Assessing Interactions of Multiple Agrichemicals by Using Bacterial Assemblages in a Wetland Mesocosm System
Abstract
:Introduction
Materials and Methods
Description of Agrichemicals
Experimental Design and Sampling
Microbial Biomass and Activity Measurements
Statistical Analysis
Results and Discussion
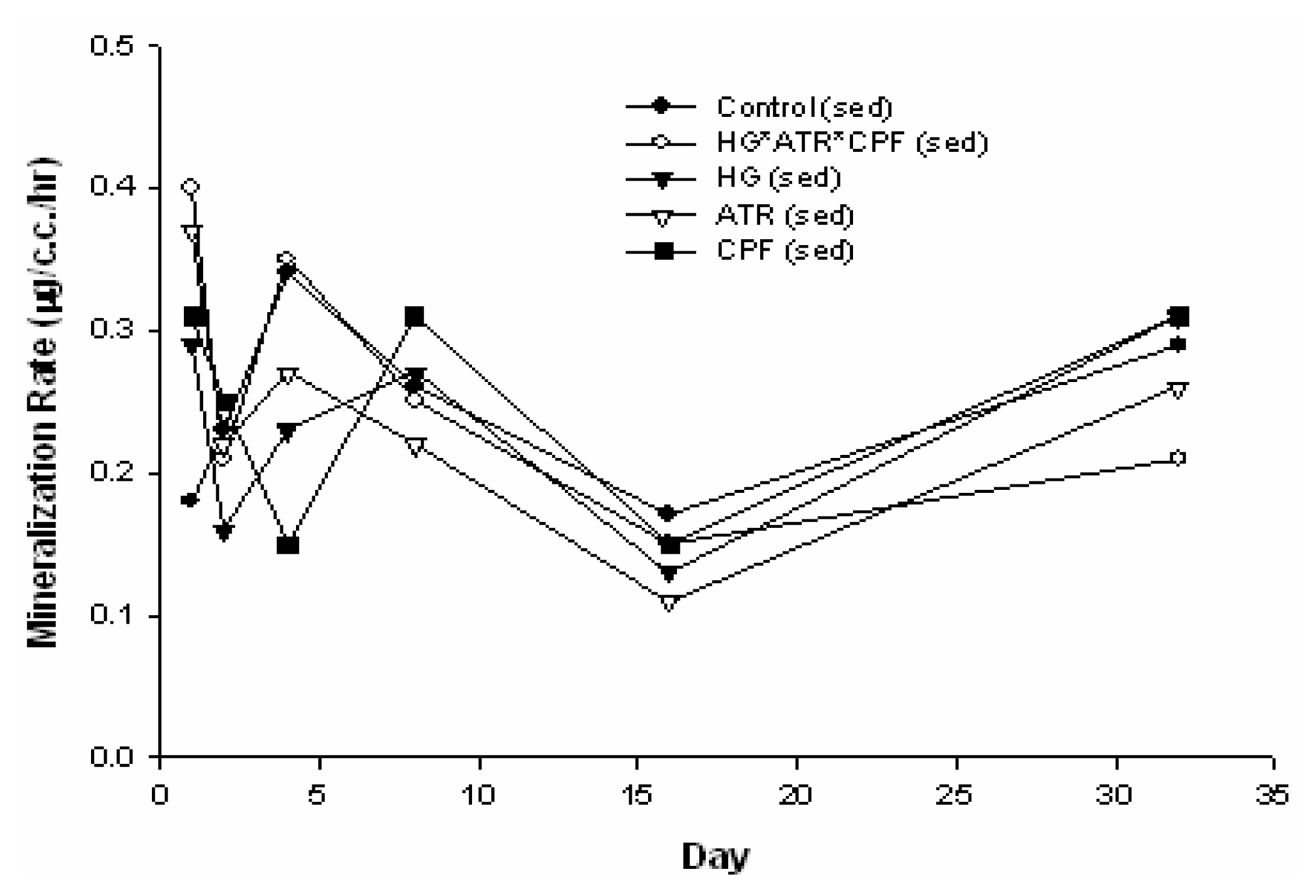
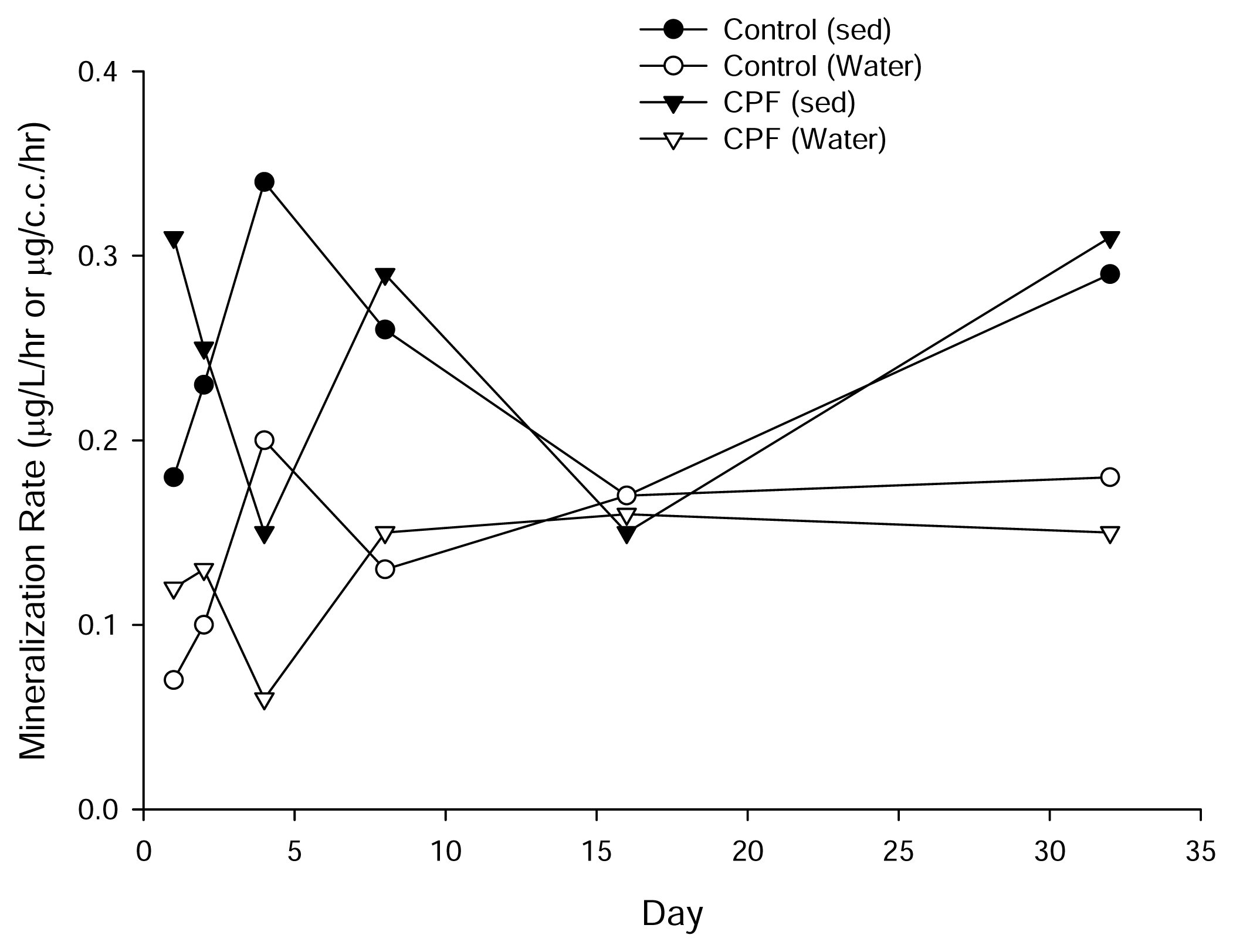
| Source | DF | Type III SS | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| HG | 1 | 0.003 | 0.003 | 0.26 | 0.62 |
| ATR | 1 | 0.006 | 0.006 | 0.43 | 0.52 |
| HG*ATR | 1 | 0.002 | 0.002 | 0.15 | 0.70 |
| AS | 1 | 0.000 | 0.000 | 0.00 | 0.99 |
| HG*AS | 1 | 0.024 | 0.024 | 1.82 | 0.18 |
| ATR*AS | 1 | 0.015 | 0.015 | 1.09 | 0.30 |
| HG*ATR*AS | 1 | 0.008 | 0.008 | 0.56 | 0.46 |
| CPF | 1 | 0.007 | 0.007 | 0.49 | 0.49 |
| HG*CPF | 1 | 0.000 | 0.000 | 0.01 | 0.94 |
| ATR*CPF | 1 | 0.015 | 0.015 | 1.10 | 0.30 |
| HG*ATR*CPF | 1 | 0.054 | 0.054 | 4.06 | 0.05* |
| AS*CPF | 1 | 0.014 | 0.014 | 1.02 | 0.32 |
| HG*AS*CPF | 1 | 0.000 | 0.000 | 0.03 | 0.86 |
| ATR*AS*CPF | 1 | 0.037 | 0.037 | 2.73 | 0.10 |
| HG*ATR*AS*CPF | 1 | 0.007 | 0.007 | 0.53 | 0.47 |
| Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| HG | ATR | CPF | Day 1 | Day 2 | Day 4 | Day 8 | Day 16 | Day 32 | Overall |
| 1 | 1 | 1 | 0.59 | 0.37 | 0.45 | 0.42 | 0.25 | 0.37 | 0.41 |
| 1 | 1 | −1 | 0.21 | 0.40 | 0.47 | 0.29 | 0.23 | 0.39 | 0.33 |
| 1 | −1 | 1 | 0.39 | 0.40 | 0.39 | 0.53 | 0.23 | 0.47 | 0.40 |
| 1 | −1 | −1 | 0.50 | 0.37 | 0.36 | 0.44 | 0.25 | 0.31 | 0.37 |
| −1 | 1 | 1 | 0.26 | 0.30 | 0.43 | 0.43 | 0.26 | 0.43 | 0.35 |
| −1 | 1 | −1 | 0.57 | 0.41 | 0.41 | 0.39 | 0.17 | 0.34 | 0.38 |
| −1 | −1 | 1 | 0.58 | 0.42 | 0.30 | 0.51 | 0.25 | 0.51 | 0.43 |
| −1 | −1 | −1 | 0.40 | 0.41 | 0.49 | 0.47 | 0.26 | 0.42 | 0.41 |
| 3-way Effect Size | 0.25 | 0.02 | −0.06 | 0.01 | −0.01 | −0.04 | 0.03 | ||
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| CPF | Day 1 | Day 2 | Day 4 | Day 8 | Day 16 | Day 32 | Overall |
| Present | 0.133 | 0.13 | 0.13 | 0.124 | 0.163 | 0.173 | 0.145 |
| Absent | 0.091 | 0.128 | 0.128 | 0.136 | 0.18 | 0.135 | 0.136 |
| Effect Size | 0.041 | 0.003 | 0.003 | −0.013 | −0.018 | 0.034 | 0.008 |
Acknowledgments
References
- Newton, R. B. The effects of stormwater surface runoff on freshwater wetlands: a review of the literature and annotated bibliography; University of Massachusetts: Amherst, MA, 1989. [Google Scholar]
- Olson, R. K. (Ed.) Created and natural wetlands for controlling nonpoint source pollution; U.S. EPA, Office of Research and Development and Office of Wetlands, Oceans, and Watersheds, C.K. Smoley, c/o CRC Press, Inc: Boca Raton, FL, 1993.
- Hwang, H.-M.; Hodson, R. E.; Lee, R. F. Photochemical and microbial degradation of 2,4, 5-trichloroaniline in a freshwater lake. Appl. Environ. Microbiol 1986, 50, 1177–1180. [Google Scholar]
- Hughes, M. N.; Poole, R. K. Metals and micro-organisms; Chapman and Hall: New York, 1989. [Google Scholar]
- Collins, Y. E.; Stotzky, G. Factors affecting the toxicity of heavy metals to microbes. Beveridge, T.J., Doyle, R. J., Eds.; In Metals and Bacteria; John Wiley & Sons: New York, 1989; pp. 31–90. [Google Scholar]
- Hwang, H.-M.; Maloney, S. W. A study of microbial transformations of trichloroaniline and p-Cresol using size fractionation technique. Bull. Environ. Contam. Toxicol 1996, 56, 343–350. [Google Scholar]
- Hwang, H.-M.; Hodson, R. E.; Lewis, D. L. Assessing interactions of organic compounds during biodegradation of complex waste mixtures by naturally occurring bacterial assemblages. Environ. Toxicol. & Chem 1989, 8, 209–214. [Google Scholar]
- Rodriguez, C. J.; Harkin, J. M. Degradation of atrazine in subsoils and groundwater mixed with aquifer sediments. Bull. Environ. Contam. Toxicol 1997, 59, 728–735. [Google Scholar]
- Solomon, K. R.; Baker, D. B.; Richards, R. P.; Dixon, K. R.; Klaine, S. J.; La Point, T. W.; Kendall, R. J.; Weisskopfs, C. P.; Giddings, J. M.; Giesy, J. P.; Hall, L. W., Jr.; Williams, W. M. Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem 1996, 15, 31–76. [Google Scholar]
- U.S. Geological Survey. Pesticides in streams of the U.S. - Initial results. In WRIR 98–4222; Sacramento, CA, 1999. [Google Scholar]
- U.S. Environmental Protection Agency, National primary drinking water regulations; EPA 816-F-02-013; Office of Water: Washington, DC, 2002.
- Schulz, A.; Wengenmayer, F.; Goodman, H. M. Genetic engineering of herbicide resistance in higher plants. Plant Sci 1990, 9, 1–15. [Google Scholar]
- De Lorenzo, M. E.; Scott, G. I.; Ross, P. E. Toxicity of pesticides to aquatic microorganisms: A Review. Environ. Toxicol. Chem 2001, 20, 84–98. [Google Scholar]
- Bolle, P.; Mastrangelo, S.; Tucci, P.; Evandri, M.G. Clastogenicity of atrazine assessed with the Allium cepa test. Environ. Mol. Mutagen 2004, 43, 137–141. [Google Scholar]
- Environmental Working Group (EWG). New EPA study elevates cancer rating for top U.S. weed killer. 2000. http://www.ewg.org/pub/home/reports/atrazine/atrazine.html.
- Winkelmann, D. A.; Klaine, S. J. Atrazine metabolite behavior in soil-core microcosms: formation, disappearance, and bound residues. In Pesticide transformation products: fate and significance in the environment; Somasundaram, L., Coats, J. R., Eds.; American Chemical Society: Washington, DC, 1991; pp. 74–92. [Google Scholar]
- Berg, G. L. (Ed.) Farm Chemicals Handbook; Willoughby, OH; Meister Publishing Company, 1986.
- U.S. Environmental Protection Agency, Registration Standard (2nd round review) for the Reregistration of Pesticide Products Containing Chlorpyrifos; Office of Pesticide Programs, US EPA: Washington, DC, June 1989.
- Somasundaram, L.; Coats, J. R. Influence of pesticide metabolites on the development of enhanced biodegradation. In Enhanced biodegradation of pesticides in the environment; Racke, K. D., Coats, J. R., Eds.; American Chemical Society: Washington, DC, 1990; pp. 128–140. [Google Scholar]
- Howard, P. H. (Ed.) Handbook of Environmental Fate and Exposure Data for Organic Chemicals. Lewis Publishers: Chelsea, MI; In Pesticides; 1989; Volume III.
- Racke, K. D. The environmental fate of chlorpyrifos. Rev. Environ. Contam. Toxicol 1992, 131, 150–158. [Google Scholar]
- Moore, M. M.; Harrington-Brock, K.; Doerr, C. L. Genotoxicity of arsenic and its methylated metabolites. Chap in Arsenic Exposure and Health. Science and Technology Letters 1994. [Google Scholar]
- Beveridge, T. J.; Doyle, R. J. (Eds.) Metal ions and bacteria; John Wiley & Sons: New York, 1989.
- Lyman, W. J. Transport and transformation processes. In Fundamentals of Aquatic Toxicology, 2nd ed; Rand, G. M., Ed.; Taylor &Francis: Washington, DC, 1995; pp. 449–492. [Google Scholar]
- Chang, L. W. (Ed.) Toxicology of Metals; CRC Press: Boca Raton, FL, 1996.
- Atlas, R. M.; Bartha, R. Microbial Ecology: Fundamentals and Applications, 3rd ed; The Benjamin Cummings Publishing Company, Inc: Redwood City, California, 1993. [Google Scholar]
- Britson, C. A.; Threlkeld, S. T. Abundance, metamorphosis, developmental, and behavioral abnormalities in Hyla chrysoscelis Tadpoles following exposure to three agrichemicals and methyl mercury in outdoor mesocosms. Bull. Environ. Contam. Toxicol 1998, 61, 154–161. [Google Scholar]
- Hobbie, J. E.; Daley, R. J.; Jasper, S. Use of Nucleopore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol 1977, 33, 1225–1228. [Google Scholar]
- SAS Institute, Inc, SAS/STAT User’s Guide; Release 6.03 Ed; Cary, NC, 1988.
- Hwang, H.-M.; Balarezo, A. L.; Jones, V. N.; Yu, H. Effect of river humic acid on 1-aminopyrene ecotoxicity in a dynamic solar photolysis process. Bull. Environ. Contam. & Toxicol 2004, 72, 1059–1066. [Google Scholar]
- Landis, W. G.; Yu, M.-H. (Eds.) Introduction to environmental toxicology: impacts of chemicals upon ecological systems; Lewis Publishers: Boca Raton, FL, 1995.
- Marrs, K. A. The function and regulation of glutathione s-transferases in plants. Ann. Rev. Plant Physiol. and Plant Molecul. Biol 1996, 47, 127–158. [Google Scholar]
© 2005 MDPI. All rights reserved.
Share and Cite
Hwang, H.-M.; McArthur, N.; Ochs, C.; Libman, B. Assessing Interactions of Multiple Agrichemicals by Using Bacterial Assemblages in a Wetland Mesocosm System. Int. J. Environ. Res. Public Health 2005, 2, 328-334. https://doi.org/10.3390/ijerph2005020019
Hwang H-M, McArthur N, Ochs C, Libman B. Assessing Interactions of Multiple Agrichemicals by Using Bacterial Assemblages in a Wetland Mesocosm System. International Journal of Environmental Research and Public Health. 2005; 2(2):328-334. https://doi.org/10.3390/ijerph2005020019
Chicago/Turabian StyleHwang, Huey-Min, Neisee McArthur, Clifford Ochs, and Bruce Libman. 2005. "Assessing Interactions of Multiple Agrichemicals by Using Bacterial Assemblages in a Wetland Mesocosm System" International Journal of Environmental Research and Public Health 2, no. 2: 328-334. https://doi.org/10.3390/ijerph2005020019




