky aluminium reagents for selective organic synthesis. Chem. Commun. 1997, 1585–1592. [Google Scholar] [CrossRef]
- Macedo, C.C.S.; Abreu, F.R.; Tavares, A.P.; Alves, M.B.; Zara, L.F.; Rubin, J.C.; Suares, P.A.Z. New Heteregoneous Metal-Oxides Based Catalyst for Biodiesel oil Transesterification. J. Braz. Chem. Soc. 2006, 17, 1291–1296. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Dimiccoli, M.; Cammarota, F.; Nastasi, M.; Santacesaria, E. Synthesis of biodiesel via homogeneous Lewis acid catalyst. J. Mol. Catal. A 2005, 239, 111–117. [Google Scholar] [CrossRef]
- Jousseaume, B.; Laporte, C.; Rascle, M.-C.; Toupance, T. Investigations in the catalytic species of the distannoxane-catalyzed transcarbamoylation. Chem. Commun. 2003, 1428–1429. [Google Scholar] [CrossRef]
- Baumhof, P.; Mazitschek, R.; Giannis, A. A Mild Effective Method for the Transesterification of Carboxylic Acid Esters. Angew. Chem. Int. Ed. 2001, 40, 3672–3678. [Google Scholar] [CrossRef]
- Mascaretti, O.A.; Furlán, L.E. Esterifications, Transesterifications, and Deesterifications Mediated by Organotin Oxydes, Hydroxydes and Alkoxydes. Aldrichim. Acta 1997, 30, 55–68. [Google Scholar]
- Otera, J.; Dan-oh, N.; Nozaki, H. Novel Template Effects of Distannoxane Catalysts in Highly Efficient Transesterification and Esterification. J. Org. Chem. 1991, 56, 5307–5311. [Google Scholar] [CrossRef]
- Hoydonckx, H. E.; De Vos, D.E.; Chavan, S.A.; Jacobs, P.A. Esterification and transesterification of renewables chemicals. Topics Catal. 2004, 27, 83–88. [Google Scholar] [CrossRef]
- Abreu, F.R.; Lima, D.G.; Hamú, E.H.; Einloft, S.; Rubim, J.; Suarez, P.A.Z. New Metals Catalysts for Soybean Oil Transesterification. J. Am. Oil Chem. Soc. 2003, 80, 601–604. [Google Scholar] [CrossRef]
- Abreu, F.R.; Lima, D.G.; Hamú, E.H.; Wolf, C.R.; Suarez, P.A.Z. New multi-phase catalytic systems based on tin compounds active for vegetable oil transesterification reaction. J. Mol. Catal. A 2005, 227, 263–268. [Google Scholar] [CrossRef]
- Silva, M.J.; Augusti, R.; Cardoso, A.L. Investigations on the esterification of fatty acids catalyzed by heteropolyacids H3PW12O40. J. Am. Oil Chem. Soc. 2008, 85, 555–560. [Google Scholar]
- Berrios, M.; Siles, J.; Martın, M.A. A kinetic Study of the Esterification of Free fatty Acids (FFA) in Sunflower Oil. Fuel 2007, 86, 2383–2388. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Sawant, S.B. Kinetics of the Catalytic Esterification of Castor Oil with Lauric Acid Using n-Butyl Benzene as a Water Entrainer. J. Am. Oil. Chem. Soc. 2003, 80, 1033–1038. [Google Scholar] [CrossRef]
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cardoso, A.L.; Neves, S.C.G.; Da Silva, M.J. Esterification of Oleic Acid for Biodiesel Production Catalyzed by SnCl2: A Kinetic Investigation. Energies 2008, 1, 79-92. https://doi.org/10.3390/en1020079
Cardoso AL, Neves SCG, Da Silva MJ. Esterification of Oleic Acid for Biodiesel Production Catalyzed by SnCl2: A Kinetic Investigation. Energies. 2008; 1(2):79-92. https://doi.org/10.3390/en1020079
Chicago/Turabian StyleCardoso, Abiney L., Soraia Cristina Gonzaga Neves, and Marcio J. Da Silva. 2008. "Esterification of Oleic Acid for Biodiesel Production Catalyzed by SnCl2: A Kinetic Investigation" Energies 1, no. 2: 79-92. https://doi.org/10.3390/en1020079




