The Effects of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) on the Mortality and Growth of Two Amphibian Species (Xenopus laevis and Pseudacris triseriata)
Abstract
:Introduction
Materials and Methods
Egg Collection and Exposure Protocol
Measurements and Data Analysis
Results
Egg Mortality
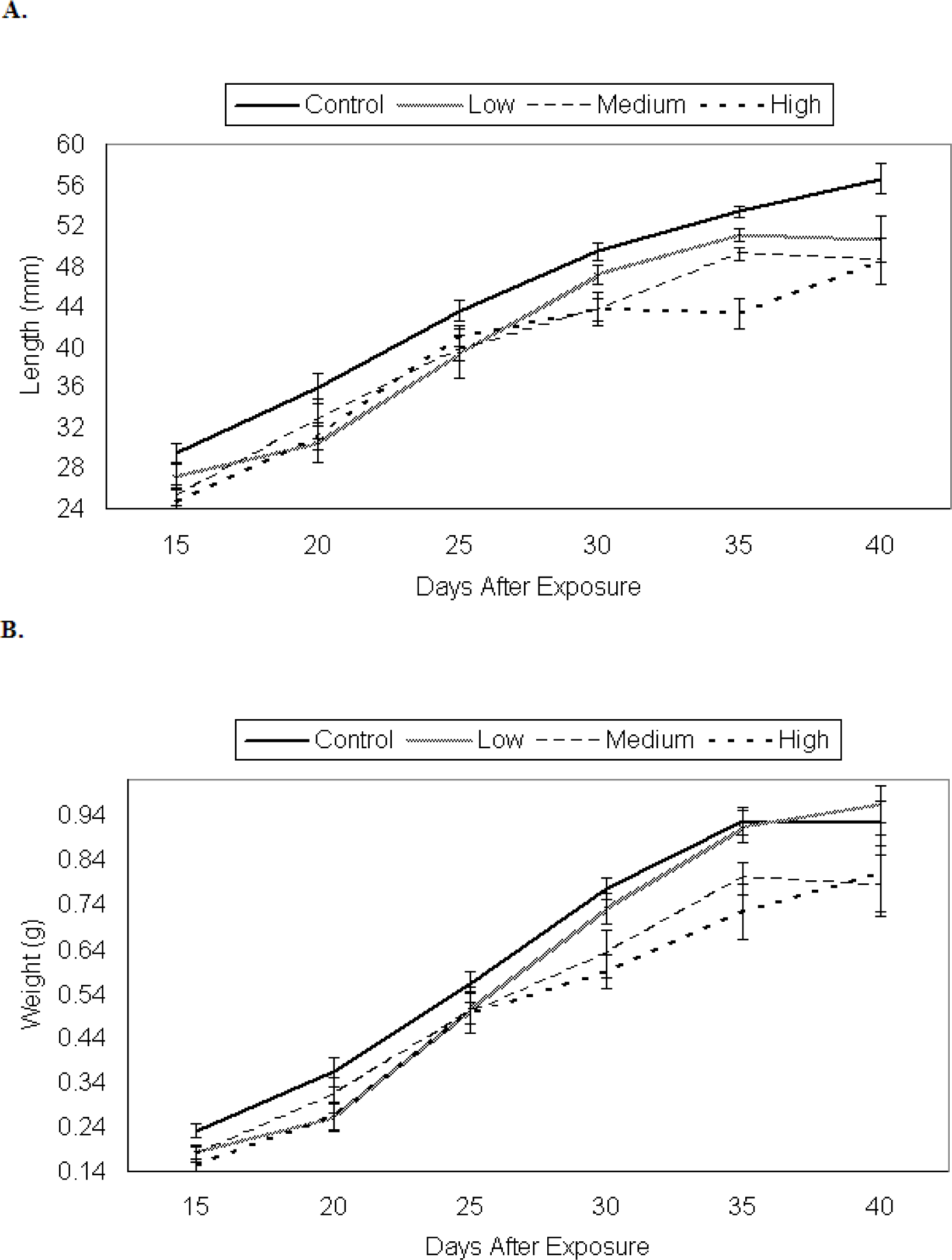
Growth of P. triseriata
Growth of X. laevis
Discussion
TCDD and Egg Mortality
TCDD Effects on Larval Growth and Metamorphosis
Conclusions



| 24 h X. laevis | Control | Low | Medium | High |
| (Replicate 1) | 6 | 9 | 12 | 12 |
| (Replicate 2) | 9 | 12 | 12 | 21 |
| 48 h X. laevis | Control | Low | Medium | High |
| (Replicate 1) | 9 | 15 | 18 | 24 |
| (Replicate 2) | 6 | 24 | 15 | 30 |
| 48 h P. triseriata | Control | Low | Medium | High |
| (Replicate 1) | 25 | 16 | 14 | 15 |
| (Replicate 2) | 10 | 17 | 20 | 10 |
| X. laevis (24 h) | X. laevis (48 h) | ||||||||
| Day 22 | Day 22 | ||||||||
| Stage | C | L | M | H | Stage | C | L | M | H |
| Stage 49 | - | 1 | - | 1 | Stage 49 | - | 1 | - | - |
| Stage 50 | 1 | 1 | 1 | - | Stage 50 | 1 | 1 | 2 | 2 |
| Stage 51 | 1 | 2 | 3 | 1 | Stage 51 | 3 | 2 | 2 | 2 |
| Stage 52 | 3 | 1 | 1 | 3 | Stage 52 | 1 | 1 | 1 | 1 |
| Day 32 | Day 32 | ||||||||
| Stage | C | L | M | H | Stage | C | L | M | H |
| Stage 51 | - | 1 | - | - | Stage 51 | - | - | - | - |
| Stage 52 | 1 | 1 | 1 | 1 | Stage 52 | - | 1 | 1 | 2 |
| Stage 53 | - | 2 | - | 1 | Stage 53 | 1 | 1 | - | - |
| Stage 54 | 3 | - | 3 | 2 | Stage 54 | 2 | 2 | 3 | 2 |
| Stage 55 | 1 | 1 | 1 | 1 | Stage 55 | 2 | 1 | 1 | 1 |
| P. triseriata (48 h) | P. triseriata (48 h) | ||||||||
| Day 20 | Day 40 | ||||||||
| Stage | C | L | M | H | Stage | C | L | M | H |
| Stage 31 | - | 1 | 1 | 1 | Stage 37 | − | 2 | 2 | 3 |
| Stage 32 | 1 | 3 | 3 | 3 | Stage 38 | 1 | 4 | 2 | 3 |
| Stage 33 | 3 | 3 | 4 | 4 | Stage 39 | 2 | 2 | 4 | 3 |
| Stage 34 | 2 | 2 | 1 | 1 | Stage 40 | 4 | 2 | 2 | 1 |
| Stage 35 | 4 | 1 | 1 | 1 | Stage 41 | 3 | - | - | - |
Acknowledgments
References
- Davidson, D; Shaffer, HB; Jennings, MR. Declines of the California red-legged frog: Climate, UV-B, habitat, and pesticides hypotheses. Ecological Applications 2001, 11, 464–479. [Google Scholar]
- Marsh, DM; Trenham, PC. Metapolulation dynamics and amphibian conservation. Conservation Biology 2001, 15, 40–49. [Google Scholar]
- Blaustein, AR; Romansic, JM; Kiesecker, JM; Hatch, AC. Ultraviolet radiation, toxic chemicals and amphibians population declines. Diversity and Distributions 2003, 9, 123–140. [Google Scholar]
- Daszak, PA; Cunningham, AA; Hyatt, AD. Infectious disease and amphibian population declines. Diversity and Distributions 2003, 9, 141–150. [Google Scholar]
- Ankley, GT; Tietge, JE; Defoe, DL; Jensen, KM; Holcombe, GW; Durhan, EJ; Diamond, SA. Effects of ultraviolet light and methoprene on survival and development of Rana pipiens. Environ. Toxicol. Chem 1998, 17, 2530–2542. [Google Scholar]
- Berrill, M; Coulson, D; McGillivray, L; Pauli, B. Toxicity of endosulfan to aquatic stages of anuran amphibians. Environ. Toxicol. Chem 1998, 17, 1738–1744. [Google Scholar]
- La Clair, JJ; Bantle, JA; Dumont, J. Photoproducts and metabolites of a common insect growth regulator produce developmental deformities in Xenopus. Environ. Sci. Technol 1998, 32, 1453–1461. [Google Scholar]
- Mann, RM; Bidwell, JR. The toxicity of glyphosphate and several glyphosphate formulations to four species of southwestern Australian frogs. Arch. Environ. Contam. Toxicol. 1999, 36, 193–199. [Google Scholar]
- Saka, M. Acute toxicity tests on Japanese amphibian larvae using thiobencarb, a component of rice paddy herbicides. Herpetological Journal 1999, 9, 73–81. [Google Scholar]
- Kiesecker, JM; Blaustein, AR; Belden, LK. Complex causes of amphibian population declines. Nature 2001, 410, 681–684. [Google Scholar]
- Carey, C; Alexander, MA. Climate change and amphibian declines: is there a link? Diversity and Distributions 2003, 9, 111–121. [Google Scholar]
- Jennings, MR; Hayes, MP. Pre-1900 overharvest of California (USA) Red-legged frogs (Rana aurora draytoni): The inducement for bullfrog (Rana catesbeiana) introduction. Herpetologica 1985, 41, 94–103. [Google Scholar]
- Lannoo, MJ; Lank, K; Waltz, T; Phillips, GS. An altered amphibian assemblage: Dickinson County, Iowa, 70 years after Frank Blanchard’s survey. American Midland Naturalist 1994, 131, 311–319. [Google Scholar]
- van den Berg, M; Birnbaum, L; Bosveld, BTC; Brunstrom, B; Cook, P; Feeley, M; Giesy, JP; Hanberg, A; Hasegawa, R; Kennedy, SW; et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, and PCDFs for humans and wildlife. Environ. Health Persp 1998, 106, 775–792. [Google Scholar]
- Olie, K. Chlorodibenzo-p-dioxins and chlorodibenzofurans are trace components of fly ash and flue gas of some municipal incinerators in the Netherlands. Chemosphere 1980, 9, 501–522. [Google Scholar]
- Environmental Protection Agency (EPA). Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds: National Academy of Sciences (NAS) review draft. Accessed March 25, 2008, from http://www.epa.gov/ncea/pdfs/dioxin/nas-review/.
- Hahn, ME; Stegeman, JJ. Phylogenic distribution of the Ah receptor in non-mammalian species: Implications for dioxin toxicity and Ah receptor evolution. Chemosphere 1992, 25, 931–937. [Google Scholar]
- Rowlands, JC; Gustafsson, JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit. Rev. Toxicol. 1997, 27, 109–134. [Google Scholar]
- Hoffman, EC; Reyes, H; Chu, FF; Sander, F; Conley, LH; Brooks, BA; Hankinson, O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 1991, 252, 954–958. [Google Scholar]
- Hahn, ME. Mechanisms of innate and acquired resistance to dioxin-like compounds. Rev. Toxicol 1998, 2, 395–443. [Google Scholar]
- Lavine, JA; Rowatt, AJ; Klimova, T; Withington, AJ; Dengler, E; Beck, C; Powell, WH. Aryl hydrocarbon receptors in the frog Xenopus laevis: Two AhR1 paralogs exhibit low affinity for 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD). Toxicol. Sci 2005, 60–72. [Google Scholar]
- Vadja, AM; Norris, DO. Effects of steroids and dioxin (2,3,7,8-TCDD) on the developing wolffian ducts of the tiger salamander (Ambystoma tigrinum). Gen. Comp. Endocrinol 2005, 141, 1–11. [Google Scholar]
- Zimmermann, AL; King, E; Dengler, E; Scogin, SR; Powell, WH. An aryl hydrocarbon receptor repressor from Xenopus laevis: Function, expression and role in dioxin responsiveness during frog development. Toxicol. Sci. 2008, 104(1), 124–134. [Google Scholar]
- Hankinson, O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch. Biochem. Biophys 2005, 433, 379–386. [Google Scholar]
- Petrulis, JR; Kusnadi, A; Ramadoss, P; Hollingshead, B; Perdew, GH. The hsp90 co-chaperone XAP2 alters importin β recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J. Biol. Chem. 2003, 278(4), 2677–2685. [Google Scholar]
- Puga, A; Tomlinson, CR; Xia, Y. Ah receptor signals cross-talk with multiple developmental pathways. Biochem. Pharmacol. 2005, 69, 199–207. [Google Scholar]
- Puga, A; Marlowe, J; Barnes, S; Chang, C; Maier, A; Tan, Z; Kerzee, KJ; Chang, X; Strobeck, M; Knudsen, ES. Role of the aryl hydrocarbon receptor in cell cycle regulation. Toxicol 2002, 181–182, 171–177. [Google Scholar]
- Schmidt, JV; Bradfield, CA. Ah receptor signaling pathways. Annu. Rev. Cell. Dev. Biol 1996, 12, 55–89. [Google Scholar]
- Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol 1995, 2, 395–443. [Google Scholar]
- Birnbaum, LS. Developmental effects of dioxins and related endocrine disrupting chemicals. Toxicol. Lett 1995, 35, 307–340. [Google Scholar]
- Pohjanvirta, R; Tuomisto, J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: Effects, mechanisms, and animal models. Pharmacol. Rev. 1994, 46, 483–549. [Google Scholar]
- Salisbury, TB; Marcinkiewicz, JL. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-pdioxin and 2,3,4,7,8-pentachlorodibenzofuran reduces growth and disrupts reproductive parameters in female rats. Biol. Repro 2002, 66, 1621–1626. [Google Scholar]
- Spitsbergen, JM; Walker, MK; Olson, JR; Peterson, RE. Pathologic alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquat. Toxicol. 1991, 19, 41–72. [Google Scholar]
- Cantrell, SM; Lutz, LH; Tillitt, DE; Hannink, M. Embryotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): The embryonic vasculature is a physiological target for TCDD-induced changes in DNA damage and apoptotic cell death in medaka (Oryzias latipes). Toxicol. Appl. Pharmacol. 1996, 141, 23–34. [Google Scholar]
- Youngchul, K; Cooper, KR. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and polychlorinated biphenyls (PCBs) in the embryos and newly hatched larvae of the Japanese medaka (Oryzias latipes). Chemosphere 1999, 39(3), 527–538. [Google Scholar]
- Ivnitski, I; Elmaoued, R; Walker, MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary development is preceeded by a decrease in myocyte proliferation and an increase in cardiac apoptosis. Teratology 2001, 64, 201–212. [Google Scholar]
- Ivnitski-Steele, ID; Walker, MK. Vascular endothelial growth factor rescues 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibition of coronary vaculogenesis. Birth Defects Res. A Clin. Mol. Teratol. 2003, 67, 496–503. [Google Scholar]
- Ivnitski-Steele, ID; Friggens, M; Chavez, M; Walker, MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary vasculogenesis is mediated, in part, by reduced responsiveness to endogenous angiogenic stimuli, including vascular endothelial growth factor A (VEGF-A). Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 440–446. [Google Scholar]
- Mima, S; Sakamoto, MK; Tanimura, T. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on development in Xenopus laevis after continuous exposure from blastula to larval stage. Environ. Sci. 1993, 6, 221–227. [Google Scholar]
- Jung, RE; Walker, MK. Effects of 2,3,7,8-tetrachlorodibenzo-p-Dioxin (TCDD) on Development of Anuran Amphibian Environ. Toxicol. Chem. 1997, 16(2), 230–240. [Google Scholar]
- Sakamoto, MK; Mima, S; Tanimura, T. A morphological study of liver lesions in Xenopus larvae exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with special reference to apoptosis of hepatocytes. J. of Env. Path. Toxicol. Oncol. 1995, 14(2), 69–82. [Google Scholar]
- Sakamoto, MK; Mima, S; Takahashi, KP; Tanimura, T. Apoptotic cell death of erythrocytes in Xenopus larvae exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Pathol. 1997, 25, 398–402. [Google Scholar]
- Sakamoto, MK; Mima, S; Tanimura, TA. Apoptosis of the intestinal principal cells of Xenopus larvae exposed to 2,3,7,8-tetrachlorodibenzo-pdioxin. J. Environ. Pathol. Toxicol. Oncol. 1999, 18, 289–295. [Google Scholar]
- McKinney, JD; Fawkes, J; Jordan, S; Kae, K; Oatley, S; Coleman, RE; Briner, W. 2,3,7,8-Tetrachloro-p-dioxin (TCDD) as a potent and persistent thyroxine agonist: A mechanistic model for toxicity based on molecular reactivity. Env. Health Pers 1985, 61, 41–53. [Google Scholar]
- Dell’Orto, N; Cantelli, D; Urani, C. Cellular targets in response to dioxin exposure. Chemosphere 1998, 37(14–15), 2809–2821. [Google Scholar]
- Mann, RM; Bidwell, JR. The acute toxicity of agricultural surfactants to the tadpoles of four Australian and two exotic frogs. Environ. Pollut. 2001, 114, 195–205. [Google Scholar]
- Environmental Protection Agency (EPA). Ground water and drinking water. Technical factsheet on: Dioxin (2,3,7,8-TCDD). Accessed March 25. 2008. from http://www.epa.gov/cgi-bin/epaprintonly.cgi.
- Duellman, WE; Trueb, L. Part 1 Life History: Ch. 6 Larvae. In The Biology of Amphibians; The Johns Hopkins University Press: Baltimore, 1986; p. 167. [Google Scholar]
- Huang, Y; Karasov, WH; Patnode, KA; Jefcoate, CR. Exposure of northern leopard frogs in the Green Bay ecosystem to polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans is measured by direct chemistry but not hepatic ethoxyresorufin-odeethylase activity. Environ. Chem. 1999, 18(10), 2123–2130. [Google Scholar]
- Kleinow, K; Baker, J; Nichols, J; Gobas, F; Parkerton, T; Muir, D; Monteverdi, G; Mastrodone, P. Exposure, uptake and disposition of chemicals in reproduction and developmental stage of selected oviparous vertebrates. In Reproductive and Developmental Effects of Contaminants in Oviparous Vertebrates; Di Giulio, RT, Tillitt, DE, Eds.; SEATAC Press: Pensacola, Florida, 1999; pp. 9–111. [Google Scholar]
- Russell, RW; Gobas, FAPC; Haffner, GD. Maternal transfer and in ovo exposure of organochlorines in oviparous organisms: A model and field verification. Environ. Sci. Technol. 1999, 33, 416–420. [Google Scholar]
- Kadokami, K; Takeishi, M; Kuramoto, M; Ono, Y. Maternal transfer of organochlorine pesticides, polychlorinated dibenzo-p-dioxins, dibenzofurans, and coplanar polychlorinated buphenyls in frogs to their eggs. Chemosphere. 2004, 57, 383–389. [Google Scholar]
- Gudernatsch, JF. Feeding experiments on tadpoles. I. The influence of specific organs given as food on growth and differentiation. A contribution to the knowledge of organs with internal secretion. Arch. Entwickl. Org 1912, 35, 457. [Google Scholar]
- Mondou, PM; Kaltenbach, JC. Thyroxine concentrations in blood serum and pericardial fluid of metamorphosing tadpoles and of adult frogs. Gen. Comp. Endocrinol. 1979, 39, 343. [Google Scholar]
- Gutleb, AC; Appelman, J; Bronkhorst, MC; van den Berg, JHJ; Spenkelink, A; Brouwer, A; Murk, AJ. Delayed effects of pre- and early-life time exposure to polychlorinated biphenyls (PCBs) on tadpoles of two amphibian species (Xenopus laevis and Rana temporaria). Environ. Toxicol. Pharmacol. 1999, 8(1), 1–14. [Google Scholar]
- Gutleb, AC; Appleman, J; Bronkhorst, M; van den Berg, JHJ; Murk, AJ. Effects of oral exposure to polychlorinated biphenyls (PCBs) on the development and metamorphosis of two amphibian species (Xenopus laevis and Rana temporaria). Sci. Total Env. 2000, 262, 147–157. [Google Scholar]
- Werner, EE. Amphibian metamorphosis: Growth rate, predation rate and optimal size at metamorphosis. Am. Nat. 1986, 128, 319–341. [Google Scholar]
- ASTM (American Society for Testing and Materials). Standard Guide for Conducting the Frog Embryo Teratogenesis Assay—Xenopus(FETAX). 1998; In ASTM E1439-98; Annual Book of ASTM Standards: Philadelphia. [Google Scholar]
- Mably, TA; Moore, RW; Peterson, RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Effects on androgenic status. Toxicol. Appl. Pharmacol. 1992, 114, 97–107. [Google Scholar]
- Mably, TA; Moore, RW; Peterson, RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Effects on sexual behavior and the regulation of luteinizing hormone secretion in adulthood. Toxicol. Appl. Pharmacol. 1992, 114, 108–117. [Google Scholar]
- Sive, HL; Grainger, RM; Harland, RM. Early Development of Xenopus laevis: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, 2000. [Google Scholar]
- Gosner, N. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
- Nieuwkoop, PD; Faber, J. Normal Tables of Xenopus laevis(Daudin); Garland Publishing, Inc.: New York and London, 1994. [Google Scholar]
- Fujita, Y; Ohi, H; Murayama, N; Saguchi, K; Higuchi, S. Molecular cloning and sequence analysis of cDNAs coding for 3-methyl-cholanthreneinducible cytochromes P450 in Xenopus laevis liver. Arch. Biochem. Biophys. 1999, 371, 24–28. [Google Scholar]
- Bollerot, K; Angelier, N; Coumailleau, P. Molecular cloning and embryonic expression of the Xenopus Arnt gene. Mech. Dev. 2001, 108, 227–231. [Google Scholar]
- Rowatt, AJ; DePowell, JJ; Powell, WH. ARNT gene multiplicity in amphibians: Characterization of ARNT2 from the frog Xenopus laevis. J. Exp. Zool. 2003, 300B, 48–57. [Google Scholar]
- Ohi, H; Fujita, Y; Miyao, M; Saguchi, K; Murayama, N; Higuchi, S. Molecular cloning and expression analysis of the aryl hydrocarbon receptor of Xenopus laevis. Biochem. Biophys. Res. Commun. 2003, 307, 595–599. [Google Scholar]
- Beaty, PW; Holscher, MA; Neal, RA. Toxicology of 2,3,7,8-tetra-chlorodibenzo-p-dioxin in larvae and adult forms of Rana catesbeiana. Bull. Environ. Contam. Toxicol 1976, 16, 578–581. [Google Scholar]
- Schuytema, GS; Nebeker, AV; Griffis, WL. Comparative toxicity of Guthion and Guthion 2S to Xenopus laevis and Pseudacris regilla tadpoles. Bull. Environ. Contam. Toxicol. 1995, 54, 382–388. [Google Scholar]
- Schuytema, GS; Nebeker, AV. Comparative toxicity of diuron on survival and growth of Pacific treefrog, bullfrog, red-legged frog and African clawed frog embryos and tadpoles. Arch. Environ. Contam. Toxicol. 1998, 34, 370–376. [Google Scholar]
- Mima, S. Effects of continuous exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) from the cleavage to the larval stageon the development of the Xenopus laevis. Acta. Med. Kinki. Univ. 1997, 22, 217–233. [Google Scholar]
- Travis, J. Variation in growth and survival of Hyla gratiosa larvae in experimental enclosures. Copeia 1983, 1, 232–237. [Google Scholar]
- Richards, SJ; Bull, CM. Size-limited predation on tadpoles of three Australian frogs. Copeia 1990, 1041. [Google Scholar]
- Carey, C; Bryant, CJ. Possible interrelations among environmental toxicants, amphibian development, and decline of amphibian populations. Env. Health Pers. 1995, 103 Supp.4, 13–17. [Google Scholar]
- Korfmacher, WA; Hansen, EB, Jr; Rowland, KL. Tissue distribution of 2,3,7,8-TCDD in bullfrogs obtained from a 2,3,7,8-TCDD–contaminated area. Chemosphere 1986, 15, 121–126. [Google Scholar]
- Young, AL; Cockerham, LG. Fate of TCDD in field ecosystems-Assesment and significance for human exposures. In Dioxins in the Environment; Kamrin, MA, Rodgers, PW, Eds.; Hemisphere Publ. Corp.: New York, 1985; pp. 153–171. [Google Scholar]
- Watson, MR; Stone, WB; Okoniewski, JC; Smith, LM. Wildlife as monitors of the movement of polychlorinated biphenyls and other organochlorine compounds from a hazardous waste site. Trans. Northeast Fish Wildlife Conf. 1985, Hartford, Conneticut. 91–104. [Google Scholar]
- Fontenot, LW; Noble, GP; Atkins, JM; Stephens, MD; Cobb, GP. Bioaccumulation of polychlorinated biphenyls in ranid frogs and northern water snakes from a hazardous waste site and a contaminated watershed. Chemosphere 2000, 40, 803–809. [Google Scholar]
- Cantrell, SM; Joy-Schlezinger, J; Stegeman, JJ; Tillitt, DE; Hannink, M. Correlation of 2,3,7,8-tetrachlorodibenzo-p-diozin-induced apoptotic cell death in the embryonic vasculature with embryotoxicity. Toxicol. Appl. Pharmacol. 1998, 148, 24–34. [Google Scholar]
- Spitsbergen, JM; Walker, MK; Olson, JR; Peterson, RE. Pathologic alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquat. Toxicol. 1990, 19, 41–72. [Google Scholar]
- Elonen, GE; Spehar, RL; Holcombe, G; Johnson, RD; Fernandez, JD; Erickson, RJ; Tietge, JE; Cook, PM. Comparative toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to seven freshwater fish species during early life-stage development. Environ. Toxicol. Chem 1998, 17(3), 472–483. [Google Scholar]
- Relyea, RA; Mills, N. Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles. Proc. Nat. Acad. Sci 2001, 98(5), 2491–2496. [Google Scholar]
© 2008 MDPI All rights reserved.
Share and Cite
Collier, A.; Orr, L.; Morris, J.; Blank, J. The Effects of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) on the Mortality and Growth of Two Amphibian Species (Xenopus laevis and Pseudacris triseriata). Int. J. Environ. Res. Public Health 2008, 5, 368-377. https://doi.org/10.3390/ijerph5050368
Collier A, Orr L, Morris J, Blank J. The Effects of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) on the Mortality and Growth of Two Amphibian Species (Xenopus laevis and Pseudacris triseriata). International Journal of Environmental Research and Public Health. 2008; 5(5):368-377. https://doi.org/10.3390/ijerph5050368
Chicago/Turabian StyleCollier, Alex, Lowell Orr, Julie Morris, and James Blank. 2008. "The Effects of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) on the Mortality and Growth of Two Amphibian Species (Xenopus laevis and Pseudacris triseriata)" International Journal of Environmental Research and Public Health 5, no. 5: 368-377. https://doi.org/10.3390/ijerph5050368




