Preclinical Assessment of Vernonia amygdalina Leaf Extracts as DNA Damaging Anti-cancer Agent in the Management of Breast Cancer
Abstract
:Introduction
Materials and Methods
Chemicals and Media
Vernonia Amygdalina Preparation
Cell Culture
Cell Viability Assay
Cell Treatment for Comet/Genotoxicity Assay
Statistical Analysis
Results
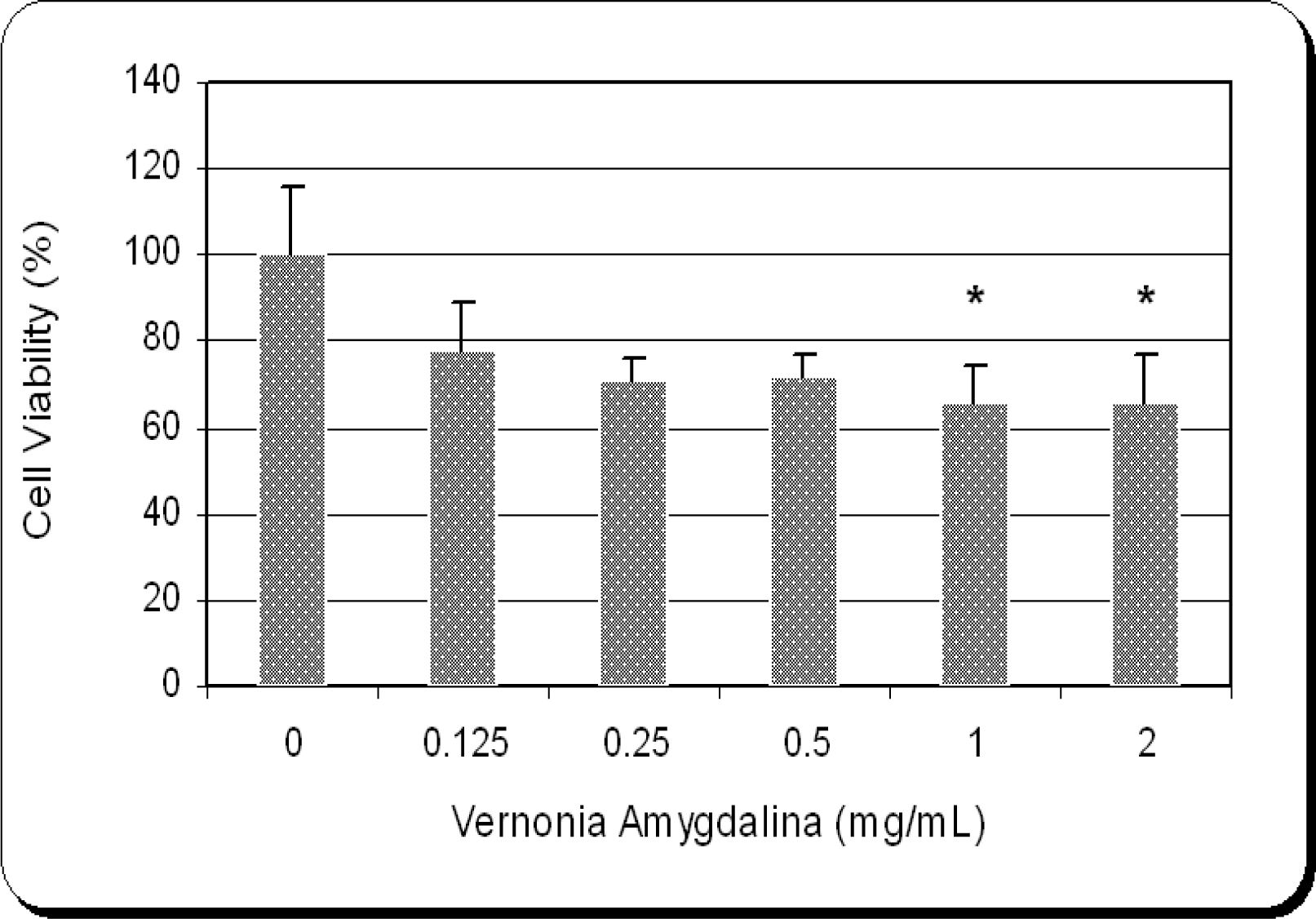
Cytotoxic Efficacy of Vernonia amygdalina
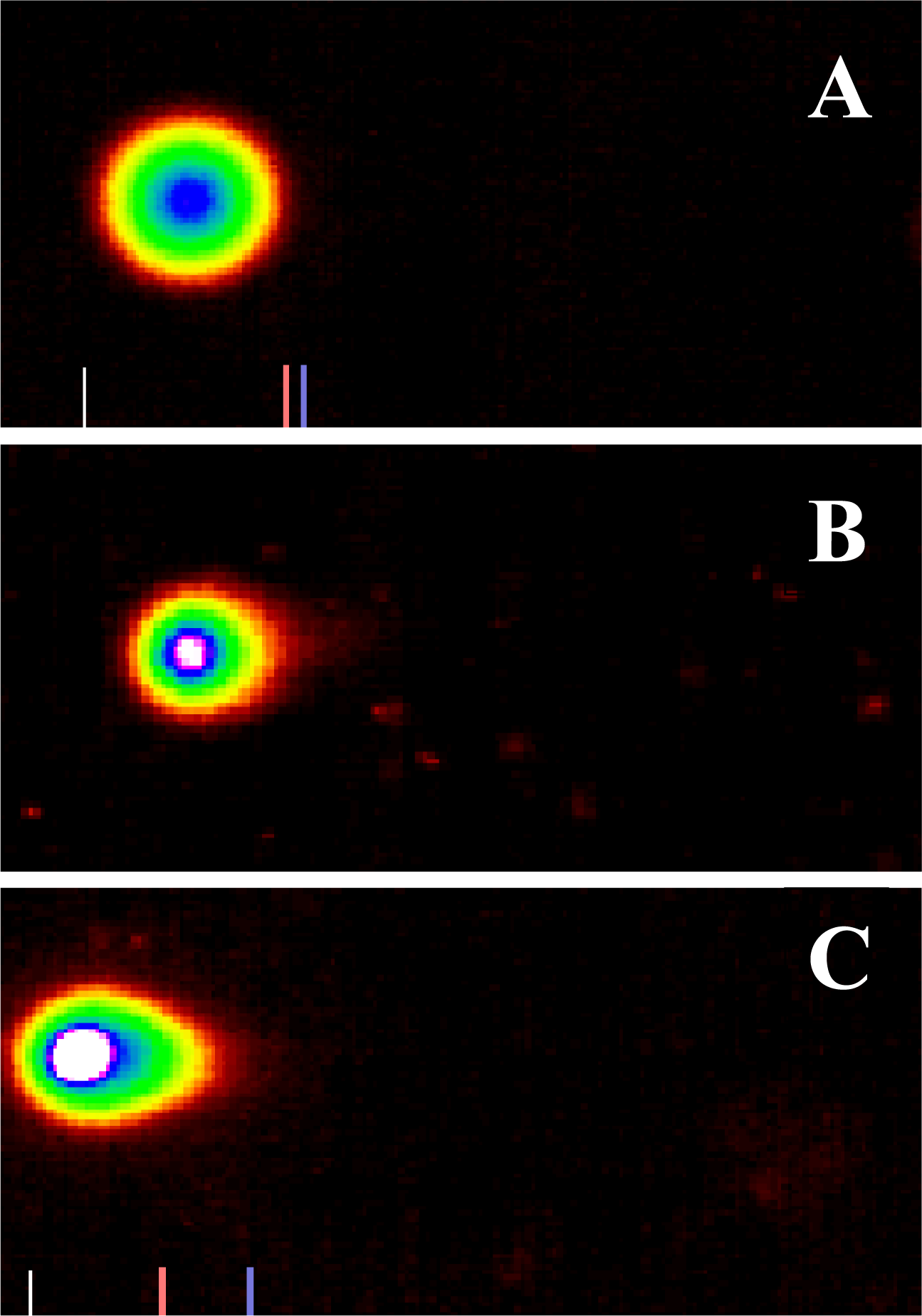
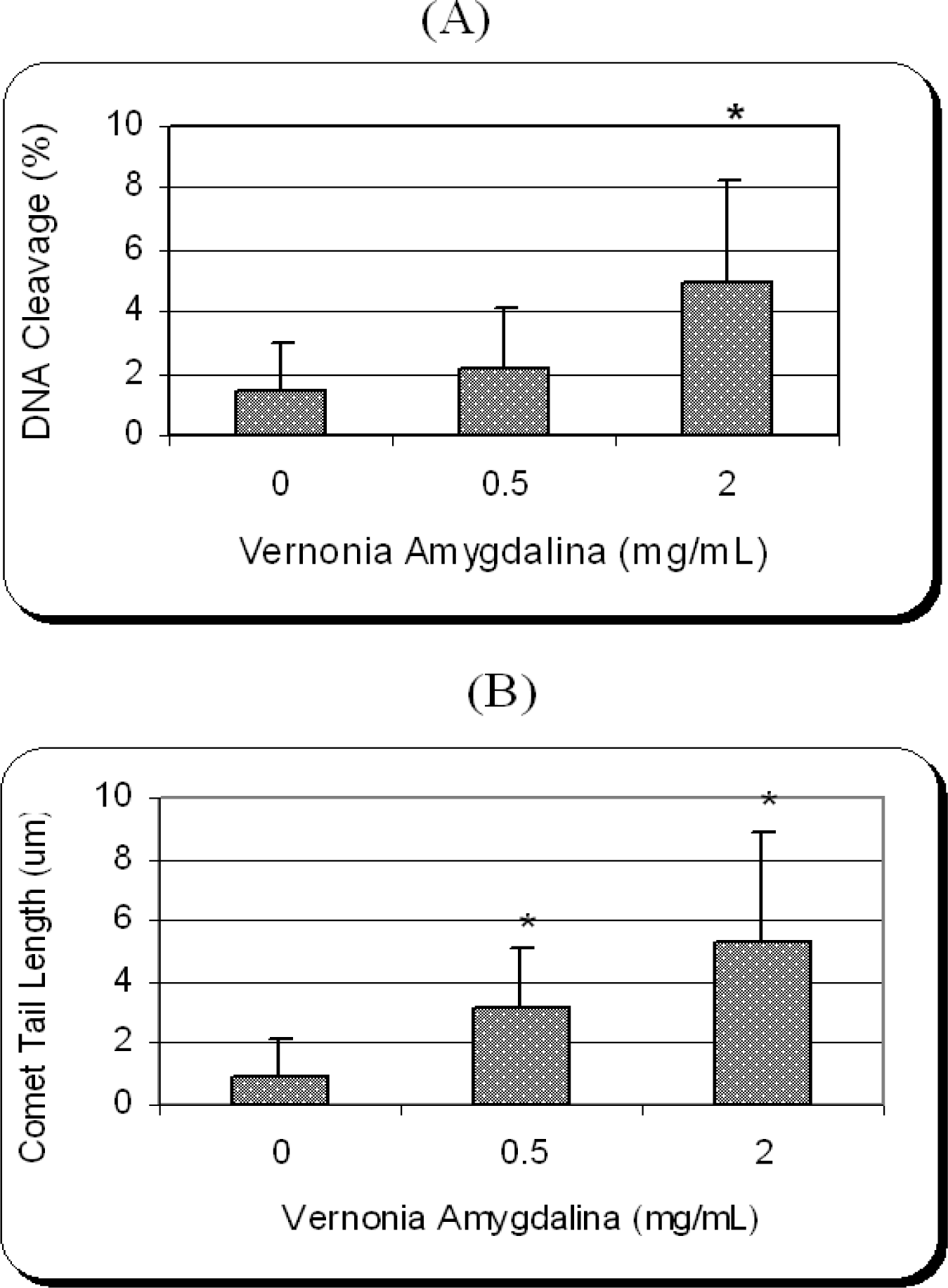
Genotoxic Efficacy of Vernonia amygdalina
Discussions
Cytotoxic Efficacy of Vernonia amygdalina
Genotoxic Efficacy of Vernonia amygdalina
Conclusion
Acknowledgments
References
- Ainslie, JR. List of plants used in native medicine in Nigeria; Imperial Forestry Institute: Oxford, UK, 1973; p. 42. [Google Scholar]
- Burkill, HM. The useful plants of West tropical Africa, 2d ed; Kew; England; Royal Botanical Gardens, 1985; Volume 1. [Google Scholar]
- Izevbigie, EB. Discovery of water-soluble anticancer agents (edotides) from a vegetable found in Benin City, Nigeria. Exp. Biol. Med 2003, 228, 293–298. [Google Scholar]
- Tadesse, A; Gebre-Hiwot, A; Asres, K; Djote, M; Frommel, D. The in vitro activity of Vernonia amygdalina on Leishmania aethiopica. Ethiop. Med. J. 1993, 31, 183–189. [Google Scholar]
- Hamowia, AM; Saffaf, AM. Pharmacological studies on Vernonia amygdalina (Del)and tithonia diversifolia (Gray). Vet. Med. J. 1994, 2, 91–97. [Google Scholar]
- Akah, PA; Okafor, CI. Hypoglycaemic effect of vernonia amygdalina Del, in experimental rabbits. Plant Med. Res. 1992, 1, 6–10. [Google Scholar]
- Onwuka, CFI; Akinsoyinu, AO; Tewe, OO. Feed value of some Nigerian browse plants: chemical composition and in vitro digestibility. East African Agriculture and Forestry J 1989, 54, 157–163. [Google Scholar]
- Aregheore, EMK; Makkar, HPS; Becker, K. Feed value of some browse plants from the central zone of Delta State. Nig. Trop. Sci. 1998, 38, 97–104. [Google Scholar]
- Iwalokun, BA. Enhanced antimalarial effects of chloroquine by aqueous Vernonia amygdalina leaf extract in mice infected with chloroquine resistant sensitive. Plasmodium berghei strains. Afr. Health Sci 2008, 8, 25–35. [Google Scholar]
- Ijeh, L; Nwugo, VO; Obidoa, O. Comparative studies on the nutritive Phyto-chemical antimicrobial and properties of two varieties of Vernomia amygdalina. Plant Pros. Res. Comm 1996, 1, 71–75. [Google Scholar]
- Babalola, OO; Anetor, JI; Adeniyi, FA. Amelioration of carbon tetrachlorine-induced hepatotoxicity by terpenoid extract leaves of Vernonia amygdalina. Afr. J. Med. Sci 2001, 30, 91–3. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth survival: applications to proliferation and cytotoxicity assays. J. Immunol. Method 1983, 65, 55–63. [Google Scholar]
- Tchounwou, PB; Yedjou, CG; Dorsey, WC. Arsenic Trioxide-Induced Transcriptional Activation and Expression of Stress Genes in Human Liver Carcinoma Cells (HepG2). Cell. Mol. Biol. 2003, 49, 1071–1079. [Google Scholar]
- Yedjou, CG; Rogers, C; Brown, E; Tchounwou, PB. Differential effect of ascorbic acid and n-acetyl-l-cysteine on arsenic trioxide mediated oxidative stress in human leukemia (HL-60) cells. J. Biochem. Mol. Toxicol 2008, 22, 85–92. [Google Scholar]
- Adaramoye, O; Ogyngbenro, B; Anyaegou, O; Fafunso, M. Protective effects of extracts of vernonia amygdalina hisbicus. sabdaiffa and vitamin C against radiation-induced liver damage in rats. J. Radiat. Res. 2008, 49, 123–131. [Google Scholar]
- Tekobo, AM; Onabanjo, AO; Amole, OO; Emeka, PM. Analgesic antipyretic effects of the aqueous extract of Vernonia amygdalina. West Afri. J. Pharm 2002, 16, 68–74. [Google Scholar]
- Hsiang, YH; Lihou, MG; Liu, LF. Arrest of replication forks by drug-stabilized topoisomerase IDNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989, 49, 5077–5082. [Google Scholar]
- Zhang, H; D’Arpa, P.; Liu, LF. A model for tumor cell killing by topoisomerase poisons. Cancer Cells. 1990, 2, 23–27. [Google Scholar]
- Toxicological profile for arsenic (update); Agency for Toxic Substances and Disease Registry: Atlanta, GA, 1999.
- Wei, M; Wanibuchi, H.; Yamamoto, S; Li, W.; Fukushima, S. Urinary bladder carcinogenicity of dimethylarsinic acid in male F344 rats. Carcinogenesis. 1999, 20, 1873–1876. [Google Scholar]
- Yedjou, CG; Tchounwou, PB. In vitro cytotoxic genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT alkaline single cell gel electrophoreis (comet) assays. Mol Cell Biochem 2007, 301, 123–130. [Google Scholar]
© 2008 MDPI All rights reserved.
Share and Cite
Yedjou, C.; Izevbigie, E.; Tchounwou, P.B. Preclinical Assessment of Vernonia amygdalina Leaf Extracts as DNA Damaging Anti-cancer Agent in the Management of Breast Cancer. Int. J. Environ. Res. Public Health 2008, 5, 337-341. https://doi.org/10.3390/ijerph5050337
Yedjou C, Izevbigie E, Tchounwou PB. Preclinical Assessment of Vernonia amygdalina Leaf Extracts as DNA Damaging Anti-cancer Agent in the Management of Breast Cancer. International Journal of Environmental Research and Public Health. 2008; 5(5):337-341. https://doi.org/10.3390/ijerph5050337
Chicago/Turabian StyleYedjou, Clement, Ernest Izevbigie, and Paul B. Tchounwou. 2008. "Preclinical Assessment of Vernonia amygdalina Leaf Extracts as DNA Damaging Anti-cancer Agent in the Management of Breast Cancer" International Journal of Environmental Research and Public Health 5, no. 5: 337-341. https://doi.org/10.3390/ijerph5050337



