Apparent Temperature and Cause-Specific Mortality in Copenhagen, Denmark: A Case-Crossover Analysis
Abstract
:1. Introduction
2. Methods
2.1. Mortality and Hospital Admission Data
2.2. Meteorological and Air Pollution Data
2.3. Influenza Data
2.4. Effect Modifier Data
2.5. Ethics
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Information
ijerph-08-03712-s001.pdfAcknowledgments
- Conflict of InterestNo competing interests are declared.
References
- Parry, ML; Canziani, OF; Palutikof, JP; van der Linden, PJ; Hanson, CE (Eds.) Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 2007; Cambridge University Press: Cambridge, UK and New York, NY, USA, 2007. Available online: http://www.ipcc-wg2.gov/publications/AR4/index.html accessed on 17 August 2011.
- Kovats, RS; Hajat, S. Heat stress and public health: A critical review. Ann Rev Public Health 2008, 29, 41–55. [Google Scholar]
- Basu, R; Samet, JM. Relation between elevated ambient temperature and mortality: A review of the epidemiologic evidence. Epidemiol Rev 2002, 24, 190–202. [Google Scholar]
- Basu, R. High ambient temperature and mortality: A review of epidemiologic studies from 2001 to 2008. Environ Health 2009, 8. [Google Scholar] [CrossRef]
- Braga, A; Zanobetti, A; Schwartz, J. The effect of weather on respiratory and cardiovascular deaths in 12 US cities. Environ Health Perspect 2002, 110, 859–863. [Google Scholar]
- Medina-Ramon, M; Zanobetti, A; Cavanagh, DP; Schwartz, J. Extreme temperatures and mortality: Assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ Health Perspect 2006, 114, 1331–1336. [Google Scholar]
- Barnett, AG. Temperature and cardiovascular deaths in the US elderly: Changes over time. Epidemiology 2007, 18, 369–372. [Google Scholar]
- Nafstad, P; Skrondal, A; Bjertness, E. Mortality and temperature in Oslo, Norway, 1990–1995. Euro J Epidemiol 2001, 17, 621–627. [Google Scholar]
- Näyhä, S. Heat mortality in Finland in the 2000s. Int J Circum Health 2007, 66, 418–424. [Google Scholar]
- Rocklöv, J; Forsberg, B. The effect of temperature on mortality in Stockholm 1998–2003: A study of lag structures and heatwave effects. Scand J Public Health 2008, 36, 516–523. [Google Scholar]
- Reichert, TA; Simonsen, L; Sharma, A; Pardo, SA; Fedson, DS; Miller, MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol 2004, 160, 492–502. [Google Scholar]
- Eurowinter Group. Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet 1997, 349, 1341–1346.
- Analitis, A; Katsouyanni, K; Biggeri, A; Baccini, M; Forsberg, B; Bisanti, L; Kirchmayer, U; Ballester, F; Cadum, E; Goodman, PG; et al. Effects of cold weather on mortality: Results from 15 European cities within the PHEWE project. Am J Epidemiol 2008, 168, 1397–1408. [Google Scholar]
- O’Neill, MS; Zanobetti, A; Schwartz, J. Modifiers of the temperature and mortality association in seven US cities. Am J Epidemiol 2003, 157, 1074–1082. [Google Scholar]
- Stafoggia, M; Forastiere, F; Agostini, D; Biggeri, A; Bisanti, L; Cadum, E; Caranci, N; de’Donato, F; De Lisio, S; De Maria, M; et al. Vulnerability to heat-related mortality: A multicity, population-based, case-crossover analysis. Epidemiology 2006, 17, 315–323. [Google Scholar]
- Ellermann, T; Nordstr⊘m, C; Brandt, J; Christensen, J; Ketzel, M; Jensen, SS. The Danish Air Quality Monitoring Programme. In Annual Summary for 2010, Technical Report No 836; National Environmental Research Institute, Aarhus University: Aarhus, Denmark, 2011. [Google Scholar]
- Barnett, AG; Tong, S; Clements, ACA. What measure of temperature is the best predictor of mortality? Environ Res 2010, 110, 604–611. [Google Scholar] [Green Version]
- Michelozzi, P; Accetta, G; De Sario, M; D’Ippoliti, D; Marino, C; Baccini, M; Biggeri, A; Anderson, HR; Katsouyanni, K; Ballester, F; et al. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med 2009, 179, 383–389. [Google Scholar]
- Danish Health Review for Regions and Communes. Sundhedsprofil for Region og Kommuner. 2008; Forskningscenter for Forebyggelse og Sundhed: Glostrup, Denmark, 2008. Available online: http://www.regionh.dk/NR/rdonlyres/8DC6D62D-DBC3-4219-8B1E-6113226066E9/0/Sundhedsprofil2008_for_RegionH.pdf accessed on 17 August 2011. in Danish.
- Maclure, M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am J Epidemiol 1991, 133, 144–153. [Google Scholar]
- Bateson, TF; Schwartz, J. Control for seasonal variation and time trend in case-crossover studies of acute effects of environmental exposures. Epidemiology 1991, 10, 539–544. [Google Scholar]
- Lee, JT; Kim, H; Schwartz, J. Bidirectional case-crossover studies of air pollution: Bias from skewed and incomplete waves. Environ Health Perspect 2002, 108, 1107–1111. [Google Scholar]
- Bateson, TF; Schwartz, J. Selection bias and confounding in case-crossover analyses of environmental time-series data. Epidemiology 2001, 12, 654–661. [Google Scholar]
- Levy, D; Lumley, T; Sheppard, L; Kaufman, J; Checkoway, H. Referent selection in case crossover analyses of acute health effects of air pollution. Epidemiology 2001, 12, 186–192. [Google Scholar]
- Andersen, ZJ; Wahlin, P; Raaschou-Nielsen, O; Scheike, T; Loft, S. Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J Expo Sci Environ Epidemiol 2007, 17, 625–663. [Google Scholar]
- Anderson, HR; Atkinson, RW; Peacock, JL; Marston, L; Konstantinou, K. Meta-Analysis of Time Series Studies and Panel Studies of Particulate Matter (PM) and Ozone (O3). 2004; World Health Organization: Copenhagen, Denmark, 2004. Available online: http://www.euro.who.int/__data/assets/pdf_file/0004/74731/e82792.pdf accessed on 17 August 2011.
- European Commission Environment DG. Directive on Ambient Air Quality and Cleaner Air for Europe (Directive 2008/50/EC); European Commission Environment DG: Brussels, Belgium, 2008. Available online: http://ec.europa.eu/environment/air/quality/legislation/existing_leg.htm accessed on 17 August 2011.
- Mercer, JB. Cold—An underrated risk factor for health. Environ Res 2003, 92, 8–13. [Google Scholar]
- Baccini, M; Biggeri, A; Accetta, G; Kosatsky, T; Katsouyanni, K; Analitis, A; Anderson, HR; Bisanti, L; D’Ippoliti, D; Danova, J; et al. Heat effects on mortality in 15 European cities. Epidemiology 2008, 19, 711–719. [Google Scholar]
- Baccini, M; Tom, K; Biggeri, A. Impact of heat on mortality in 15 European cities: Attributable deaths under different weather scenarios. J Epidemiol Community Health 2011, 65, 64–70. [Google Scholar]
- O’Neill, MS; Ebi, KL. Temperature extremes and health: Impacts of climate variability and change in the United States. J Occup Environ Med 2009, 51, 13–25. [Google Scholar]
- McArthur, K; Dawson, J; Walters, M. What is it with weather and stroke? Expert Rev Neurotherap 2010, 10, 243–249. [Google Scholar]
- Mathers, CD; Fat, DM; Inoue, M; Rao, C; Lopez, AD. Counting the dead and what they died from: An assessment of the global status of cause of death data. Bull World Health Organ 2005, 83, 171–177. [Google Scholar]
- Helweg-Larsen, K. The Danish register of causes of death. Scand J Public Health 2011, 39(Suppl 7), 26–29. [Google Scholar]
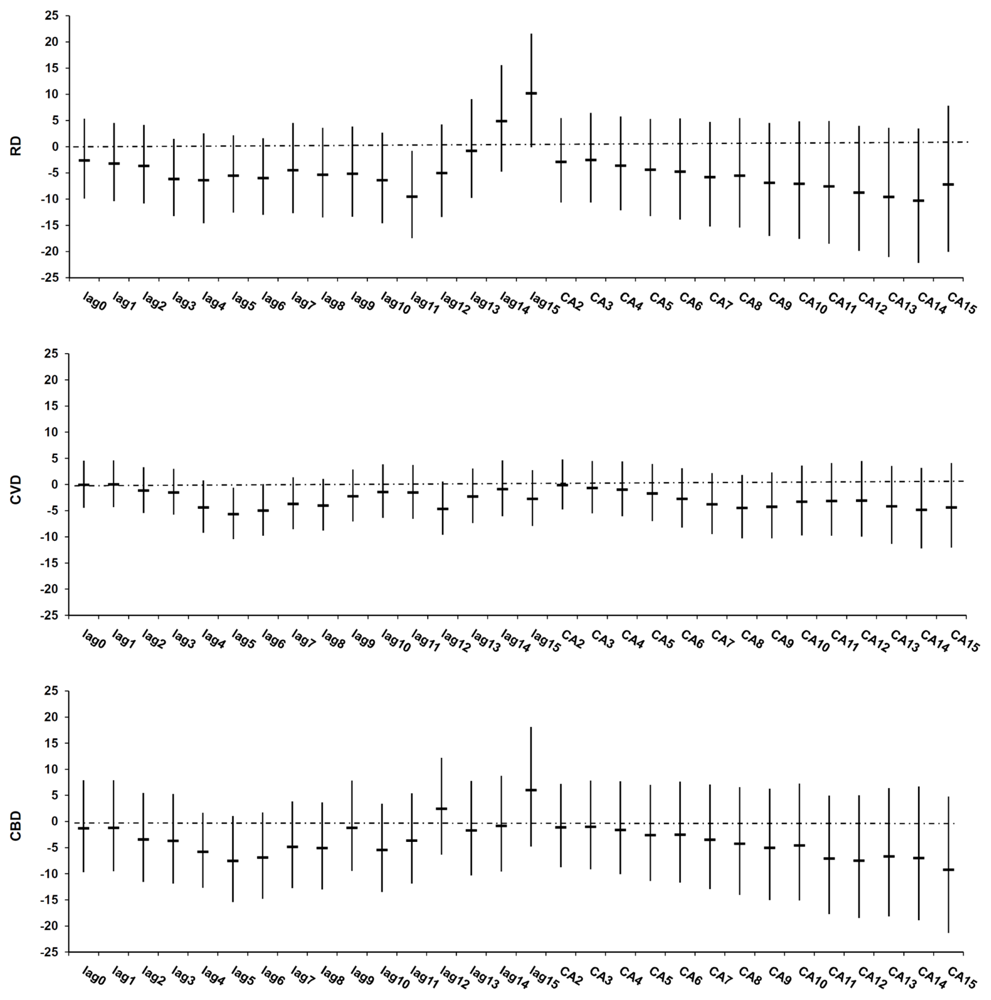
| All year | Warm period | Cold period | |
|---|---|---|---|
| Number of days | 2,922 | 1,464 | 1,458 |
| Respiratory deaths | |||
| Mean ± SD | 2 ± 2 | 2 ± 1 | 2 ± 2 |
| Range | 0–10 | 0–7 | 0–10 |
| Cardiovascular deaths | |||
| Mean ± SD | 6 ± 3 | 6 ± 3 | 7 ± 3 |
| Range | 0–18 | 0–15 | 0–18 |
| Cerebrovascular deaths | |||
| Mean ± SD | 2 ± 2 | 2 ± 2 | 2 ± 2 |
| Range | 0–10 | 0–9 | 0–10 |
| Tappmax (°C) | |||
| Number of days with missing data | 114 | 32 | 82 |
| Mean ± SD | 10 ± 8 | 16 ± 6 | 4 ± 5 |
| Range | −8–30 | 0–30 | −8–18 |
| Percentiles | |||
| 25th | 3 | 12 | 0 |
| 50th | 9 | 16 | 3 |
| 75th | 16 | 20 | 7 |
| Inter-quartile range | 13 | 8 | 7 |
| PM10 (μg/m3) | |||
| Number of days with missing data | 454 | 266 | 188 |
| Mean ± SD | 27 ± 16 | 27 ± 14 | 28 ± 17 |
| Range | 0–284 | 1–284 | 0–248 |
| NO2 (ppb) | |||
| Number of days with missing data | 164 | 109 | 55 |
| Mean ± SD | 12 ± 5 | 11 ± 4 | 13 ± 5 |
| Range | 2–41 | 3–33 | 2–41 |
| NO2max (ppb) | |||
| Number of days with missing data | 137 | 97 | 40 |
| Mean ± SD | 22 ± 9 | 21 ± 10 | 23 ± 9 |
| Range | 4–78 | 4–78 | 5–60 |
| CO (ppm) | |||
| Number of days with missing data | 129 | 81 | 48 |
| Mean ± SD | 0.28 ± 0.10 | 0.23 ± 0.07 | 0.33 ± 0.10 |
| Range | 0.08–0.92 | 0.08–0.58 | 0.13–0.92 |
| Weekly GP visits due to influenza in Denmark (%) | |||
| Number of weeks with missing data | 0 | 0 | 0 |
| Mean ± SD | 1.12 ± 1.50 | 0.28 ± 0.61 | 1.96 ± 1.65 |
| Range | 0–9.70 | 0–3.40 | 0–9.70 |
| In-hospital deaths | Out-of-hospital deaths | Total * | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Repiratory deaths | 3,089 | 100.0 | 2,883 | 100.0 | 5,973 | 100.0 |
| Simple and mucopurulent chronic bronchitis | 0 | 0.0 | 7 | 0.2 | 7 | 0.1 |
| Unspecified chronic bronchitis | 156 | 5.1 | 313 | 10.9 | 469 | 7.9 |
| Emphysema | 44 | 1.4 | 73 | 2.5 | 117 | 2.0 |
| Chronic obstructive pulmonary disease | 2,857 | 92.5 | 2,192 | 76.0 | 5,049 | 84.5 |
| Asthma | 26 | 0.8 | 288 | 10.0 | 314 | 5.3 |
| Status astmaticus | 6 | 0.2 | 10 | 0.3 | 16 | 0.3 |
| Cardiovascular deaths | 6,310 | 100.0 | 12,502 | 100.0 | 18,816 | 100.0 |
| Angina pectoris | 26 | 0.4 | 61 | 0.5 | 87 | 0.5 |
| Acute myocardial infarction | 2,517 | 39.9 | 3,064 | 24.5 | 5,581 | 29.7 |
| Subsequent myocardial infarction | 154 | 2.4 | 165 | 1.3 | 319 | 1.7 |
| Other acute ischemic heart diseases | 8 | 0.1 | 8 | 0.1 | 16 | 0.1 |
| Chronic ischemic heart disease | 1,387 | 22.0 | 5,244 | 41.9 | 6,631 | 35.2 |
| Pulmonary embolism | 337 | 5.3 | 304 | 2.4 | 641 | 3.4 |
| Cardiac arrest | 143 | 2.3 | 1,013 | 8.1 | 1,156 | 6.1 |
| Atrial fibrillation and flutter | 544 | 8.6 | 496 | 4.0 | 1,040 | 5.5 |
| Other cardiac arrhythmias | 27 | 0.4 | 92 | 0.7 | 119 | 0.6 |
| Heart failure | 1,167 | 18.5 | 2,055 | 16.4 | 3,222 | 17.1 |
| Cerebrovascular deaths | 3,469 | 100.0 | 3,082 | 100.0 | 6,558 | 100.0 |
| Intracerebral haemorrhage | 1,128 | 32.5 | 389 | 12.6 | 1,517 | 23.1 |
| Cerebral infarction | 595 | 17.2 | 345 | 11.2 | 940 | 14.3 |
| Stroke, not specified as haemorrhage or infarction | 1,746 | 50.3 | 2,348 | 76.2 | 4,094 | 62.4 |
| Total | 12,868 | 18,467 | 31,347 | |||
| Respiratory disease | Cardiovascular disease | Cerebrovascular disease | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IQR | n | % | 95% CI | IQR | n | % | 95% CI | IQR | n | % | 95% CI | ||||
| All | 7 | 2,431 | 6.3 | −5.4 | 19.4 | 7 | 7,976 | −6.9 | −12.7 | −0.6 | 7 | 2,834 | 2.0 | −8.6 | 13.8 |
| Age categories | |||||||||||||||
| ≤ 65 years | 8 | 234 | −3.7 | −36.5 | 46.1 | 7 | 871 | 7.5 | −11.3 | 30.4 | 7 | 249 | 21.3 | −16.7 | 76.7 |
| 66–80 years | 7 | 1,145 | 5.3 | −11.1 | 24.8 | 7 | 2,225 | −9.0 | −19.5 | 2.9 | 7 | 788 | 7.1 | −12.8 | 31.6 |
| > 80 years | 8 | 1,052 | 12.3 | −8.4 | 37.6 | 7 | 4,880 | −8.3 | −15.7 | −0.4 | 7 | 1,797 | −2.6 | −15.2 | 11.9 |
| Sex | |||||||||||||||
| Women | 7 | 1,422 | 1.0 | −13.3 | 17.7 | 7 | 4,286 | −4.9 | −13.0 | 3.9 | 7 | 1,780 | 1.0 | −12.2 | 16.2 |
| Men | 8 | 1,009 | 16.3 | −5.2 | 42.8 | 7 | 3,690 | −9.1 | −17.4 | 0.0 | 7 | 1,054 | 3.9 | −13.0 | 24.0 |
| Socio-economic status | |||||||||||||||
| Lowest | 7 | 882 | 4.8 | −13.7 | 27.4 | 7 | 2,667 | −11.2 | −20.7 | −0.6 | 7 | 876 | 13.6 | −6.8 | 38.3 |
| Second lowest | 8 | 677 | −12.5 | −31.9 | 12.4 | 7 | 2,000 | −6.7 | −17.9 | 6.0 | 7 | 725 | −5.4 | −23.7 | 17.4 |
| Second highest | 8 | 572 | 30.2 | −0.7 | 70.7 | 8 | 2,166 | −1.5 | −14.7 | 13.6 | 7 | 801 | −10.5 | −27.3 | 10.2 |
| Highest | 8 | 265 | 20.8 | −19.6 | 81.5 | 7 | 1,034 | −7.0 | −22.5 | 11.6 | 8 | 396 | 16.0 | −17.5 | 63.1 |
| Place of death | |||||||||||||||
| In-hospital | 8 | 1,242 | 1.1 | −16.0 | 21.7 | 7 | 2,629 | −9.4 | −19.1 | 1.4 | 7 | 1,488 | −7.0 | −20.2 | 8.4 |
| Out-of-hospital | 7 | 1,188 | 12.1 | −5.1 | 32.5 | 7 | 5,345 | −5.6 | −12.8 | 2.2 | 7 | 1,342 | 11.5 | −4.8 | 30.7 |
| Respiratory disease | Cardiovascular disease | Cerebrovascular disease | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IQR | n | % | 95% CI | IQR | n | % | 95% CI | IQR | n | % | 95% CI | ||||
| All | 6 | 2,854 | −4.8 | −13.9 | 5.4 | 6 | 8,777 | −2.7 | −8.2 | 3.1 | 6 | 3,010 | −2.5 | −11.7 | 7.6 |
| Age categories | |||||||||||||||
| ≤ 65 years | 6 | 318 | 4.7 | −22.7 | 41.8 | 6 | 927 | −13.7 | −28.0 | 3.5 | 7 | 221 | 26.1 | −15.5 | 88.1 |
| 66–80 years | 6 | 1,349 | 1.9 | −12.1 | 18.2 | 6 | 2,415 | 5.9 | −5.2 | 18.2 | 6 | 885 | −1.6 | −18.1 | 18.1 |
| > 80 years | 6 | 1,187 | −13.4 | −25.9 | 1.2 | 6 | 5,435 | −4.5 | −11.3 | 2.8 | 6 | 1,904 | −5.7 | −16.8 | 6.8 |
| Sex | |||||||||||||||
| Women | 6 | 1,720 | −1.7 | −13.6 | 12.0 | 6 | 4,769 | −0.3 | −7.8 | 7.9 | 6 | 1,970 | −0.3 | −11.9 | 12.8 |
| Men | 6 | 1,134 | −9.3 | −22.8 | 6.5 | 6 | 4,008 | −5.6 | −13.3 | 2.9 | 6 | 1,040 | −6.0 | −20.3 | 10.8 |
| Socio-economic status | |||||||||||||||
| Lowest | 6 | 1,034 | −6.2 | −20.2 | 10.4 | 6 | 2,996 | −2.1 | −11.3 | 8.1 | 6 | 937 | −19.2 | −32.4 | −3.5 |
| Second lowest | 6 | 744 | −1.2 | −19.7 | 21.5 | 6 | 2,121 | 0.5 | −10.7 | 13.0 | 6 | 807 | 11.8 | −7.6 | 35.3 |
| Second highest | 6 | 701 | −10.8 | −27.2 | 9.4 | 6 | 2,362 | 0.3 | −10.3 | 12.1 | 6 | 806 | −2.8 | −19.9 | 17.8 |
| Highest | 6 | 341 | 4.5 | −22.1 | 40.2 | 6 | 1,144 | −16.2 | −28.5 | −1.7 | 6 | 419 | 11.1 | −14.3 | 44.2 |
| Place of death | |||||||||||||||
| In-hospital | 6 | 1,494 | 5.0 | −8.7 | 20.7 | 6 | 2,978 | −6.8 | −15.6 | 2.8 | 6 | 1,603 | −4.5 | −16.6 | 9.2 |
| Out-of-hospital | 6 | 1,360 | −14.4 | −26.0 | −0.9 | 6 | 5,797 | −0.6 | −7.4 | 6.8 | 6 | 1,406 | 0.0 | −13.5 | 15.7 |
| Respiratory disease | Cardiovascular disease | Cerebrovascular disease | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IQR | n* | % | 95% CI | IQR | n | % | 95% CI | IQR | n | % | 95% CI | ||||
| Warm | 7 | 1,342 | 3.3 | −5.4 | 12.8 | 7 | 1,342 | −4.4 | −8.9 | 0.4 | 7 | 1,342 | −1.3 | −9.1 | 7.2 |
| Cold | 6 | 1,271 | −8.0 | −14.1 | −1.5 | 6 | 1,271 | −7.5 | −10.9 | −4.0 | 6 | 1,271 | −0.9 | −7.3 | 6.0 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wichmann, J.; Andersen, Z.J.; Ketzel, M.; Ellermann, T.; Loft, S. Apparent Temperature and Cause-Specific Mortality in Copenhagen, Denmark: A Case-Crossover Analysis. Int. J. Environ. Res. Public Health 2011, 8, 3712-3727. https://doi.org/10.3390/ijerph8093712
Wichmann J, Andersen ZJ, Ketzel M, Ellermann T, Loft S. Apparent Temperature and Cause-Specific Mortality in Copenhagen, Denmark: A Case-Crossover Analysis. International Journal of Environmental Research and Public Health. 2011; 8(9):3712-3727. https://doi.org/10.3390/ijerph8093712
Chicago/Turabian StyleWichmann, Janine, Zorana Jovanovic Andersen, Matthias Ketzel, Thomas Ellermann, and Steffen Loft. 2011. "Apparent Temperature and Cause-Specific Mortality in Copenhagen, Denmark: A Case-Crossover Analysis" International Journal of Environmental Research and Public Health 8, no. 9: 3712-3727. https://doi.org/10.3390/ijerph8093712
APA StyleWichmann, J., Andersen, Z. J., Ketzel, M., Ellermann, T., & Loft, S. (2011). Apparent Temperature and Cause-Specific Mortality in Copenhagen, Denmark: A Case-Crossover Analysis. International Journal of Environmental Research and Public Health, 8(9), 3712-3727. https://doi.org/10.3390/ijerph8093712





