Responses of Antioxidant Enzymes in Catfish Exposed to Liquid Crystals from E-Waste
Abstract
:Introduction
Materials and Methods
Test Animals
Liquid Crystal Stock Solution
Fish Food
Exposure Test
Sample Preparation
Sample Analysis
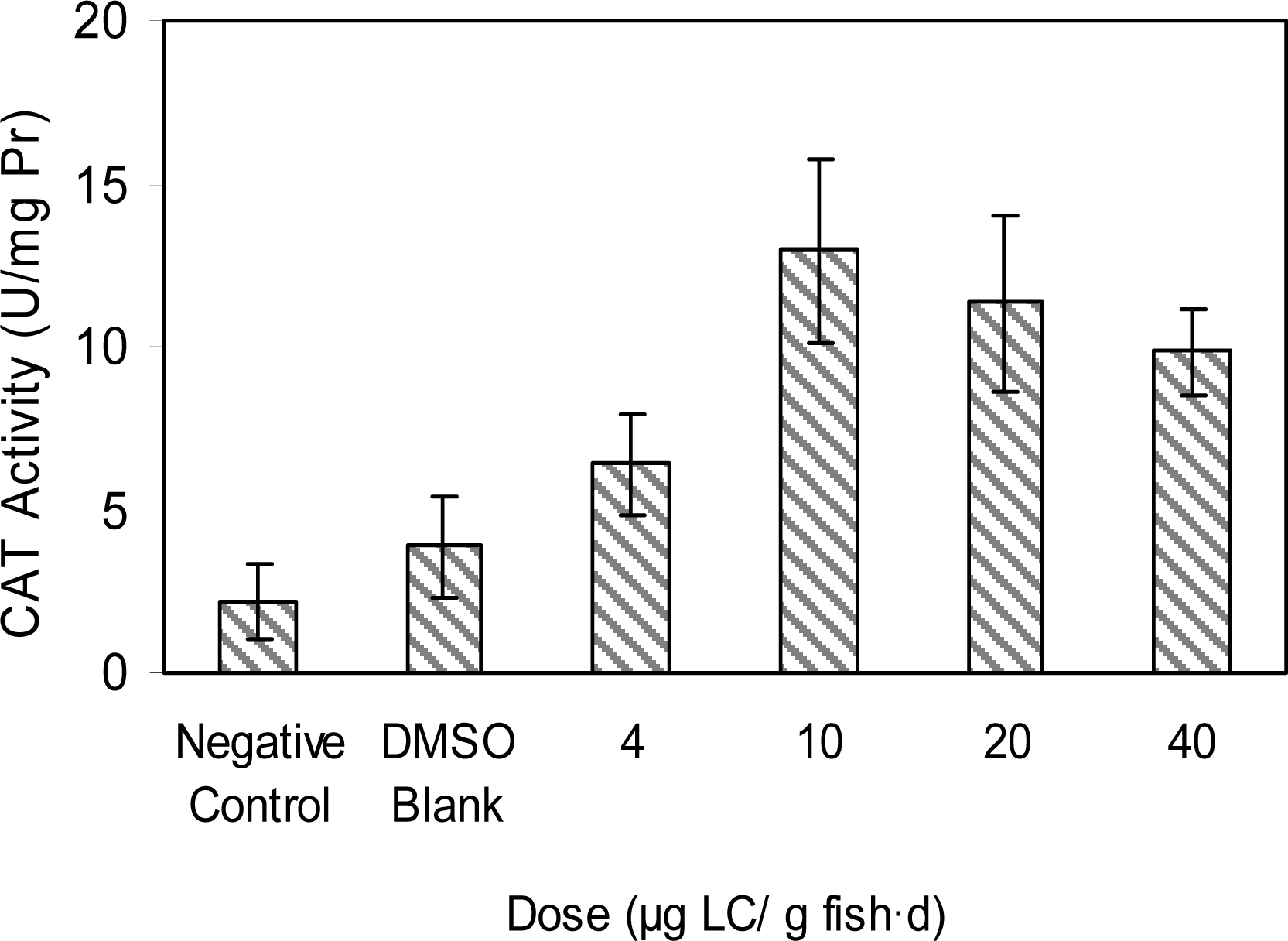
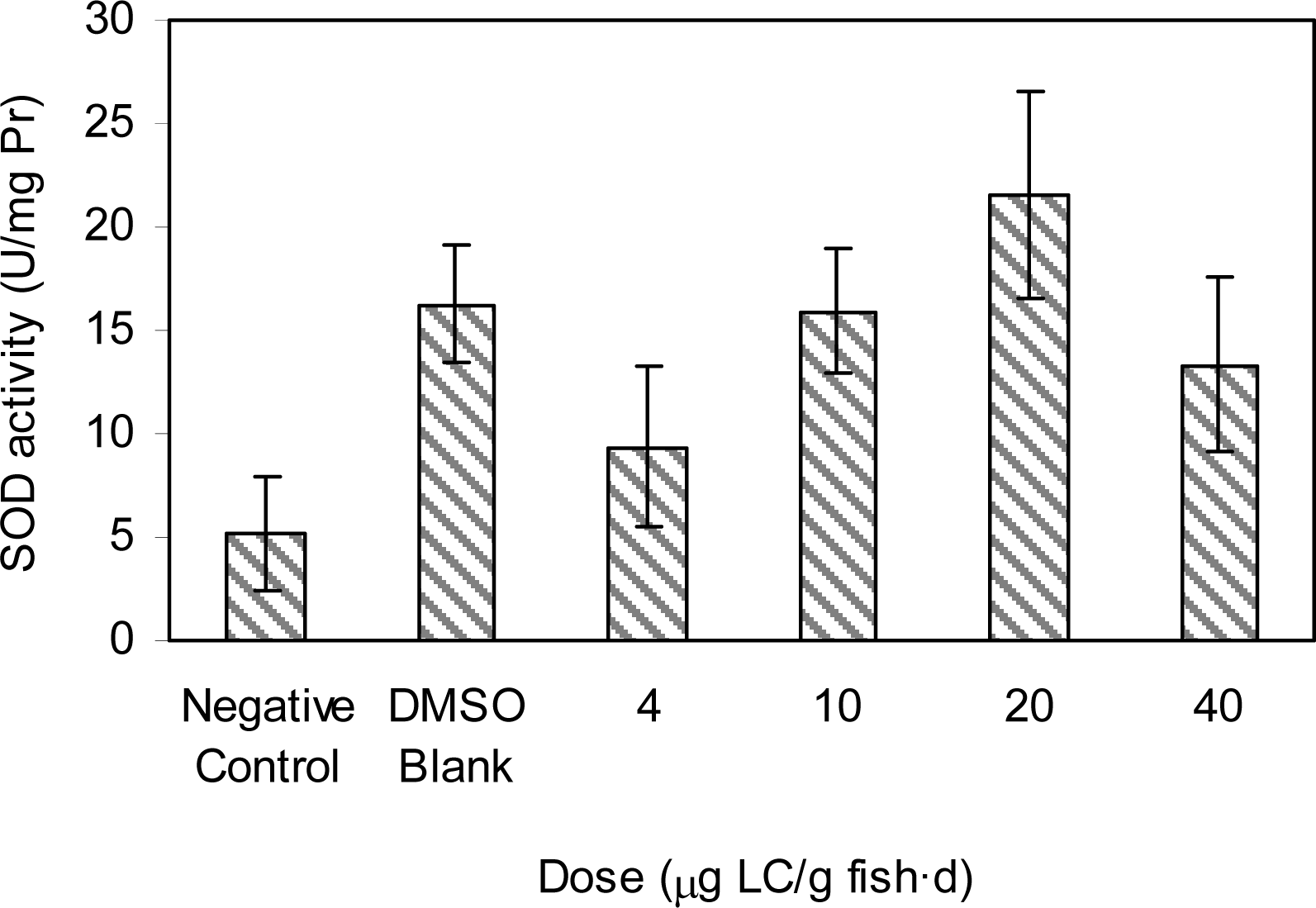
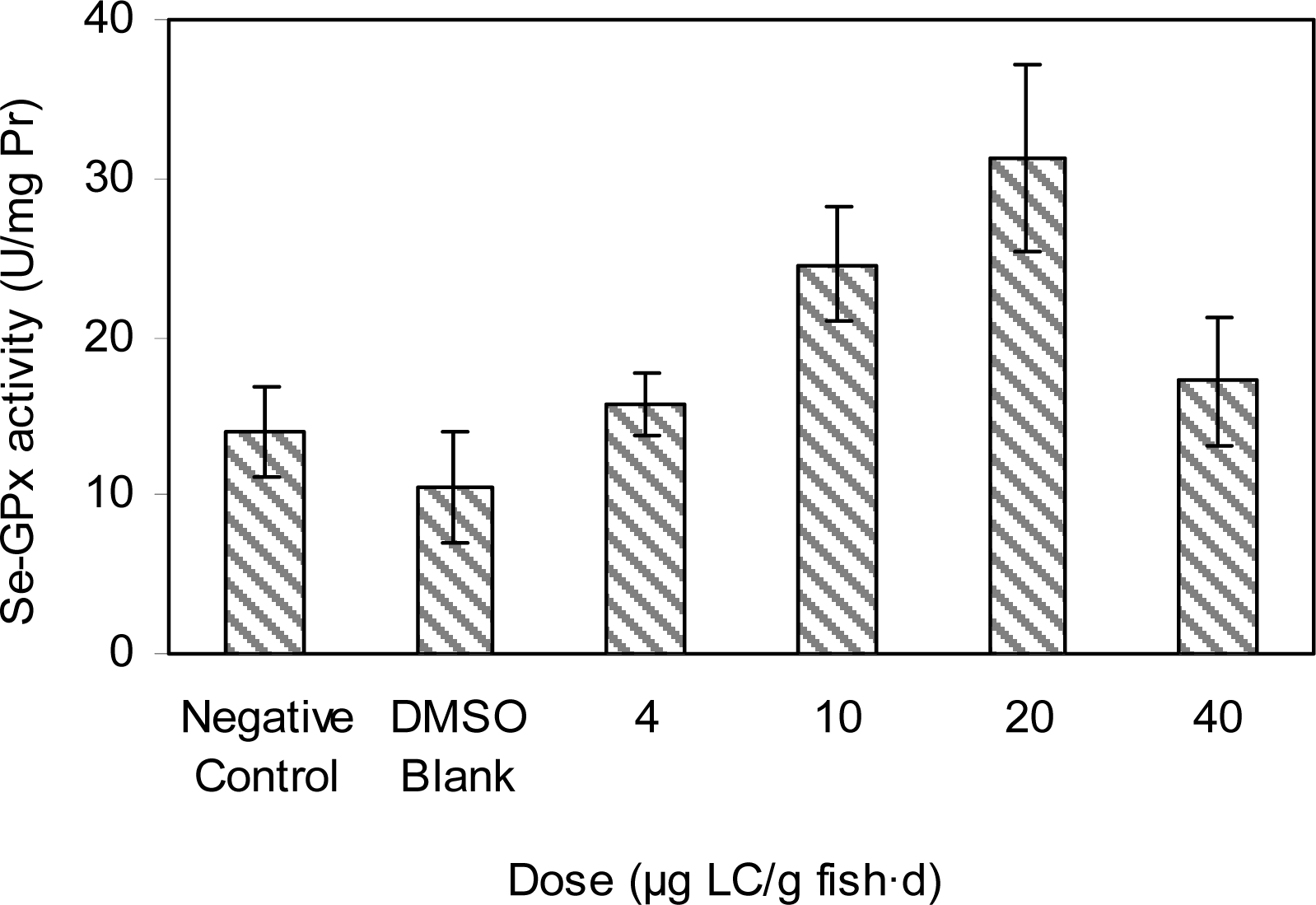
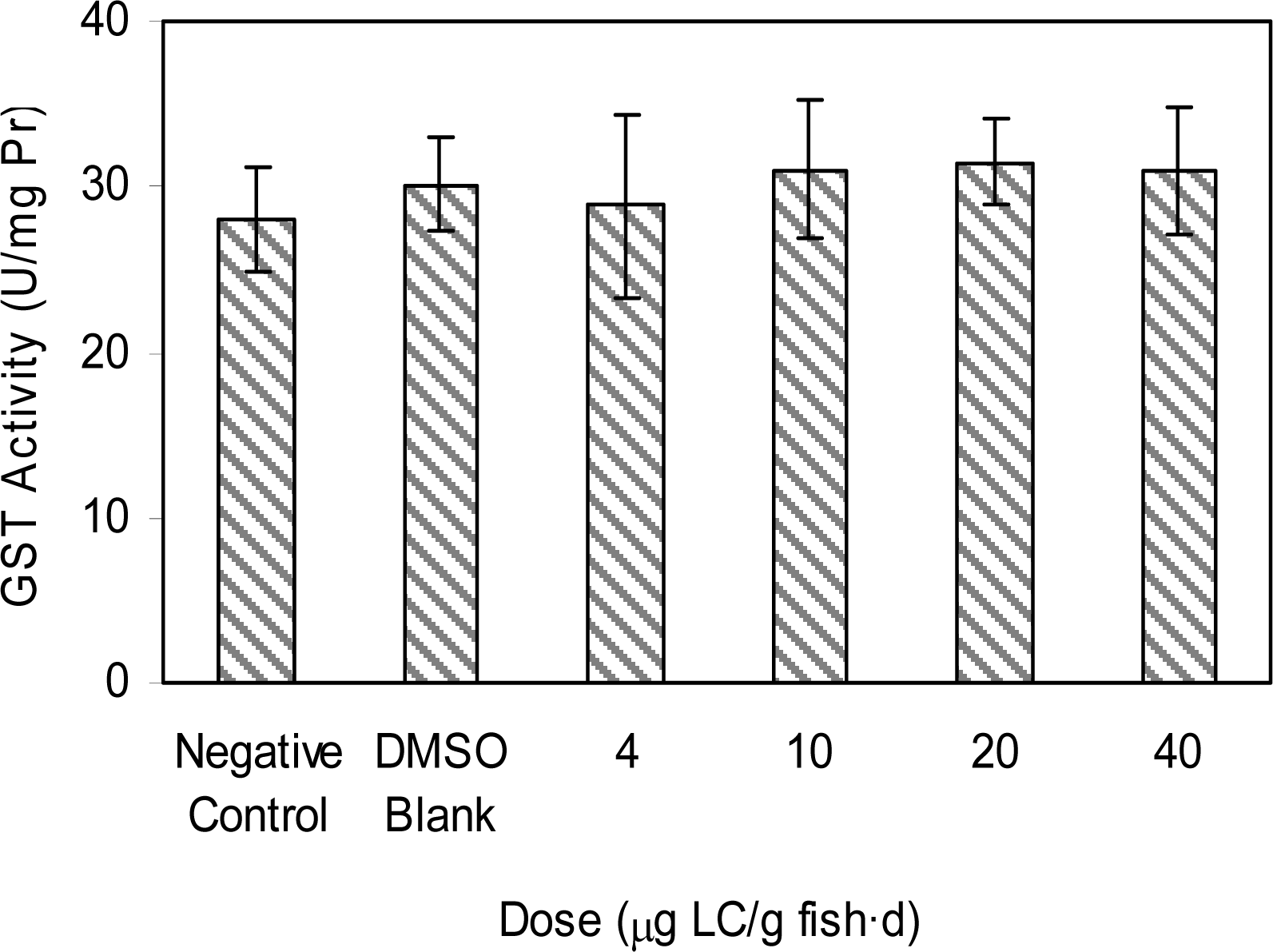
Results and Discussion
Doses of Test Groups
Conclusions
Acknowledgments
References
- Werner, B; Simon-Hettich, B; Hoenicke, P. Toxicological and Ecotoxicological Investigations of Liquid Crystals. MERCK. http://www.MERCK.com. 2002.
- Geracitano, L; Monserrat, JM; Bianichini, A. Physiological and antioxidant enzyme responses to acute and chronic exposure of Laeonereis acuta (Polychaeta Nereididae) to copper. J Exp Marine Biol Ecol 2002, 277, 145–156. [Google Scholar]
- Webb, NA; Shaw, JR; Moran, J; Hogstrand, C; Wood, CM. Acute and Chronic physiological effects of silver exposure in three marine teleosts. Aquat Toxicol 2001, 54, 161–178. [Google Scholar]
- Ronisz, D; Finne, EF; Karlsson, H; Forlin, L. Effects of HBCDD and TBBPA on hepatic enzymes and other biomarkers in juvenile rainbow trout and feral eelpout. Aquat Toxicol 2004, 69, 229–245. [Google Scholar]
- Frei, B. Molecular and biological mechanisms of antioxidant action. FASEB J 1999, 13, 963–964. [Google Scholar]
- Di Guilio, RT; Washburn, PC; Wenning, RJ; Winston, GW; Jewell, CS. Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem 1989, 8, 1103–1123. [Google Scholar]
- Storey, KB. Oxidative stress: animal adaptations in nature. Braz. J Med Biol Res 1996, 29, 1715–1733. [Google Scholar]
- Zhang, J; Shen, H; Wang, X; Wu, J; Xue, Y. Effects of chronic exposure of 2,4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemoshpere 2004, 55, 167–174. [Google Scholar]
- Pedrajas, JR; Lopez-Barea, J; Peinado, J. Dieldrin induces peroxisomal enzymes in fish (sparus aurata) liver. Comp Biochem Physiol 1996, 115C, 125–131. [Google Scholar]
- Wijeyaratne, WMDN; Pathiratne, A. Acetylcholinesterase inhibition and gill lesions in Rasbora caverii, and indigenous fish inhabiting ric field associated waterbodies in Sri Lanka. Ecotoxicology 2006, 15, 609–619. [Google Scholar]
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72, 248–254. [Google Scholar]
- Xu, J; Yuan, X; Lang, P. Determination of catalase activity and catalase inhibition by ultraviolet spectrophotometry. Chin Environ Chem 1997, 16, 73–76. [Google Scholar]
- Zou, G; Gui, X; Zhong, X; Zhu, R. A new method for SOD activity measurement. Prog Biochem Biophy (in Chinese). 1986, 4, 71–73. [Google Scholar]
- Hafeman, DG; Sunde, RA; Hoekstra, WG. Effect of dietary selenium and erythrocyte and liver glutathione peroxidase in the rat. J Nutrit 1973, 104, 580. [Google Scholar]
- Habig, WH; Pabst, MJ; Jakoby, WB. Glutathione S-transferases: the first step in mercapturic acid formation. J Biol Chem 1974, 249, 7130–7139. [Google Scholar]
- Rendon-von Osten, J; Ortiz-Arana, A; Guilhermino, L; Soares, A. In vivo evaluation of three biomarkers in the mosquitofish exposed to pesticides. Chemosphere 2005, 58, 627–636. [Google Scholar]
- Van Erp, S; Brooth, L; Gooneratne, R; O’Halloran, K. Sublethal responses of wolf spiders to organophosphorous insecticides. Environ Toxicol 2002, 7, 449–456. [Google Scholar]
© MDPI. All rights reserved
Share and Cite
An, R.; Li, Y.; Niu, X.; Yu, H. Responses of Antioxidant Enzymes in Catfish Exposed to Liquid Crystals from E-Waste. Int. J. Environ. Res. Public Health 2008, 5, 99-103. https://doi.org/10.3390/ijerph5020099
An R, Li Y, Niu X, Yu H. Responses of Antioxidant Enzymes in Catfish Exposed to Liquid Crystals from E-Waste. International Journal of Environmental Research and Public Health. 2008; 5(2):99-103. https://doi.org/10.3390/ijerph5020099
Chicago/Turabian StyleAn, Ran, Yadong Li, Xiaojun Niu, and Hongtao Yu. 2008. "Responses of Antioxidant Enzymes in Catfish Exposed to Liquid Crystals from E-Waste" International Journal of Environmental Research and Public Health 5, no. 2: 99-103. https://doi.org/10.3390/ijerph5020099



