Evaluating Pharmacokinetic and Pharmacodynamic Interactions with Computational Models in Supporting Cumulative Risk Assessment
Abstract
:1. Introduction
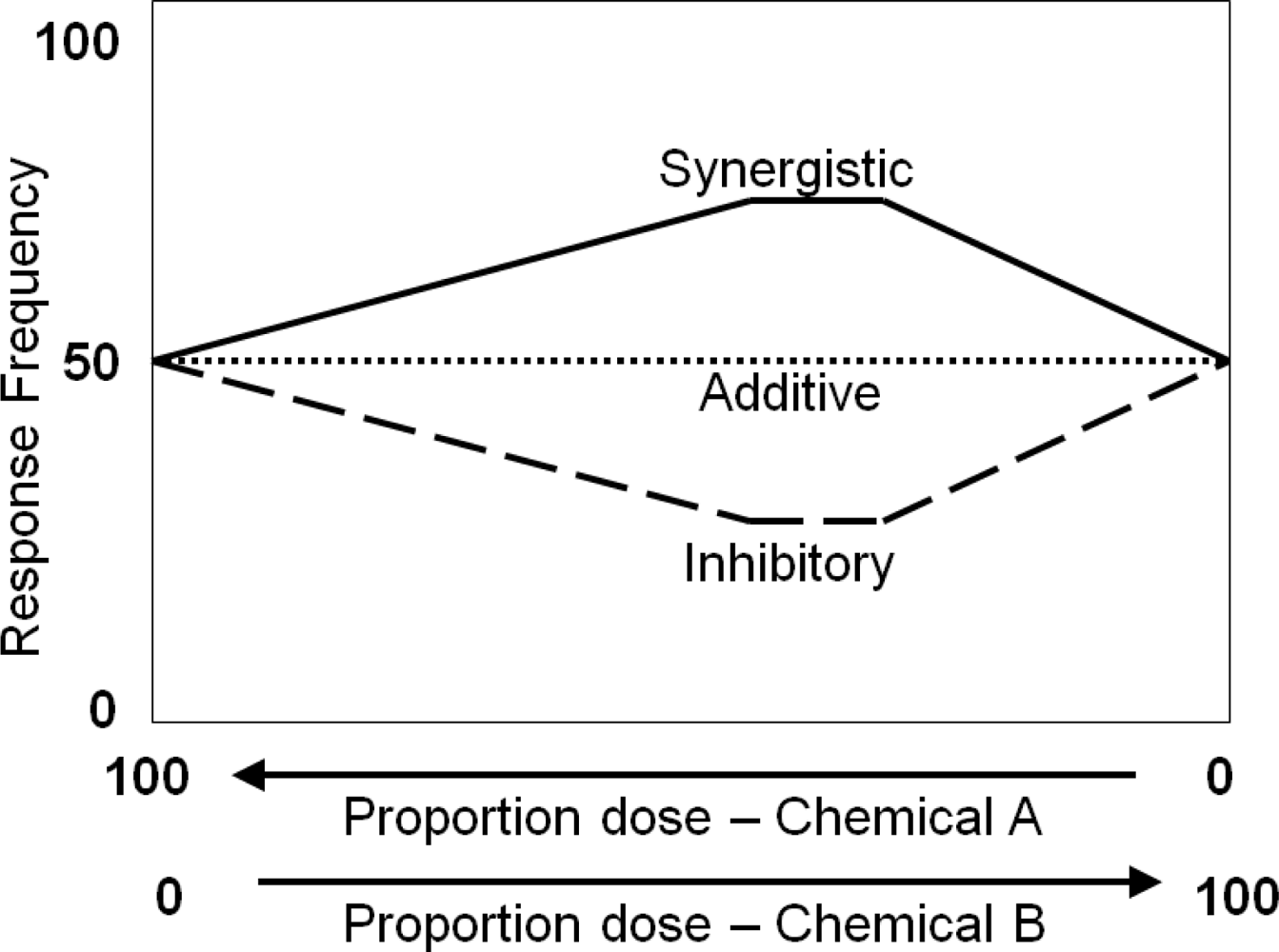
2. Computational Modeling of Chemical Interactions
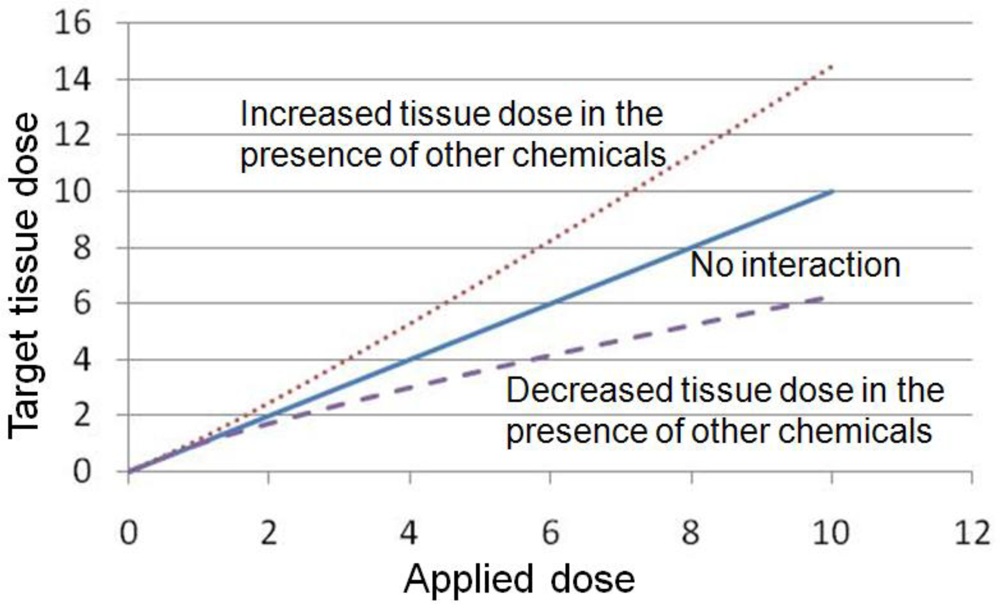
3. Examples of Pharmacokinetic Interactions among Mixtures
3.1. Decreased Tissue Dose in the Presence of Other Chemicals
3.1.1. Trichloroethylene (TCE) in a Mixture
3.1.2. Toluene in a Mixture
3.2. Increased Tissue Dose in the Presence of Other Chemicals
3.2.1. Carbon Tetrachloride (CCl4) and Methanol
3.2.2. Mirex, Phenobarbital, Chlordecone and Bromotrichloromethane (BrCCl3)
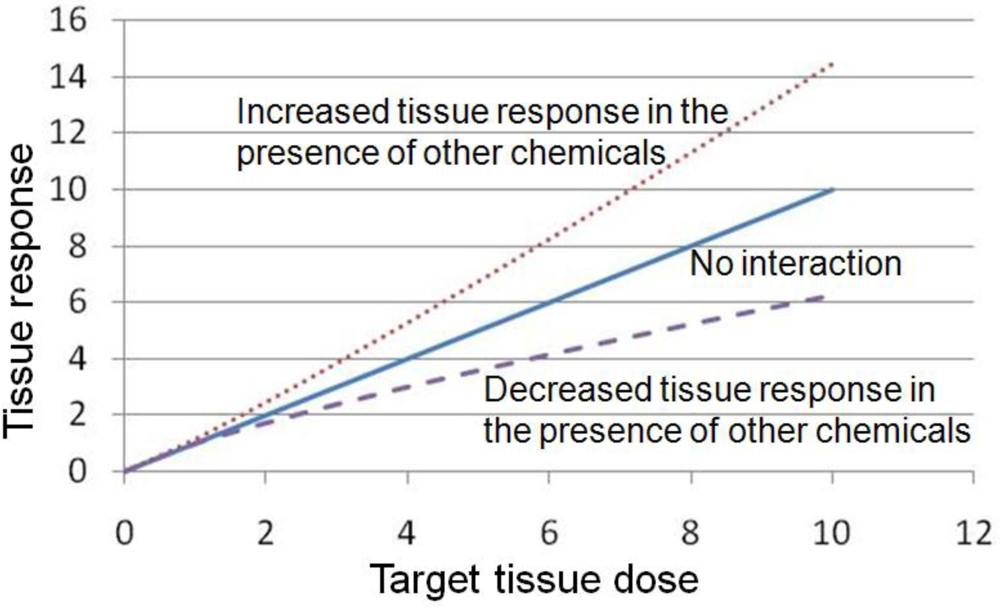
4. Examples of Pharmacodynamic Interactions among Mixtures
4.1. Decreased Tissue Response in the Presence of Other Chemicals
4.2. Increased Tissue Response in the Presence of Other Chemicals
5. Applications in Assessing Human Health Risks
6. Conclusions
Acknowledgments
- DisclaimerThe United States Environmental Protection Agency through its Office of Research and Development collaborated in the research described here. It has been subjected to Agency review and approved for publication.
References and Notes
- Stork, LG; Gennings, C; Carchman, RA; Carter, WH; Pounds, J; Muntaz, M. Testing for additivity at select mixture groups of interest based on statistical equivalence testing methods. Risk Anal 2006, 26, 1601–1612. [Google Scholar]
- ATSDR, Gudiance Manual for the Assessment of Joint Toxic Action of Chemical Mixtures; U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2002.
- Ragas, MJ; Oldenkamp, R; Preeker, NL; Wernicke, J; Schlink, U. Cumulative risk assessment of chemical exposures in urban environments. Environ. Int 2011, 37, 872–881. [Google Scholar]
- Kortenkamp, A; Faust, M. Combined exposures to anti-androgenic chemicals: Steps towards cumulative risk assessment. Int. J. Androl 2010, 33, 463–474. [Google Scholar]
- Luciene da Silva, M; Charest-Tardif, G; Krishnan, K; Tardif, R. Influence of oral administration of a quaternary mixture of triahlomethanes on their blood kinetics in the rat. Toxicol. Lett 1999, 106, 49–57. [Google Scholar]
- Andersen, ME; Dennison, JE. Mechanistic approaches for mixture risk assessments—present capabilities and future directions. Environ. Toxicol. Pharmacol 2004, 16, 1–11. [Google Scholar]
- Krishnan, K; Brodeur, J. Toxicological consequences of combined exposure to environmental pollutants. Arch. Complex Environ. Stud 1991, 3, 1–106. [Google Scholar]
- Dennison, JE; Bigelow, PL; Andersen, ME. Occupational exposure limits in the context of solvent mixtures, consumption of ethanol, and target tissue dose. Toxicol. Ind. Health 2004, 20, 165–175. [Google Scholar]
- Dekant, W. The role of biotransformation and bioactivation in toxicity. Experientia Suppl 2009, 99, 57–86. [Google Scholar]
- Reddy, M; Yang, RSH; Clewell, HJ; Andersen, ME. Physiologically Based Pharmacokinetic Modeling: Science and Applications; John Wiley & Sons Inc: Hoboken, NJ, USA, 2005. [Google Scholar]
- Tardif, R; Charest-Tardif, G. The importance of measured end-points in demonstrating the occurrence of interactions: A case study with methylchloroform and m-xylene. Toxicol. Sci 1999, 49, 321–317. [Google Scholar]
- Jollow, DJ; Bruckner, JV; McMillan, DC; Fisher, JW; Hoel, DG; Mohr, LC. Trichloroethylene risk assessment: A review and commentary. Crit. Rev. Toxicol 2009, 39, 782–797. [Google Scholar]
- Caldwell, JC; Keshava, N; Evans, MV. Difficulty of mode of action determination for trichloroethylene: An example of complex interactions of metabolites and other chemical exposures. Environ. Mol. Mutagen 2008, 49, 142–154. [Google Scholar]
- Dobrev, ID; Andersen, ME; Yang, RS. Assessing interaction thresholds for trichloroethylene in combination with tetrachloroethylene and 1,1,1-trichloroethane using gas uptake studies and PBPK modeling. Arch. Toxicol 2001, 75, 134–144. [Google Scholar]
- De Rosa, CT; El-Masri, HA; Pohl, H; Cibulas, W; Mumtaz, MM. Implications of chemical mixtures in public health practice. J. Toxicol. Environ. Health B Crit. Rev 2004, 7, 339–350. [Google Scholar]
- Dekant, W; Martens, G; Vamvakas, S; Metzler, M; Henschler, D. Bioactivation of tetrachloroethylene. Role of glutathione S-transferase-catalyzed conjugation versus cytochrome P-450-dependent phospholipid alkylation. Drug Metabol. Dispos 1987, 15, 702–709. [Google Scholar]
- Bruckner, JV; Kyle, GM; Luthra, R; Acosta, D; Mehta, SM; Sethuraman, S; Muralidhara, S. Acute, short-term and subchronic oral toxicity of 1,1,1-trichloroethane in rats. Toxicol. Sci 2001, 60, 363–372. [Google Scholar]
- Andersen, ME; Gargas, ML; Clewell, HJ; Severyn, KM. Quantitative evaluation of the metabolic interaction between trichloroethylene and 1,1-dichloroethylene in vivo using gas uptake methods. Toxicol. Appl. Pharmacol 1987, 89, 149–157. [Google Scholar]
- Jaeger, RJ; Conolly, RB; Murphy, SD. Effect of 18 hr fast and glutathione depletion on 1,1-dichloroehtylene-induced hepatotoxicity and lethality in rats. Exp. Mol. Pathol 1974, 20, 187–198. [Google Scholar]
- Watanabe, PG; Zempel, JA; Pegg, DG; Gehring, PJ. Hepatic macromolecular binding following exposure to vinyl chloride. Toxicol. Appl. Pharmacol 1978, 77, 571–579. [Google Scholar]
- Purcell, KJ; Cason, GH; Gargas, ML; Andersen, ME; Travis, CC. In vivo metabolic interactions of benzene and toluene Toxicol. Lett 1990, 52, 141–152. [Google Scholar]
- Tardif, R; Charest-Tardif, G; Brodeur, J; Krishnan, K. Physiologically based pharmacokinetic modeling of a ternary mixture of alkyl benzenes in rats and humans. Toxicol. Appl. Pharmacol 1997, 144, 120–134. [Google Scholar]
- Haddad, S; Charest-Tardif, G; Tardif, R; Krishnan, K. Physiological modeling of the toxicokinetic interactions in a quaternary mixture of aromatic hydrocarbons. Toxicol. Appl. Pharmacol 1999, 161, 249–257. [Google Scholar]
- Haddad, S; Charest-Tardif, G; Tardif, R; Krishnan, K. Validation of a physiological modeling framework for simulating the toxicokinetics of chemicals in mixtures. Toxicol. Appl. Pharmacol 2000, 167, 199–209. [Google Scholar]
- Price, K; Krishnan, K. An integrated QSAR-PBPK modeling approach for predicting the inhalation toxicokinetics of mixtures of volatile organic chemicals in the rat. SAR QSAR Environ. Res 2011, 22, 107–128. [Google Scholar]
- Dennison, JE; Andersen, ME; Yang, RSH. Characterization of the pharmacokinetics of gasoline using PBPK modeling with a complex mixtures chemical lumping approach. Inhal. Toxicol 2003, 15, 964–986. [Google Scholar]
- ATSDR, Toxicological Profile of Carbon Tetrachloride—Draft; U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2003.
- Mehendale, HM. Role of hepatocellular regeneration and hepatolobular healing in the final outcome of liver injury. A two-stage model of toxicity. Biochem. Pharmacol 1991, 42, 1155–1162. [Google Scholar]
- Evans, MV; Simmons, JE. Physiologically based pharmacokinetic estimated metabolic constants and hepatotoxicity of carbon tetrachloride after methanol pretreatment in rats. Toxicol. Appl. Pharmacol 1996, 140, 245–253. [Google Scholar]
- Simmons, JE; McDonald, A; Seely, JC; Sey, YM. Potentiation of carbon tetrachloride hepatotoxicity by inhaled methanol: Time course of injury and recovery. J. Toxicol. Environ. Health 1995, 46, 203–216. [Google Scholar]
- Thakore, KN; Gargas, ML; Andersen, ME; Mehendale, HM. PB-PK derived metabolic constants, hepatotoxicity, and lethality of BrCCl3 in rats pretreated with chlordecone, phenobarbital or mirex. Toxicol. Appl. Pharmacol 1991, 109, 514–528. [Google Scholar]
- El-Masri, H; Mumtaz, M; Yushak, M. Application of physiologically-based pharmacokinetic modeling to investigate the toxicological interaction between chlorpyrifos and parathion in the rat. Environ. Toxicol. Pharmacol 2004, 16, 57–71. [Google Scholar]
- ATSDR, Toxicological Profile of Chlorpyrifos; U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1997.
- Sultatos, LG; Minor, LD; Murphy, SD. Metabolic activation of phosphorothioate pesticides: Role of the liver. J. Pharmacol. Exp. Ther 1985, 232, 624–628. [Google Scholar]
- Sams, C; Mason, HJ; Rawbone, R. Evidence for the activation of organophosphate pesticides by cytochromes P450 3A4 and 2D6 in human liver microsomes. Toxicol. Lett 2000, 116, 217–221. [Google Scholar]
- Huff, RA; Corcoran, JJ; Anderson, JK; Abou-Donia, MB. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J. Pharmacol. Exp. Ther 1994, 269, 329–335. [Google Scholar]
- Costa, LG; McDonald, BE; Murphy, SD; Omenn, GS; Richter, RJ; Motulsky, AG; Furlong, CE. Serum paraoxonase and its influence on paraoxon and chlorpyrifos-oxon toxicity in rats. Toxicol. Appl. Pharmacol 1990, 103, 66–76. [Google Scholar]
- Mutch, E; Daly, AK; Leathart, JB; Blain, PG; Williams, FM. Do multiple cytochrome P450 isoforms contribute to parathion metabolism in man? Arch. Toxicol 2003, 77, 313–320. [Google Scholar]
- Timchalk, C; Poet, TS. Development of a physiologically based pharmacokinetic and pharmacodynamic model to determine dosimetry and cholinesterase inhibition for a binary mixture of chlorpyrifos and diazinon in the rat. Neurotoxicol. Teratol 2008, 29, 428–443. [Google Scholar]
- Timchalk, C; Nolan, RJ; Mendrala, AL; Dittenber, DA; Brzak, KA; Mattsson, JL. A physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol. Sci 2002, 66, 34–53. [Google Scholar]
- Nong, A; Tan, Y; Krolski, ME; Wang, J; Lunchick, C; Conolly, RB; Clewell, HJ. Bayesian calibration of a physiologically based pharmacokinetic/pharmacodynamic model of carbaryl cholinesterase inhibition. J. Toxicol. Environ. Health 2008, 71, 1363–1381. [Google Scholar]
- Campbell, JL; Krishnan, K; Clewell, HJ; Andersen, ME. Kinetic interactions of chemical mixtures. In Principles and Practice of Mixtures Toxicology; Mumtaz, M, Ed.; Wiley: Weinheim, Germany, 2010. [Google Scholar]
- Mehendale, HM. Amplified interactive toxicity of chemicals at nontoxic levels: Mechanistic considerations and implications to public health. Environ Health Perscept 1994, 102(Suppl 9), 139–149. [Google Scholar]
- Curtis, LR; Williams, WL; Mehendale, HM. Potentiation of the hepatotoxicity of carbon tetrachloride following preexposure to chlordecone (kepone) in the male rat. Toxicol. Appl. Pharmacol 1979, 51, 283–293. [Google Scholar]
- Agarwal, AK; Mehendale, HM. Potentiation of CCl4 hepatotoxicity and lethality by chlordecone in female rats. Toxicology 1983, 26, 231–242. [Google Scholar]
- Lockard, VG; Mehendale, HM; O’Neal, RM. Chlordecone-induced potentiation of carbon tetrachloride heaptotoxicity: A light and electron microscopic study. Exp. Mol. Pathol 1983, 39, 230–245. [Google Scholar]
- Lockard, VG; Mehendale, HM; O’Neal, RM. Chlordecone-induced potentiation of carbon tetrachloride heaptotoxicity: A morphometric and biochemical study. Exp. Mol. Pathol 1983, 39, 246–255. [Google Scholar]
- Mehendale, HM. Mechanism of the lethal interaction of chlordecone and CCl4 at non-toxic doses. Toxicol. Lett 1989, 49, 215–241. [Google Scholar]
- Kodavanti, PR; Kodavanti, UP; Faroon, OM; Mehendale, HM. Pivotal role of hepatocellular regeneration in the ultimate hepatotoxicity of CCl4 in chlordecone-, mirex-, or Phenobarbital-pretreated rats. Toxicol. Pathol 1992, 20, 556–569. [Google Scholar]
- Cai, Z; Mehendale, HM. Resiliency to amplification of carbon tetrachloride hepatotoxicity by chlordecone during postnatal development in rats. Pediatr. Res 1993, 33, 225–232. [Google Scholar]
- El-Masri, HA; Thomas, RS; Sabados, GR; Phillips, JK; Constan, AA; Benjamin, SA; Andersen, ME; Mehendale, HM; Yang, RS. Physiologically based pharmacokinetic/pharmacodynamic modeling of the toxicologic interaction between carbon tetrachloride and kepone. Arch. Toxicol 1996, 70, 704–713. [Google Scholar]
- 29 CFR 1910.1000—Air Contaminants. Code of Federal Regulations—Title 29: Labor; Occupational Safety and Health Administration (OSHA): Washington, DC, USA, 2004.
- Dennison, JE; Bigelow, PL; Mumtaz, MM; Andersen, ME; Dobrev, ID; Yang, RSH. Evaluation of potential toxicity from co-exposure to three CNS depressants (toluene, ethylbenzene, and xylene) under resting and working conditions using PBPK modeling. J. Occup. Environ. Hyg 2005, 2, 127–135. [Google Scholar]
- Jang, JY; Droz, PO; Kim, S. Biological monitoring of workers exposed to ethylbenzene and co-exposed to xylene. Int. Arch. Occup. Environ. Health 2001, 74, 31–37. [Google Scholar]
- Dobrev, ID; Andersen, ME; Yang, RSH. In silico toxicology: Simulating interaction thresholds for human exposure to mixtures of trichloroethylene, tetrachloroethylene, and 1,1,1-trichloroethane. Environ. Health Perspect 2002, 110, 1031–1039. [Google Scholar]
- Lash, LH; Fisher, JW; Lipscomb, JC; Parker, JC. Metabolism of trichloroethylene. Environ. Health Perspect 2000, 108, 177–200. [Google Scholar]
- Haddad, S; Béliveau, M; Tardif, R; Krishnan, K. A PBPK modeling-based approach to account for interactions in the health risk assessment of chemical mixtures. Toxicol. Sci 2001, 63, 125–131. [Google Scholar]
- Haddad, S; Poulin, P; Funk, C. Extrapolating in vitro metabolic interactions to isolated perfused liver: Predictions of metabolic interactions between R-Bufuralol, Bunitrolol, and Debrisoquine. J. Pharm. Sci 2010, 99, 4406–4426. [Google Scholar]
- Vossen, M; Sevestre, M; Niederalt, C; Jang, I; Willmann, S; Edginton, A. Dynamically simulating the interaction of midazolam and the CYP3A4 inhibitor itraconazole using individual coupled whole-body physiologically-based pharmacokinetic (WB-PBPK) models. Theor. Biol. Med. Model 2007, 4, 13. [Google Scholar]
- Kato, M; Shitara, Y; Sato, H; Yoshisue, K; Hirano, M; Ikeda, T; Sugiyama, Y. The quantitative prediction of CYP-mediated drug interaction by physiologically based pharmacokinetic modeling. Pharmacol. Res 2008, 25, 1891–1901. [Google Scholar]
- Rowland, M; Peck, C; Tucker, G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu. Rev. Pharmacol. Toxicol 2011, 51, 45–73. [Google Scholar]
- Ideker, T; Galitski, T; Hood, L. A new approach to decoding life: Systems biology. Annu. Rev. Genom. Hum. Genet 2001, 2, 343–372. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tan, Y.-M.; Clewell, H.; Campbell, J.; Andersen, M. Evaluating Pharmacokinetic and Pharmacodynamic Interactions with Computational Models in Supporting Cumulative Risk Assessment. Int. J. Environ. Res. Public Health 2011, 8, 1613-1630. https://doi.org/10.3390/ijerph8051613
Tan Y-M, Clewell H, Campbell J, Andersen M. Evaluating Pharmacokinetic and Pharmacodynamic Interactions with Computational Models in Supporting Cumulative Risk Assessment. International Journal of Environmental Research and Public Health. 2011; 8(5):1613-1630. https://doi.org/10.3390/ijerph8051613
Chicago/Turabian StyleTan, Yu-Mei, Harvey Clewell, Jerry Campbell, and Melvin Andersen. 2011. "Evaluating Pharmacokinetic and Pharmacodynamic Interactions with Computational Models in Supporting Cumulative Risk Assessment" International Journal of Environmental Research and Public Health 8, no. 5: 1613-1630. https://doi.org/10.3390/ijerph8051613
APA StyleTan, Y.-M., Clewell, H., Campbell, J., & Andersen, M. (2011). Evaluating Pharmacokinetic and Pharmacodynamic Interactions with Computational Models in Supporting Cumulative Risk Assessment. International Journal of Environmental Research and Public Health, 8(5), 1613-1630. https://doi.org/10.3390/ijerph8051613



