Photochemical Reaction of 7,12-Dimethylbenz[a]anthracene (DMBA) and Formation of DNA Covalent Adducts
Abstract
:Introduction
Materials and Methods
Materials
Light Source
Photo-Irradiation of DMBA
Photo-Irradiation of DMBA, 7-HOCH2-12-MBA, 12-HOCH2-7-MBA, 7-CHO-12-MBA, and 12-CHO-7-MBA in the Presence of Calf thymus DNA
Reaction of DMBA Metabolites with Calf Thymus DNA and Analysis of DNA Adducts by 32P-postlabeling/TLC
Instrumentation
Results
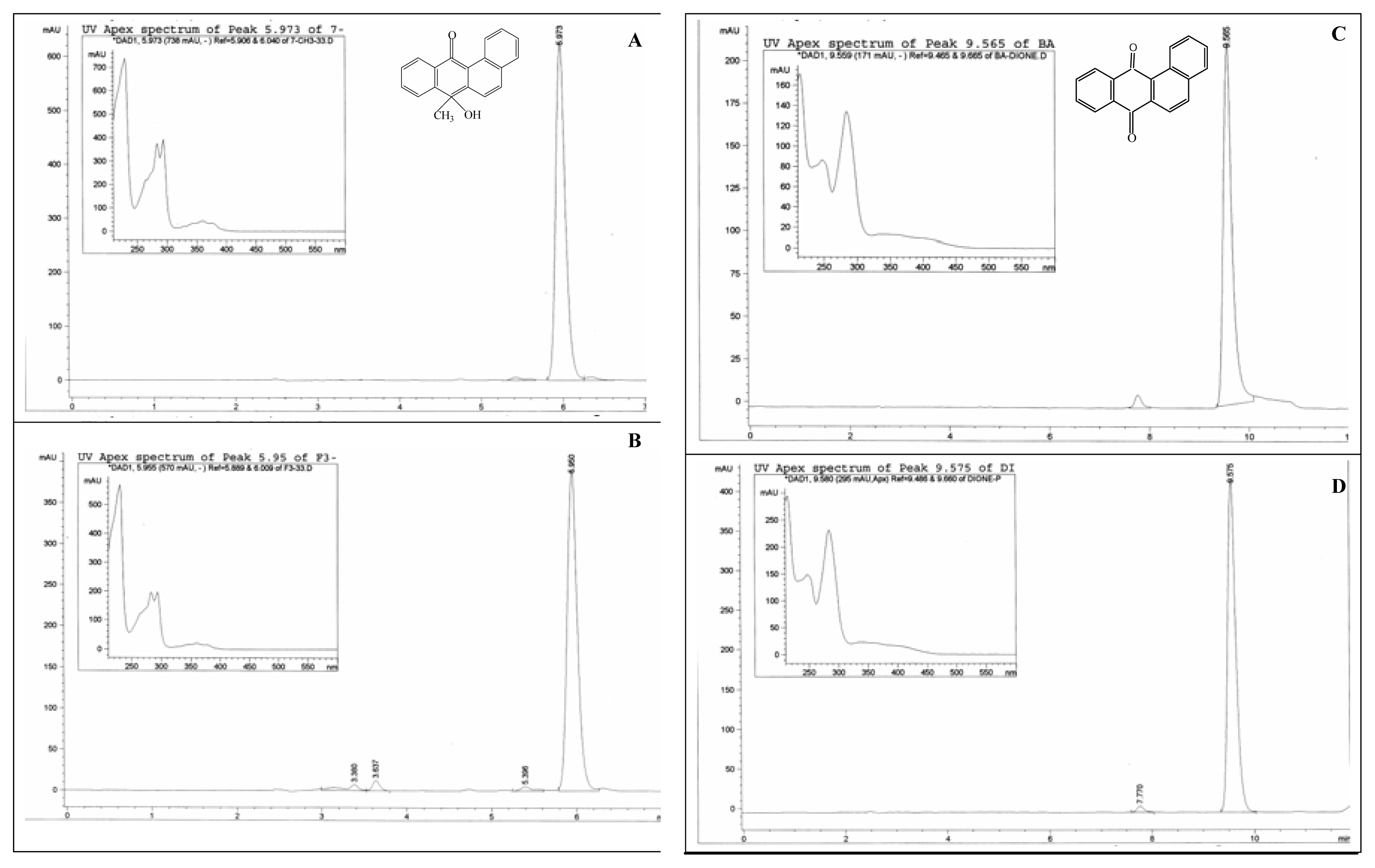
DMBA Photoproduct Analysis
Kinetics for the Photodecomposition of DMBA and Photoproduct Formation
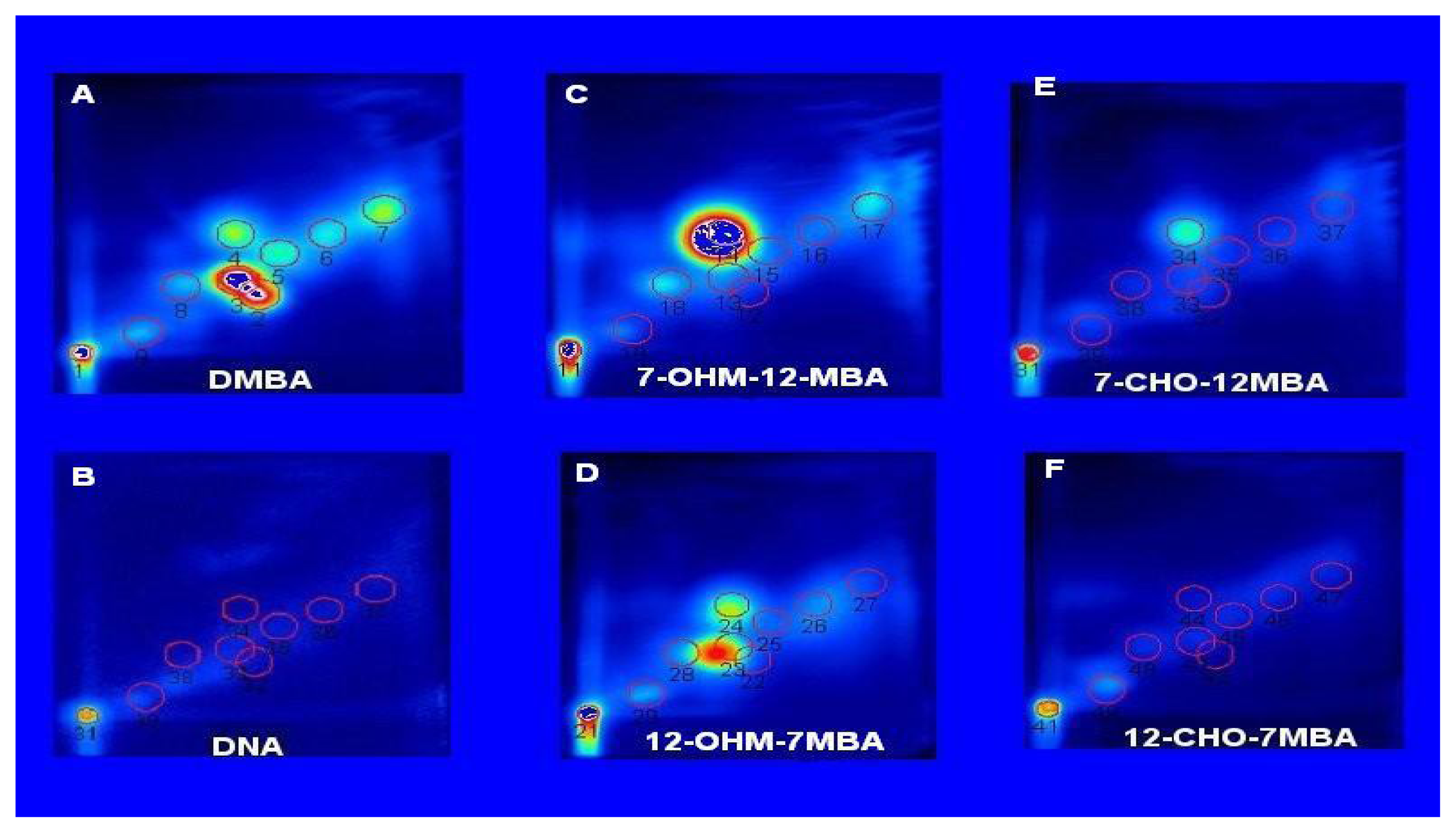
Photo-Irradiation of DMBA, 7-HOCH2-12-MBA, 12-HOCH2-7-MBA, 7-CHO-12-MBA, and 12-CHO-7-MBA in the Presence of Calf thymus DNA
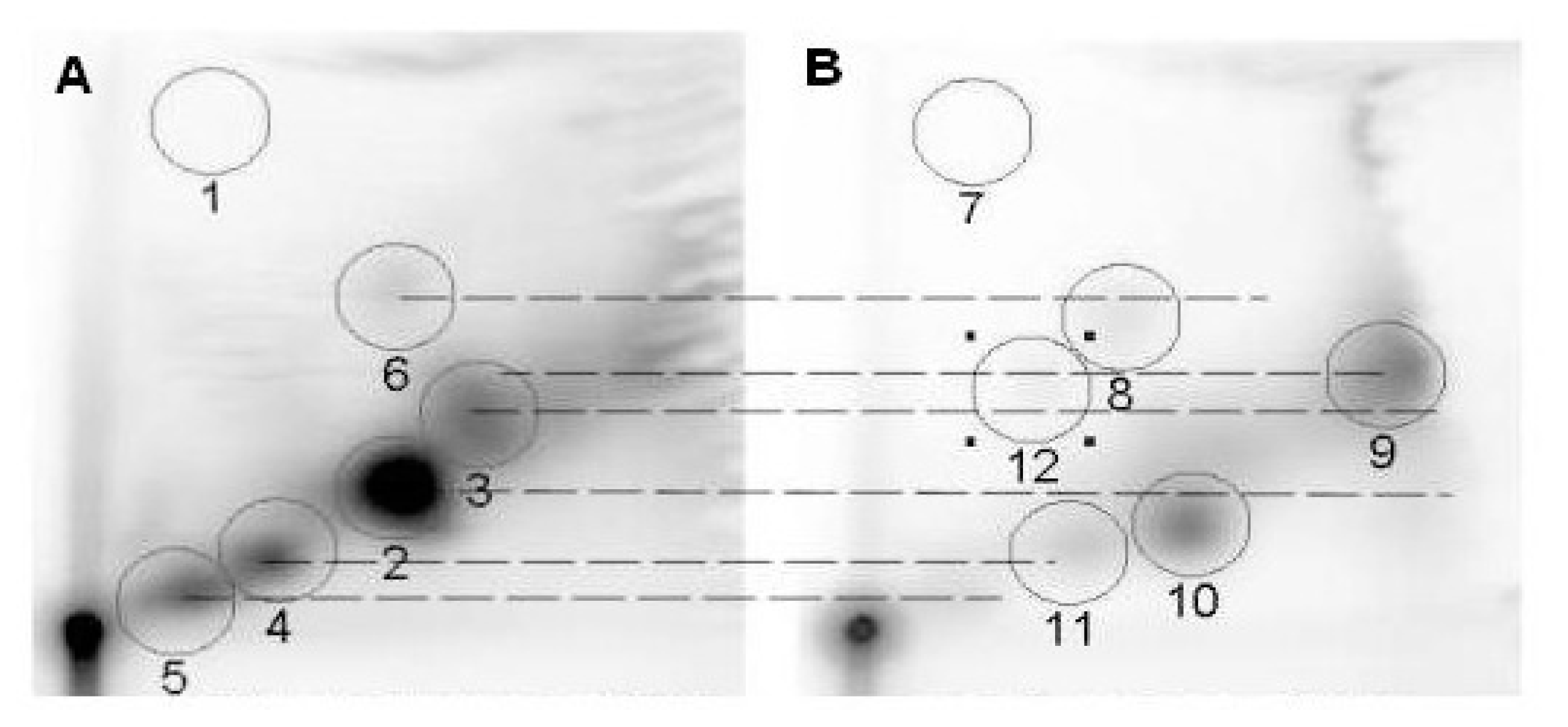
Reaction of Metabolites of DMBA with Calf thymus DNA and DNA Adducts Analyzed by 32P-postlabeling/TLC
Discussion





Acknowledgements
References
- Dipple, A.; Moschel, R. C.; Bigger, C. A. H. Polynuclear aromatic carcinogens. American Chemical Society: Washington, DC; Searle, C. E., Ed.; 1984; Volume 1, pp. 41–163. [Google Scholar]
- Yang, S. K.; Silverman, B. D. (Eds.) Polycyclic Aromatic Hydrocarbon Carcinogenesis: Structure-Activity Relationships; CRC Press: Boca Raton, FL, 1988; Volume I and II.
- Harvey, R. G. Polycyclic Aromatic Hydrocarbons; Wiley-VCH: New York, 1997. [Google Scholar]
- Conney, A. H. Induction of Microsomal Enzymes by Foreign Chemicals and Carcinogenesis by Polycyclic Aromatic Hydrocarbons. Cancer Res 1982, 42, 4875–4917. [Google Scholar]
- Arfsten, D. P.; Schaeffer, D. J.; Mulveny, D. C. The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: A review. Ecotoxicol. Environ. Safety 1996, 33, 1–24. [Google Scholar]
- Dabestani, R.; Ivanov, I. N. A comparison of physical, spectroscopic and photophysical properties of polycyclic aromatic hydrocarbons. Photochem. Photobiol 1999, 70, 10–34. [Google Scholar]
- Yu, H. Environmental carcinogenic polycyclic aromatic hydrocarbons: Photochemistry and phototoxicity. J. Environ. Sci. Health C-Environ. Carcinog. & Ecotoxic. Rev 2002, 20, 149–183. [Google Scholar]
- Pelletier, M. C.; Burgess, R. M.; Ho, K. T.; Kuhn, A.; McKinney, R. A.; Ryba, S. A. Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrate larvae and juveniles. Environ. Toxicol. Chem 1997, 16, 2190–2199. [Google Scholar]
- Swartz, R. C.; Ferraro, S. P.; Lamberson, J. O.; Cole, F. A.; Ozretich, R. J.; Boese, B. L.; Schults, D. W.; Behrenfeld, M.; Ankley, G. T. Photoactivation and toxicity of mixtures of polycyclic aromatic hydrocarbon compounds in marine sediment. Environ. Toxicol. Chem 1997, 16, 2151–2157. [Google Scholar]
- Kagan, J.; Tuveson, R. W.; Gong, H. H. The light-dependent cytotoxicity of benzo[a]pyrene: effect on human erythrocytes, Escherichia coli cells, and Haemophilus influenzae transforming DNA. Mutat. Res 1989, 216, 231–242. [Google Scholar]
- Fernandez, M.; L’Haridon, J. Influence of lighting conditions on toxicity and genotoxicity of various PAH in the newt in vivo. Mutat. Res 1992, 298, 31–41. [Google Scholar]
- Mukhtar, H. (Ed.) Skin Cancer: Mechanisms and Human Relevance; CRC Press: Boca Raton, 1995.
- Ahmad, H.; Mukhtar, H. Toxicology of the skin: new and emerging concepts. Toxicol Applied Pharm 2004, 195, 265–266. [Google Scholar]
- Dong, S.; Hwang, H.-M.; Harrison, C.; Holloway, L.; Shi, X.; Yu, H. UVA light-induced DNA single strand cleavage by selected polycyclic aromatic hydrocarbons. Bull. Environ. Contam. Toxicol 2000, 64, 467–474. [Google Scholar]
- Dong, S.; Wang, S.; Stewart, G.; Hwang, H.-M.; Fu, P. P.; Yu, H. Effect of organic solvents and biologically relevant ions on the light-induced DNA cleavage by pyrene and its amino and hydroxy derivatives. Intl. J. Mol. Sci 2002, 3, 937–947. [Google Scholar]
- Dong, S.; Fu, P. P.; Hwang, H.-M.; Yu, H. Effect of histidine on light-induced DNA single strand cleavage by selected polycyclic aromatic hydrocarbons. Polycycl. Arom. Compd 2002, 22, 451–458. [Google Scholar]
- Yu, H.; Dong, S.; Fu, P. P.; Hwang, H.-M. UVA light-induced DNA single strand cleavage by hydroxybenzo[a]pyrenes. Polycycl. Arom. Compd 2002, 22, 861–870. [Google Scholar]
- Dong, S.; Fu, P. P.; Shirsat, R. N.; Hwang, H.-M.; Leszczynski, J.; Yu, H. UVA Light-Induced DNA cleavage by isomeric methylbenz[a]anthracenes. Chem. Res. Toxicol 2002, 15, 400–407. [Google Scholar]
- Zeng, K.; Hwang, H.-M.; Fu, P. P.; Yu, H. Identification of 1-hydroxypyrene photoproducts and study of the effect of humic substances on its photolysis. Polycycl. Arom. Compd 2002, 22, 459–467. [Google Scholar]
- Wang, L.; Yan, J.; Cohly, H.; Hwang, H.-M.; Wang, S.; Fu, P. P.; Yu, H. Photo-Acute toxicity and genotoxicity of azulene on human Jurkat T-cells. Mutat. Res 2004, 562(1–2), 143–150. [Google Scholar]
- Wang, L.; Yan, J.; Fu, P. P.; Parekh, K. A.; Yu, H. Photomutagenicity of cosmetic ingredient chemicals azulene and guaiazulene. Mutat.. Res 2003, 530, 19–26. [Google Scholar]
- Yan, J.; Wang, L.; Fu, P. P.; Yu, H. Photomutagenicity of sixteen polycyclic aromatic hydrocarbons from the US EPA’s priority pollutants. Mutat. Res 2004, 557, 99–108. [Google Scholar]
- Dong, S.; Hwang, H.-M.; Shi, X.; Holloway, L.; Yu, H. UVA-induced DNA single strand cleavage by 1-hydroxypyrene and formation of covalent adducts between DNA and 1-hydroxypyrene. Chem. Res. Toxicol 2002, 13, 585–593. [Google Scholar]
- Boyland, E.; Sims, P. Metabolism of polycyclic compounds. The metabolism of 7,12-dimethylbenz(alpha) anthracene by rat-liver homogenates. Biochem. J 1965, 95, 780–787. [Google Scholar]
- Pataki, J.; Wlos, R.; Cho, Y.-C. Adrenocorticolytic derivatives of benz[.alpha.] anthracene. J. Med. Chem 1968, 11, 1083–1090. [Google Scholar]
- Wood, J.; Barker, C. L.; Grubbs, C. J. Photooxidation products of 7,12-dimethylbenz[a]anthracene. Chem.-Biol. Interact 1979, 26, 339–347. [Google Scholar]
- Gupta, R. C. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen:DNA adducts. Cancer Res 1985, 45, 5656–5662. [Google Scholar]
- Boyland, E.; Sims, P.; Huggins, C. Induction of adrenal damage and cancer with metabolites of 7,12-dimethylbenz[a]anthracene. Nature 1969, 207, 816–817. [Google Scholar]
- Warshawsky, D.; Kerns, E.; Bissell, M. J.; Calvin, M. Characterization of a photoproduct of 7,12-dimethylbenz[a]anthracene and its effects on chick embryo cells in culture. Biochem. J 1977, 164, 481–48. [Google Scholar]
© 2005 MDPI. All rights reserved.
Share and Cite
Yu, H.; Yan, J.; Jiao, Y.; Fu, P.P. Photochemical Reaction of 7,12-Dimethylbenz[a]anthracene (DMBA) and Formation of DNA Covalent Adducts. Int. J. Environ. Res. Public Health 2005, 2, 114-122. https://doi.org/10.3390/ijerph2005010114
Yu H, Yan J, Jiao Y, Fu PP. Photochemical Reaction of 7,12-Dimethylbenz[a]anthracene (DMBA) and Formation of DNA Covalent Adducts. International Journal of Environmental Research and Public Health. 2005; 2(1):114-122. https://doi.org/10.3390/ijerph2005010114
Chicago/Turabian StyleYu, Hongtao, Jian Yan, Yuguo Jiao, and Peter P. Fu. 2005. "Photochemical Reaction of 7,12-Dimethylbenz[a]anthracene (DMBA) and Formation of DNA Covalent Adducts" International Journal of Environmental Research and Public Health 2, no. 1: 114-122. https://doi.org/10.3390/ijerph2005010114



