Abstract
Physical activity (PA) and sport play an essential role in promoting body development and maintaining optimal health status both in the short and long term. Despite the benefits, a long-lasting heavy training can promote several detrimental physiological changes, including transitory immune system malfunction, increased inflammation, and oxidative stress, which manifest as exercise-induced muscle damages (EIMDs). Meat and derived products represent a very good source of bioactive molecules such as proteins, lipids, amino acids, vitamins, and minerals. Bioactive molecules represent dietary compounds that can interact with one or more components of live tissue, resulting in a wide range of possible health consequences such as immune-modulating, antihypertensive, antimicrobial, and antioxidative activities. The health benefits of meat have been well established and have been extensively reviewed elsewhere, although a growing number of studies found a significant positive effect of meat molecules on exercise performance and recovery of muscle function. Based on the limited research, meat could be an effective post-exercise food that results in favorable muscle protein synthesis and metabolic performance.
1. Introduction
Since the last decades of the previous millennium, global interest in the issue of health and the concepts of “healthy life” is growing, thus contributing to raise public awareness on the issue of prevention and promotion of “healthy habits”. Physical activity (PA) represents an excellent, practical, and low-cost method of improving health. PA has a wide range of physiological consequences on many body systems [1,2,3,4,5,6,7].
However, despite of the large variety of positive effects of PA, some negative physiological and biomechanics changes occur. They are responsible for the reduction of muscle strength capacity and the appearance of muscle pain, swelling, and stiffness; a symptomatology commonly attributable to exercise-induced muscle damages (EIMDs) [8,9]. Hotfiel et al. [10] postulated that the ultrastructural muscle injury following intense exercise may be due to muscle membrane damage, sarcomere disorganization, protein degradation, autophagy, and local inflammatory response. It has also been suggested that EIMDs are strictly associated with perturbations of electrolyte imbalances, endocrine, and immune systems, resulting in an increased production of reactive oxygen and nitrogen species (RONS) [11] as a likely consequence of inflammatory-mediated recovery processes.
A nutritional intervention was reported to be an effective countermeasure in reducing acute and chronic inflammations and supporting the immune system [12]. It is unlikely that a dietary intervention interacts with the mechanical damage of muscle but rather that it contributes to a modulation of the damage and the subsequent healing process at the level of the oxy-inflammatory cascade in the secondary phase of EIMDs [8]. It has been widely reported that the preventive impact of a diet rich in natural antioxidants outweighs the protective effect of supplementation [13].
Therefore, the utilization of functional foods, rich in bioactive molecules, such as proteins, fatty acids, minerals, and vitamins, has received much attention recently. Bioactive molecules represent dietary compounds that can interact with one or more components of live tissue, resulting in a wide range of possible health consequences such as immune-modulating, antihypertensive, antimicrobial, and antioxidative activities [14].
Athletes consume about 4 portions of meat/week, considering that 35 g of meat provides about 10 g of protein, the meat protein intake is important [15]. Functional activity of meat and meat products is mainly due to taurine, L-carnitine, choline, alpha-lipoic acid, conjugated linoleic acid, glutathione, creatine, coenzyme Q10, minerals (heme irons, zinc), and bioactive peptides produced during post-mortem aging and cooking methods.
PA increases the need for some vitamins and minerals, some of which are highly present in an assimilable form in animal origin products. For example, Zinc intervenes in numerous very important functions such as the growth, construction, and recovery of muscle tissue [16]. Several studies revealed that both male and female athletes suffer from “sports anemia,” which can impair physical performance and is linked to a zinc deficit [17] with meat being the best food source.
For this and other purposes, meat might be useful as an intervention to reduce muscle injury signs and accelerate force recovery.
Therefore, after a summary of current knowledge relating to the EIMD, its causes and consequences; this review provides an integrated overview of the functional properties of meat molecules and the benefits of meat consumption in athletes’ nutrition.
2. The Role of Nutrition in Physical Activity
PA is a systematic sequence of physical, psychological, and tactical preparatory events [18] aimed at achieving maximum performance in a race or maintaining optimal health status.
The achievement of good athletic performance is the result of an optimization process of body functions and specific adaptation to physical effort [19]. The resolution of the adaptation process is a phenomenon that implies the restoration of a physiological equilibrium.
Considering all the factors that can influence athletic performance, such as genetics, talent, and the type of training; good nutrition is certainly the simplest to manage.
A healthy and balanced diet is essential for people who practice PA, as it offers adequate calories and nutrients to fulfill energy and nutritional demands. Good nutrition, able to satisfy the higher energy demand, helps the athlete in performance and recover quickly.
Macronutrients such as carbohydrates, and fats represent the primary energy sources; however, proteins play a key role in muscle growth and recovery. Protein and essential amino acids ingestion can stimulate muscle protein synthesis and help in the healing of injured tissues before, during, and especially after the PA. Furthermore, micronutrients such as minerals and vitamins, whose requirement increases significantly during the PA, are involved in metabolic processes, oxygen transfer and delivery, and tissue repair; while antioxidants can support adequate immune function and protect against oxidative damage exercise-induced [20,21,22].
It should therefore be emphasized that proper nutrition is a decisive factor because: (1) promotes the growth and development of the organism, (2) supplies useful energy for a performance, and (3) help with the synthesis and recovery of muscle tissue from EIMDs [23]. However, it should be considered that the entity of the EIMDs can be influenced by the intensity, type, and duration of the exercise and therefore nutrition can be remodeled according to these functions [24]. Generally, in athletes’ diet most of the daily caloric intake (55–65%) must be represented by carbohydrates, proteins must account for 10–15% of total calories (preferably a combination of proteins from foods of animal and vegetable origin), while the remaining 25–30% of the caloric intake must come from fats which, if the physical performance is long-lasting and of low intensity, are used as an energy source [15].
However, as mentioned above, the daily requirement of a sportsman varies according to the type of sport and the metabolism involved (aerobic-anaerobic) as reported in Table 1.

Table 1.
Caloric demands per hour in different sport activity [25].
Aerobic activities (marathon, cross-country and middle distance, skiers, cyclists), in fact, require a large supply of carbohydrates capable of guaranteeing a sufficient supply of glycogen to provide energy during prolonged efforts.
On the contrary, in anaerobic activities (weightlifting, shot put, hammer or discus) the protein intake is important, which favors the development of muscle mass.
The Mediterranean diet is unquestionably one of the greatest eating models to follow today [26], alongside it, new dietary trends such as the ketogenic diet [27], the Intermittent Fasting diet [28], or the alkaline diet have found widespread diffusion. Due to the restrictive regimes proposed by the aforementioned dietary plans, their implementation must be dependent on the type of physical activity performed. For example, the reduction in caloric intake associate to the Intermitted Fasting might result in a decrease in lean body mass and strength, and therefore, potentially, a decrease in the capacity to conduct resistance exercise [29,30].
It’s widely accepted that an inadequate intake of energy and hydration substances can result in decreased strength and endurance performance, increased soreness, and the consequent risk of muscle damage. Therefore, for athletes, a healthy and balanced diet must not only be a nutritional priority to meet energy demands, but above all a fulfillment of physiological needs thus ensure optimal performance during exercise.
3. The Physiology of Muscle Recovery
It is widely assumed that EIMDs arise when a person is repeatedly subjected to long-lasting heavy training characterized by an eccentric muscle contraction. EIMDs represent the main cause of the reduction of muscle strength capacity and consequently of sports performance [8,9].
Hody, et al. [31] proposed an extensive review on the mechanisms involved in the onset of EIMDs. The complex mechanisms of EIMDs could be simplified in a two-phases damage model, the initial phase starts during the exercise when excessive tension and repeated eccentric contractions lead to a greater fiber, sarcoplasmic reticulum, and membrane injury; subsequently, the release of calcium into the cytoplasm contributes to a secondary myofibrillar damage in skeletal muscle [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
Indeed, calcium is responsible for the activation of muscle proteases, it triggers a chain reaction resulting in mitochondrial dysfunction, activation of cell death signaling, and secretion of stress hormones (i.e., catecholamines, cortisol) [31,33].
As a result, immediately following exercise, an increase of leukocytes, primarily neutrophils and later macrophages, has been widely reported [31,34]. With neutrophil mobilization in damaged muscle, an oxidative-inflammatory cascade occurs; necrotic fibers are degraded, and proteases, cytotoxic enzymes and RONS are produced [35].
Specifically, the overproduction of RONS stimulates the Keap1-Nrf2 (Kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2), signaling pathway which regulates the gene expression of several antioxidants and anti-inflammatory molecules [36] and represents the main regulator system of cytoprotective responses to oxidative stress [37,38].
The occurrence of oxy-inflammatory mechanisms as a consequence of training, by activating muscle stem cells known as satellite cells [39], is therefore the basis of organism adaptations and contributes to muscle recovery and remodeling [40,41].
Since the activation of satellite cells provides the muscle with the potential to regenerate following damaging PA; boosting satellite cell activity and extending their activation may be physiological mechanisms on which dietary intervention might be used to modulate muscle recovery and performance.
4. Meat Molecules in Muscle Recovery
Meat and meat products are key components of a healthy and balanced diet. They are concentrated natural sources of high-quality proteins as well as other nutrients such as fats, vitamins (B2, B6, B12, pantothenic acid and niacin) and minerals (iron, zinc, phosphorus, and selenium) that exert a beneficial role on growth, preservation, and repair of the human body.
Furthermore, meat is rich in bioactive molecules and endogenous antioxidants with well-established beneficial properties linked to their anti-inflammatory, immunoregulatory, and oxidative stress protective activity [42,43]. Once absorbed by the ingestion of animal origin food, these molecules can affect the structure and function of organs and tissues by acting as biomolecule substrates [44].
The nutritional profile of meat (on average consisting of 75% water, 19% protein, 2.5% fat, 1.2% carbohydrates and 1.65% nitrogen compounds [45]) is heavily influenced by the species, feeding regimen, cut and cooking techniques utilized [46].
For example, the content of L-carnitine in beef steak and lamb meat ranges between 64.6–78.6 and 190 mg/100 g fresh weight, but chicken breast only reaches values of 13–34.4 mg/100 g fresh weight [47]. Table 2 provides an overview of the content of the main biologically active molecules in meat cut from different animal species.

Table 2.
Contents of major biologically active meat molecules (mg/100 g) in raw meat cuts of different animal species.
4.1. Proteins and Amino Acids
It is well accepted [61] the role of meat proteins avoids catabolism and stimulates muscle growth. On average, 100 g of meat, provides up to 20 g of useful protein to meet human daily needs [62]. This is impressive considering that the amount of protein necessary to enhance muscle and albumin protein synthesis after a single session of endurance exercise is around 20–25 g of high-quality intact protein [63].
Aside from quantity, the quality of the proteins is critical in increasing muscle protein growth. Meat proteins include well-balanced necessary amino acids that aid in damaged tissue development and repair [64,65,66]. For example, due to its immunomodulatory activity, glutamine is recognized as playing a key role in muscle recovery [67,68]. Glutamine is found in various food [69], however the best way to get it is by consuming meat and other animal products. Nakhostin-Roohi et al. [70] demonstrated that taking 1.5 g/kg/day glutamine for 7 days can lower the level of creatine kinase, an intramuscular biomarker enzyme of EIMDs. Furthermore, glutamine was found to be effective in increasing the levels of glutathione, a tripeptide of great importance in the prevention of sports injuries [71,72].
Particularly relevant in EIMDs prevention is the role of branched chain amino acid (BCAA) [73,74]; for which it was observed [75] that an intake of 200 mg/kg/day for at least 10 days can be helpful to prevent moderate muscle damage. Among all, leucine, the main BCAA in meat, was proven to be involved in the activation of skeletal muscle protein synthesis [76].
Moreover, Ra et al. [77] showed in a 2013 study that combining BCAA and taurine, compared to supplementing a single compound, might be an effective method for reducing several biomarkers of EIMDs.
4.2. Taurine and L-Carnitine
Unlike supplements, meat could be considered an effective strategy against EIMDs thanks to the balanced and complete supply of amino acids especially of functional ones (taurine, and L-carnitine) with well-defined physiological roles in anti-oxidative and anti-inflammatory reactions, as well as neurological, immunological, and cardiovascular function [78].
These compounds are abundant in all beef cuts; indeed 30 g of dry beef provides enough taurine to fulfill the daily physiological demands of a healthy 70 kg adult person [78].
The role of these molecules in muscle recovery has been extensively reviewed over the past few years. Correspondingly, Thirupathi et al. [79] and Sawicka et al. [80] discussed the link between taurine and L-carnitine with the modulation of exercise-induced RONS formation.
Taurine and L-carnitine are endogenous molecules that are typically synthesized in the body; however, when cells are under physiological stress, this quantity is insufficient [81,82].
The amino acid intake from meat consumption represents an effective strategy in maintaining the concentrations of L-carnitine and taurine at the cellular level, especially considering that glutathione (GSH) and taurine share the same precursor, and this could increase the oxidative damage induced by muscles stressful conditions [79].
According to Thirupathi et al. [79], taurine could prevent the susceptibility at subsequent pathology PA-induced by protecting cell membranes against oxidative stress and controlling inflammation [83]. L-carnitine, instead, is involved in the maintenance of mitochondrial function and metabolic processes concerning the synthesis of branched-chain amino acids, the generation of energy, and the induction of β-oxidation processes; but it is also recognized as having a transport, thermogenesis, and antioxidant function [50].
4.3. Carnosine
A central role in recovery following EIMDs is recognized to both carnosine and, its methylated metabolite, anserine: they are the most abundant antioxidants in meat [84]. Carnosine concentration in meat ranges from 500 mg/kg of the chicken thigh to 2700 mg/kg of pork shoulder [85] instead anserine is more abundant in chicken muscle [86]. Furthermore, the consumption of meat promotes the synthesis of carnosine in the human body thanks to the contribution of β–alanine, a limiting factor in carnosine biosynthesis [87,88].
Markus et al. [89] synthesized the physiological role of carnosine in enhancing exercise recovery. Varanoske and colleagues [90], found a greater reduction of fatigue when compared to the carnosine levels in recreationally trained women [89]. Thus, higher intramuscular carnosine levels were associated with greater fatigue relief, and women who consumed the most dietary protein were likewise more prone to have the highest intramuscular carnosine content.
4.4. Creatine
Compared to other foods, meat is also an excellent source of creatine, containing in beef 5,25 mg/g on average, according to Mateescu et al. [91]. Creatine effectiveness, in terms of EIMDs effects amelioration, has been widely demonstrated in several studies [91,92]. Inflammation, oxidative stress, calcium homeostasis, changes in glycogen storage, and satellite cell activity in damaged muscle are all plausible strategies creatine-associated for preventing EIMDs [92,93].
According to Jiaming and Rahimi [92] meta-analysis’ findings, creatine reduces creatine kinase (CK) and lactate dehydrogenase (LDH) concentrations immediately after 24–48–72–96 h of exercise. CK and LDH have been widely used as indicators of muscle micro-damages and, therefore, are often considered indirect biomarkers of EIMDs [94].
4.5. Glutathione (GSH)
As stated above, glutathione demonstrated [95] to be useful in reducing muscular fatigue. In fact, intense exercise results in a reduction in plasma and tissue concentrations of GSH as well as an increase in the oxidized form compared to the reduced one [96,97]. The human body, on the other hand, has been found very effective in absorbing GSH from the diet [98]. According to Aoi et al. [95], including GSH in athletes’ diets increased GSH plasma concentrations and led to an improvement of antioxidant defenses and aerobic metabolism performance.
4.6. Conjugated Linoleic Acid (CLA)
Besides the protein fraction, conjugated linoleic acid (CLA) from the meats’ lipid fraction showed an anti-fatigue effect in athletes [99].
Meats, especially red ones, have been demonized for years due to their saturated fat content. Meats contain not only saturated fat but a large amount of monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) with proven beneficial effects [100].
CLA, among all, are important for athlete general health [22]. Kim et al. [101] reviewed the impact of CLA on muscle metabolism, specifically observed, in several animal trials, an improvement in the exercise outcome with CLA treatment, however, human studies are still limited and require further investigation.
In addition to its high amino acids content, meat represents an important source of micronutrients such as minerals, vitamins, and alpha-lipoic acid.
4.7. Iron
Physical activity is associated with a constant demand for minerals, particularly in endurance or long-lasting sports, where the loss is significant, due to sweat and other factors. Iron is a necessary component of hemoglobin and myoglobin proteins, which are present in red blood cells and muscles, respectively. During exercise, hemoglobin and myoglobin transport oxygen to the tissues, and athletes’ performance greatly depends on the efficiency of this system. Sim et al. [102] widely summarized the role of iron in athlete performance. Fatigue is the main indicator of a possible iron deficiency [103]; a phenomenon to which women are particularly susceptible [104]. Iron deficiencies can be avoided by the intake of highly absorbable and usable iron, such as that contained in beef. However, dietary intake alone may not be enough to meet the higher daily iron requirements of active and trained women and therefore, supplementation may be necessary for some circumstances [104].
4.8. Coenzyme Q10 (CoQ10)
The meat’s nutritional composition is characterized by a high concentration of group B vitamins (thiamine, riboflavin, vitamin B6, niacin, pantothenic acid, biotin), they are involved in the energy conversion during PA, while folate and vitamin B12 are necessary for red blood cells and protein synthesis, tissue repair, and maintenance [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105].
The group B vitamins, besides assuming a fundamental role in human nutrition, have been proven to enhance coenzyme Q10 production (CoQ10) [106]. Meat, poultry, and fish are all excellent sources of dietary CoQ10 [107].
CoQ10, also known as ubiquinone, is a fat-soluble vitamin-like compound found in all cellular membranes [43]. Borekova et al. [60] summarized the main activities of this molecule, among all, the antioxidant properties exceed in terms of both quantity and efficiency that of recognized antioxidants. Therefore, the ingestion of CoQ10 has been observed to be effective in countering the increase in RONS and in modulating the inflammatory signaling [108,109] associated with PA. Additionally, a variety of factors are responsible for CoQ10 deficiency [110]. In sports, greater energy demands may lead to the alteration in mitochondrial metabolism characterized by decreased oxidative phosphorylation and increased physical fatigue. According to Haas [111], CoQ10 ingestion can improve energy metabolism by lowering phospholipase A2 and restoring phosphocreatine levels (a source of physical energy).
4.9. Alpha-Lipoic Acid (ALA)
Finally, different clinical trials demonstrated the antioxidants and anti-inflammatory effects of alpha-lipoic acid (ALA) in several diseases. ALA has been used to regenerate the other antioxidants, enhance glucose metabolism, and reduce EIMDs in a variety of tissues [112]. Isenmann et al. [113] found possible beneficial effects of ALA in maintaining performance and reducing muscle damage, in line with earlier studies of Morawin et al. [114], who observed significantly lower CK values after eccentric exercise when athlete received ALA supplementation.
Many of the functions described here and summarized in Table 3 have shown an impact of meat molecules on red-ox processes.

Table 3.
Major biologically active meat molecules and their functions in preventing EIMDs.
However, Margaritelis et al. [118] emphasize the critical role of oxidative stress in exercise adaptations. EIMD is a complex phenomenon, if on the one hand oxidative stress and inflammatory phenomena can increase muscle damage, on the other hand they allow the restoration of functions and adaptation thus contributing to recovery.
The alternative definition of “antioxidant paradox” [119] recognize antioxidants as inhibitors of the signaling effects of exercise induced RONS production and thus an obstacle to the mechanism of muscle adaptation and recovery.
Based on these evidence, nutritional strategies should be adopted with pragmatism. The use of the great majority of the molecules of animal origin in food formulations and as supplements in the prevention and treatment of various PA diseases should be discouraged in favor of a balanced diet which should always be considered as the main nutritional intervention strategy.
5. Importance of Meat Bioactive Molecules in Enhance Athletes’ Health and Performance
Currently, due to the growing popularity of plant-based diets, many people believe that eating meat is just harmful to health. However, giving up this essential source of bioactive molecules might really cause more damage than benefits.
Considering the tendency towards the abuse of foods of animal origin by developed Western populations and the relative negative health implications that it entails, the adoption of a more vegetarian nutritional style is today considered a directly and indirectly preventive attitude towards various pathologies and uncomfortable conditions, such as the famous “diseases of well-being”. This is because vegetarian diets bring lower levels of saturated fat, cholesterol and animal proteins indirectly related to health problems, as it is not the proteins themselves that are harmful, but the food processing methods. Vegetarian diets offer higher levels of carbohydrates, fiber, magnesium, potassium, folic acid and various types of antioxidants such as vitamin C, vitamin E, polyphenols. Data literature reported a lower risk of ischemic heart disease and cancer, but no effect on overall mortality or cerebrovascular disease in vegetarian subjects [120]. A further analysis combining two large studies found that vegetarians in the UK have an all-cause mortality like that of carnivores [120]. The positions on the beneficial or harmful effects of the vegetarian diet are controversial. In relation to sport, the athlete needs more energy which derives mainly from carbohydrates and, to a lesser extent, from lipids; more amino acids: they derive from proteins and have numerous functions. The essential ones (AAE) are especially important, necessary for the protein synthesis of structural proteins of the muscle, enzymes involved in energy processes; in addition, more minerals and water involved in sweating: especially magnesium and potassium, although their quantity varies according to the extent of sweating itself. More vitamin and mineral antioxidants such as vitamin C, vitamin E, zinc and selenium. An increase in oxidative processes inexorably determines an increase in the quantity of free radicals [121]. More precursors and enzymatic components such as the B vitamins and certain minerals. Enzymes are biological catalysts essential to cellular processes which, in sports, increase exponentially [122]. However, the vegetarian athlete also suffers from a lack of some fundamental macro and micronutrients. The intake of proteins and especially of AAE in vegetarian and especially vegan diets tends to be lower than that in omnivorous diets [123,124]. Creatine is an amino acid molecule used as a reserve of phosphate groups by muscle cells [125]. The body can synthesize it and there is no form of nutritional deficiency related to it. In vegans, internal production is maximal and some believe it is not enough. The integration of creatine in these subjects who practice strength sports, even more so if they are vegan, can increase strength performance. Supplementing with creatine is therefore recommended for vegan strength athletes and bodybuilders who follow the same diet. Despite the richness in total lipids and lipophilic molecules (such as vitamin A and vitamin C) of Western vegetarian diets, there is a lack of the two semi-essential omega 3 fatty acids: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Moreover, in the vegetarian athlete there is a lack of vitamin B12, Iron, Calcium, Zinc and Phosphorus, essential microelements for the regulation of fatty acid metabolism, Calcium is essential for muscle contraction and all have a strong anti-oxidative power [120,121,122,123,124].
In the sportsman’s diet, meat has the dual purpose of meeting energy needs and providing for the protection, repair, and growth of tissues.
Meat, although not recognized as the main energy source, contains some molecules capable of improving athletic performance, especially during intense but short-term efforts. Creatine, for example, contributes to increased phosphocreatine energy stores [126] and, thus, the capacity to perform high-intensity activities. Some B vitamins (thiamine, riboflavin, vitamin B6, niacin, pantothenic acid, biotin) are also involved in energy conversion during exercise, while folate and vitamin B12 are required for blood cell production, protein synthesis, tissue repair, and maintenance [105]. Proteins, the main and essential meat nutrient, are associated with the improvement of several biological activities. They are in-volved in the growth and recovery of various muscle groups, bones, and other organs. All the essential amino acids that athletes require to rebuild muscle tissue and perform at their best are found in meat proteins. The amount of protein required increases with age and varies based on the type of PA and the desired goal [61]. Besides protein, the potential health benefit of meat can be attributed to many bioactive molecules with proven antioxidant, anti-inflammatory, and immunomodulatory properties. Taurine, BCAAs, L-carnitine, creatine, CLA, and ALA can stimulate the immunological response in skeletal muscle, whereas iron and vitamin-derived compounds such as CoQ10 have been proved, by the attenuation of interleukin-6 (IL-6) and C-reactive proteins levels [109] in blood, to strongly support immune functions. Potential antioxidant properties are also associated with some meat molecules. Several studies have shown the role of CoQ10, ALA, and glutathione in the preservation of prooxidant/antioxidant homeostasis, in forming a complex with metal ions, and regenerating enzymes involved in RONS scavenging [43,95]. In conclusion, it is worth noting that meat, when consumed in the right proportion, provides the body with several bioactive molecules that assist athletes to stay healthy while also improving their performance and muscle repair [Figure 1].
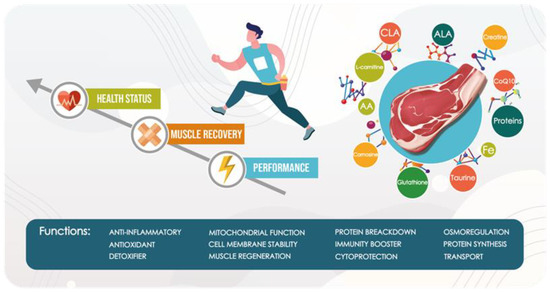
Figure 1.
Schematic representation of the functional activities of meat bioactive molecules.
It is not impossible to achieve good results in athletic performance while following a vegetarian or vegan diet. However, dietary choices must be made based on the load and the type of training, for this reason, the omnivorous diet is obviously the most complete one, with the right amount of macro and micronutrients, without absolutely demonizing meat, which like as previously reported, it has many beneficial properties both in a protein and biomolecule intake.
6. Conclusions
Nutrition also plays a fundamental role in sport as it can influence athletic performance or improve recovery after sport. Nutrition is therefore a key factor for sports performance, contributing to the athlete’s success and determining an improvement in body composition and an increase in physical/cognitive performance in view of a sporting event.
Physical activity, athletic performance and recovery from exercise are influenced and improved by a correct diet with a view to an adequate and balanced intake of macro and micronutrients. An athlete’s diet load should be like that recommended for the general population with energy intake divided into ap-proximately 55% carbohydrates, approximately 15% protein and approximately 30% lipids. This food ration must be divided into 3–4 main meals, and of course it can be modified in terms of macronutrients according to the type of sport, and also to the training period. In this scenario, it is important to say that the protein share is fundamental in an athlete, as proteins play a fundamental role in post-exercise functional recovery. In younger subjects, proteins ensure good muscle hypertrophy, which is why protein intake is generally slightly improved, especially among young athletes. Current data suggest that the amount of dietary protein needed to support metabolic adaptation, repair, remodeling, and protein turnover generally ranges from 1.2 to 2.0 g/kg body weight per day [62]. Higher intakes may be indicated in short periods during intensive training. However, in the event of an energy restriction or sudden inactivity that could occur as a result of an injury, increasing your protein intake to 2.0 g/kg per day or higher could be helpful in preventing the loss of lean mass. For these reasons, the meat consumed is a valid strategy to meet the athlete’s protein requirements. Meat consumed in the correct proportions in a healthy and balanced diet is an important food intervention to support the health and performance of athletes. Due to its compositional complexity, offer a balanced supply of nutrients and functional compounds. The molecules believed to be responsible for its functional activity have found wide application as food supplements however they should be used with caution and with specific target [9]. Molecules intake through meat allow to circumventing problems related to the specific dosage and timing of intake of food supplements. Amino acids deriving from meat proteins digestion, following absorption into the bloodstream, can interact with cells of tissue, with muscle cells, modulating biochemical and physiological cell pathways, influencing athletic performance and recovery. Certainly, protein and meat consumed plays a key role in the athlete’s diet, but further studies are useful to clarify the molecular pathways of this action.
Author Contributions
Conceptualization, M.d.C., M.A. and G.M. (Giovanni Messina); methodology, M.d.C. and R.P.; resources, N.T. and A.A.; writing—original draft preparation, M.d.C. and R.P.; writing—review and editing, A.d.M., V.C.F., M.G.C. and G.M. (Gaetana Messina); visualization, A.S., R.I.C. and M.A.; supervision, G.M. (Giovanni Messina). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article. Authors can use this data for research purposes only by citing our research article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Monda, V.; Sessa, F.; Ruberto, M.; Carotenuto, M.; Marsala, G.; Monda, M.; Cambria, M.T.; Astuto, M.; Distefano, A.; Messina, G. Aerobic Exercise and Metabolic Syndrome: The Role of Sympathetic Activity and the Redox System. Diabetes Metab. Syndr. Obes. 2020, 13, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Pan, X.; Li, G.; Chatterjee, E.; Xiao, J. Physical Exercise Protects Against Endothelial Dysfunction in Cardiovascular and Metabolic Diseases. J. Cardiovasc. Transl. Res. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Millet, G.P.; Place, N.; Kayser, B.; Zanou, N. The Muscle-Brain Axis and Neurodegenerative Diseases: The Key Role of Mitochondria in Exercise-Induced Neuroprotection. Int. J. Mol. Sci. 2021, 22, 6479. [Google Scholar] [CrossRef] [PubMed]
- Blondeel, A.; Demeyer, H.; Janssens, W.; Troosters, T. The role of physical activity in the context of pulmonary rehabilitation. COPD J. Chronic Obstr. Pulm. Dis. 2019, 15, 632–639. [Google Scholar] [CrossRef]
- Braun, B.; Hamilton, K.L.; Lark, D.S.; Newman, A. Biochemistry of Exercise Effects in Type 2 Diabetes. In The Routledge Handbook on Biochemistry of Exercise, 1st ed.; Tiidus, P.M., MacPherson, R.E.K., LeBlanc, P.J., Josse, A.R., Eds.; Routledge: New York, NY, USA, 2020; pp. 433–454. [Google Scholar]
- Tamminen, N.; Reinikainen, J.; Appelqvist-Schmidlechner, K.; Borodulin, K.; Mäki-Opas, T.; Solin, P. Associations of physical activity with positive mental health: A population-based study. Ment. Health Phys. Act. 2020, 18, 100319. [Google Scholar] [CrossRef]
- Nay, K.; Smiles, W.J.; Kaiser, J.; McAloon, L.M.; Loh, K.; Galic, S.; Oakhill, J.S.; Gundlach, A.L.; Scott, J.W. Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 4052. [Google Scholar] [CrossRef]
- Howatson, G.; van Someren, K.A. The Prevention and Treatment of Exercise-Induced Muscle Damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef]
- Hotfiel, T.; Freiwald, J.; Hoppe, M.W.; Lutter, C.; Forst, R.; Grim, C.; Bloch, W.; Hüttel, M.; Heiss, R. Advances in Delayed-Onset Muscle Soreness (DOMS): Part I: Pathogenesis and Diagnostics. Sportverletz. Sportschaden 2018, 32, 243–250. [Google Scholar] [CrossRef]
- Ji, L.L.; Yeo, D.; Kang, C.; Zhang, T. The role of mitochondria in redox signaling of muscle homeostasis. J. Sport Health Sci. 2020, 9, 386–393. [Google Scholar] [CrossRef]
- Buonocore, D.; Negro, M.; Arcelli, E.; Marzatico, F. Anti-inflammatory Dietary Interventions and Supplements to Improve Performance during Athletic Training. J. Am. Coll. Nutr. 2015, 34 (Suppl. S1), 62–67. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The antioxidant paradox. Lancet 2000, 355, 1179–1180. [Google Scholar] [CrossRef]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a Bioactive Compound? A Combined Definition for a Preliminary Consensus. Int. J. Nutr. Food Sci. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- Ministero della Salute. L’alimentazione nella Pratica Sportive e Motoria. Available online: https://www.iss.it/documents/20126/0/Libretto_Alimentazione.pdf/46cc1fe6-0f9e-9722-acf7-36dc3d350ac0?t=1582280465780 (accessed on 27 November 2021).
- Hernández-Camacho, J.D.; Vicente-García, C.; Parsons, D.S.; Navas-Enamorado, I. Zinc at the crossroads of exercise and proteostasis. Redox Biol. 2020, 35, 101529. [Google Scholar] [CrossRef]
- Nishi, Y. Anemia and zinc deficiency in the athlete. J. Am. Coll. Nutr. 1996, 15, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Rucco, R.; Ascione, A.; Di Plama, D. Motion analysis in sport training: The link between technology and pedagogy. J. Phys. Educ. Sport 2020, 20, 2337–2341. [Google Scholar]
- Schinke, R.J.; Tenenbaum, G.; Lidor, R.; Battochio, R.C. Adaptation in Action: The Transition from Research to Intervention. Sport Psychol. 2010, 24, 542–557. [Google Scholar] [CrossRef]
- Sale, C.; Elliott-Sale, K.J. Nutrition and Athlete Bone Health. Sports Med. 2019, 49, 139–151. [Google Scholar] [CrossRef]
- Walsh, N.P. Nutrition and Athlete Immune Health: New Perspectives on an Old Paradigm. Sports Med. 2019, 49, 153–168. [Google Scholar] [CrossRef]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef]
- Tipton, K.D. Nutritional Support for Exercise-Induced Injuries. Sports Med. 2015, 45, 93–104. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Bărbuică, S.I. Studies on Importance of Using Meat, Fish and Their Derivates in Athletes Diet. 2005. Available online: http://spasb.ro/index.php/spasb/article/view/1952/pdf (accessed on 14 October 2021).
- Laganà, P.; Coniglio, M.A.; Corso, C.; Turco, V.L.; Dattilo, G.; Delia, S. Mediterranean diet, sport and health. Prog. Nutr. 2020, 22. [Google Scholar]
- McSwiney, F.T.; Doyle, L.; Plews, D.J.; Zinn, C. Impact of Ketogenic Diet on Athletes: Current Insights. Open Access J. Sports Med. 2019, 10, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Chu, T. Intermittent fasting and its effects on athletic performance: A review. Curr. Sports Med. Rep. 2019, 18, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, K.; Stannard, S.R.; Ghlissi, Z.; Maughan, R.J.; Kallel, C.; Jamoussi, K.; Zeghal, K.M.; Hakim, A. Effect of fed- versus fasted state resistance training during Ramadan on body composition and selected metabolic parameters in bodybuilders. J. Int. Soc. Sports Nutr. 2013, 10, 23. [Google Scholar] [CrossRef]
- Rebai, H.; Chtourou, H.; Zarrouk, N.; Harzallah, A.; Kanoun, I.; Dogui, M.; Souissi, N.; Tabka, Z. Reducing Resistance Training Volume during Ramadan Improves Muscle Strength and Power in Football Players. Int. J. Sports Med. 2013, 35, 432–437. [Google Scholar] [CrossRef]
- Hody, S.; Croisier, J.-L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric Muscle Contractions: Risks and Benefits. Front. Physiol. 2019, 10, 536. [Google Scholar] [CrossRef]
- Gissel, H.; Clausen, T. Excitation-induced Ca2+influx and skeletal muscle cell damage. Acta Physiol. Scand. 2001, 171, 327–334. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the Immune System: Regulation, Integration, and Adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef]
- Sousa, M.; Teixeira, V.H.; Soares, J. Dietary strategies to recover from exercise-induced muscle damage. Int. J. Food Sci. Nutr. 2013, 65, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Kruger, M.J.; Smith, R.M.; Myburgh, K.H. The inflammatory response to skeletal muscle injury: Illuminating complexities. Sports Med. 2008, 38, 947–969. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1–Nrf2 system during cellular stress. J. Biol. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Jyrkkänen, H.-K.; Levonen, A.-L. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic. Biol. Med. 2012, 52, 973–982. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Chen, T.C.; Nosaka, K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc. Sport Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef]
- Baumert, P.; Lake, M.J.; Stewart, C.E.; Drust, B.; Erskine, R.M. Genetic variation and exercise-induced muscle damage: Implications for athletic performance, injury and ageing. Eur. J. Appl. Physiol. 2016, 116, 1595–1625. [Google Scholar] [CrossRef]
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Characteristics of Selected Antioxidative and Bioactive Compounds in Meat and Animal Origin Products. Antioxidants 2019, 8, 335. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Wang, M. Bioactive Substances of Animal Origin. In Handbook of Food Chemistry, 1st ed.; Cheung, P., Mehta, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Beltrán, J.A.; Bellés, M. Effect of Freezing on the Quality of Meat. Encycl. Food Secur. Sustain. 2019, 2, 493–497. [Google Scholar] [CrossRef]
- de Castro Cardoso Pereira, P.M.; dos Reis Baltazar Vicente, A.F. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Demarquoy, J.; Georges, B.; Rigault, C.; Royer, M.-C.; Clairet, A.; Soty, M.; Lekounoungou, S.; Le Borgne, F. Radioisotopic determination of l-carnitine content in foods commonly eaten in Western countries. Food Chem. 2004, 86, 137–142. [Google Scholar] [CrossRef]
- Lourenço, R.; Camilo, M.E. Taurine: A conditionally essential amino acid in humans? An overview in health and disease. Nutr. Hosp. 2002, 17, 262–270. [Google Scholar] [PubMed]
- Kalpana, A. Effects of l-carnitine [Neutraceutical] in weight management among overweight and obese adults of age between 20–45 yrs—A comparative study in Chennai and Tirupathi. Int. J. Sci. Res. Publ. 2012, 2, 1–5. [Google Scholar]
- Dayanand, C.D.; Krishnamurthy, N.; Ashakiran, S.; Shashidhar, K.N. Carnitine: A novel health factor—An overview. Int. J. Pharm. Biomed. Res. 2011, 2, 79–89. [Google Scholar]
- Aristoy, M.C.; Toldrá, F. Histidine dipeptides HPLC-based test for the detection of mammalian origin proteins in feeds for ruminants. Meat Sci. 2004, 67, 211–217. [Google Scholar] [CrossRef]
- Schmid, A. Bioactive substances in meat and meat products. Fleischwirtsch. Int. 2010, 2, 127–133. [Google Scholar]
- Gibis, M.; Weiss, J. Impact of Precursors Creatine, Creatinine, and Glucose on the Formation of Heterocyclic Aromatic Amines in Grilled Patties of Various Animal Species. J. Food Sci. 2015, 80, C2430–C2439. [Google Scholar] [CrossRef]
- Jones, D.P.; Coates, R.J.; Flagg, E.W.; Eley, J.W.; Block, G.; Greenberg, R.S.; Gunter, E.W.; Jackson, B. Glutathione in foods listed in the national cancer institute’s health habits and history food frequency questionnaire. Nutr. Cancer 1992, 17, 57–75. [Google Scholar] [CrossRef]
- Mulvihill, B. Ruminant meat as a source of conjugated linoleic acid (CLA). Nutr. Bull. 2001, 26, 295–299. [Google Scholar] [CrossRef]
- Koba, K.; Yanagita, T. Health benefits of conjugated linoleic acid (CLA). Obes. Res. Clin. Pract. 2014, 8, e525–e532. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.Y.; Bhagwat, S.A.; Williams, J.R.; Howe, J.C.; Holden, J.M. USDA Database for the Choline Content of Common Foods, Release Two; Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA: Beltsville, MD, USA, 2008.
- Lewis, E.D.; Zhao, Y.-Y.; Richard, C.; Bruce, H.L.; Jacobs, R.L.; Field, C.J.; Curtis, J.M. Measurement of the abundance of choline and the distribution of choline-containing moieties in meat. Int. J. Food Sci. Nutr. 2015, 66, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Lombardi-Boccia, G.; Martínez-Dominguez, B.; Aguzzi, A. Total Heme and Non-heme Iron in Raw and Cooked Meats. J. Food Sci. 2002, 67, 1738–1741. [Google Scholar] [CrossRef]
- Borekova, M.; Hojerova, J.; Koprda, V.; Bauerova, K. Nourishing and health benefits of coenzyme Q10—A review. Czech J. Food Sci. 2008, 26, 229–241. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Monda, V.; Valenzano, A.; Moscatelli, F.; Salerno, M.; Sessa, F.; Triggiani, A.I.; Viggiano, A.; Capranica, L.; Marsala, G.; De Luca, V.; et al. Primary Motor Cortex Excitability in Karate Athletes: A Transcranial Magnetic Stimulation Study. Front. Physiol. 2017, 8, 695. [Google Scholar] [CrossRef]
- Phillips, S.M. Dietary protein requirements and adaptive advantages in athletes. Br. J. Nutr. 2012, 108, S158–S167. [Google Scholar] [CrossRef]
- Hirsch, K.R.; Greenwalt, C.E.; Saylor, H.E.; Gould, L.M.; Harrison, C.H.; Brewer, G.J.; Blue, M.N.M.; Ferrando, A.A.; Huffman, K.M.; Mayer-Davis, E.J.; et al. High-intensity interval training and essential amino acid supplementation: Effects on muscle characteristics and whole-body protein turnover. Physiol. Rep. 2020, 9, e14655. [Google Scholar] [CrossRef]
- Atherton, P.J.; Kumar, V.; Selby, A.L.; Rankin, D.; Hildebrandt, W.; Phillips, B.E.; Williams, J.P.; Hiscock, N.; Smith, K. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clin. Nutr. 2016, 36, 888–895. [Google Scholar] [CrossRef]
- Reidy, P.T.; Rasmussen, B.B. Role of Ingested Amino Acids and Protein in the Promotion of Resistance Exercise–Induced Muscle Protein Anabolism. J. Nutr. 2016, 146, 155–183. [Google Scholar] [CrossRef]
- Street, B.; Byrne, C.; Eston, R. Glutamine Supplementation in Recovery From Eccentric Exercise Attenuates Strength Loss and Muscle Soreness. J. Exerc. Sci. Fit. 2011, 9, 116–122. [Google Scholar] [CrossRef][Green Version]
- Legault, Z.; Bagnall, N.; Kimmerly, D.S. The Influence of Oral L-Glutamine Supplementation on Muscle Strength Recovery and Soreness Following Unilateral Knee Extension Eccentric Exercise. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Lenders, C.M.; Liu, S.; Wilmore, D.W.; Sampson, L.; Dougherty, L.W.; Spiegelman, D.; Willett, W.C. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. Eur. J. Clin. Nutr. 2009, 63, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Nakhostin-Roohi, B.; Javanamani, R.; Zardoost, N.; Ramazanzadeh, R. Influence of glutamine supplementation on muscle damage and oxidative stress indices following 14 km running. Bimon. J. Hormozgan Univ. Med. Sci. 2016, 20, 323–331. [Google Scholar]
- Nemati, A.; Alipanah-Moghadam, R.; Molazadeh, L.; Baghi, A.N. The Effect of Glutamine Supplementation on Oxidative Stress and Matrix Metalloproteinase 2 and 9 After Exhaustive Exercise. Drug Des. Dev. Ther. 2019, 13, 4215–4223. [Google Scholar] [CrossRef]
- Baldelli, S.; Ciccarone, F.; Limongi, D.; Checconi, P.; Palamara, A.T.; Ciriolo, M.R. Glutathione and Nitric Oxide: Key Team Players in Use and Disuse of Skeletal Muscle. Nutrients 2019, 11, 2318. [Google Scholar] [CrossRef]
- Khemtong, C.; Kuo, C.-H.; Chen, C.-Y.; Jaime, S.J.; Condello, G. Does Branched-Chain Amino Acids (BCAAs) Supplementation Attenuate Muscle Damage Markers and Soreness after Resistance Exercise in Trained Males? A Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 1880. [Google Scholar] [CrossRef]
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Cole, N.; McCormick, J.J.; Kerksick, C.M.; Vaughan, R.A.; Dokladny, K.; et al. Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise. Nutrients 2018, 10, 1389. [Google Scholar] [CrossRef]
- Fouré, A.; Bendahan, D. Is Branched-Chain Amino Acids Supplementation an Efficient Nutritional Strategy to Alleviate Skeletal Muscle Damage? A Systematic Review. Nutrients 2017, 9, 1047. [Google Scholar] [CrossRef]
- Garlick, P.J. The Role of Leucine in the Regulation of Protein Metabolism. J. Nutr. 2005, 135, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Ra, S.-G.; Miyazaki, T.; Ishikura, K.; Nagayama, H.; Komine, S.; Nakata, Y.; Maeda, S.; Matsuzaki, Y.; Ohmori, H. Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. J. Int. Soc. Sports Nutr. 2013, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R.A.; Baker, J.S.; István, B.; Gu, Y. Taurine Reverses Oxidative Damages and Restores the Muscle Function in Overuse of Exercised Muscle. Front. Physiol. 2020, 11, 582449. [Google Scholar] [CrossRef]
- Sawicka, A.K.; Renzi, G.; Olek, R.A. The bright and the dark sides of L-carnitine supplementation: A systematic review. J. Int. Soc. Sports Nutr. 2020, 17, 49. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef]
- Pekala, J.; Patkowska-Sokoła, B.; Bodkowski, R.; Jamroz, D.; Nowakowski, P.; Lochyński, S.; Librowski, T. L-Carnitine—Metabolic Functions and Meaning in Humans Life. Curr. Drug Metab. 2011, 12, 667–678. [Google Scholar] [CrossRef]
- Fritz, I.B.; Arrigoni-Martelli, E. Sites of action of carnitine and its derivatives on the cardiovascular system: Interactions with membranes. Trends Pharmacol. Sci. 1993, 14, 355–360. [Google Scholar] [CrossRef]
- Guiotto, A.; Calderan, A.; Ruzza, P.; Borin, G. Carnosine and Carnosine-Related Antioxidants: A Review. Curr. Med. Chem. 2005, 12, 2293–2315. [Google Scholar] [CrossRef]
- Purchas, R.W.; Busboom, J.R. The effect of production system and age on levels of iron, taurine, carnosine, coenzyme Q10, and creatine in beef muscles and liver. Meat Sci. 2005, 70, 589–596. [Google Scholar] [CrossRef]
- Young, J.F.; Therkildsen, M.; Ekstrand, B.; Che, B.N.; Larsen, M.K.; Oksbjerg, N.; Stagsted, J. Novel aspects of health promoting compounds in meat. Meat Sci. 2013, 95, 904–911. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.H.; Coakley, J.J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef] [PubMed]
- Varanoske, A.N.; Hoffman, J.R.; Church, D.D.; Wang, R.; Baker, K.M.; Dodd, S.J.; Coker, N.A.; Oliveira, L.P.; Dawson, V.L.; Fukuda, D.H.; et al. Influence of Skeletal Muscle Carnosine Content on Fatigue during Repeated Resistance Exercise in Recreationally Active Women. Nutrients 2017, 9, 988. [Google Scholar] [CrossRef]
- Mateescu, R.G.; Garmyn, A.J.; O’Neil, M.A.; Tait, R.G.; Abuzaid, A.; Mayes, M.S.; Garrick, D.J.; Van Eenennaam, A.L.; Van Overbeke, D.L.; Hilton, G.G.; et al. Genetic parameters for carnitine, creatine, creatinine, carnosine, and anserine concentration in longissimus muscle and their association with palatability traits in Angus cattle. J. Anim. Sci. 2012, 90, 4248–4255. [Google Scholar] [CrossRef] [PubMed]
- Jiaming, Y.; Rahimi, M.H. Creatine supplementation effect on recovery following exercise-induced muscle damage: A systematic review and meta-analysis of randomized controlled trials. J. Food Biochem. 2021, 45, e13916. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, M.; Rahimi, R.; Ravasi, A.; Jaafari, M. The effect of rest interval between sets on markers of muscle damage in professional bodybuilders. Glob. J. Eng. Technol. 2012, 3, 9–15. [Google Scholar]
- Aoi, W.; Ogaya, Y.; Takami, M.; Konishi, T.; Sauchi, Y.; Park, E.Y.; Wada, S.; Sato, K.; Higashi, A. Glutathione supplementation suppresses muscle fatigue induced by prolonged exercise via improved aerobic metabolism. J. Int. Soc. Sports Nutr. 2015, 12, 7. [Google Scholar] [CrossRef]
- Agostini, F.; Biolo, G. Effect of physical activity on glutamine metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Gambelunghe, C.; Rossi, R.; Micheletti, A.; Mariucci, G.; Rufini, S. Physical exercise intensity can be related to plasma glutathione levels. J. Physiol. Biochem. 2001, 57, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Shimura, N.; Konishi, T.; Sauchi, Y.; Wada, S.; Aoi, W.; Nakamura, Y.; Sato, K. Increase in the Protein-Bound Form of Glutathione in Human Blood after the Oral Administration of Glutathione. J. Agric. Food Chem. 2014, 62, 6183–6189. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, N.; Okamoto, K.; Nakada, K.; Masuda, K. Effect of Conjugated Linoleic Acid Intake on Endurance Exercise Performance and Anti-fatigue in Student Athletes. J. Oleo Sci. 2017, 66, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Spener, F. Conjugated linoleic acids as functional food: An insight into their health benefits. Nutr. Metab. 2009, 6, 36. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Whang, K.-Y.; Park, Y. Impact of conjugated linoleic acid (CLA) on skeletal muscle metabolism. Lipids 2016, 51, 159–178. [Google Scholar] [CrossRef]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef]
- Pedlar, C.R.; Brugnara, C.; Bruinvels, G.; Burden, R. Iron balance and iron supplementation for the female athlete: A practical approach. Eur. J. Sport Sci. 2017, 18, 295–305. [Google Scholar] [CrossRef]
- DellaValle, D.M. Iron Supplementation for Female Athletes: Effects on iron status and performance outcomes. Curr. Sports Med. Rep. 2013, 12, 234–239. [Google Scholar] [CrossRef]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef]
- Siemieniuk, E.; Skrzydlewska, E. KoenzymQ10—biosyIteza i znaczenie biologiczneworganizmach zwiIrząt i człowieka [Coenzyme Q10—biosynthesis and biological role in animal and human organisms]. Postepy Hig. Med. Dosw. 2005, 59, 150–159. [Google Scholar]
- Weber, C.; Bysted, A.; Hølmer, G. Coenzyme Q10 in the diet-daily intake and relative bioavailability. Mol. Asp. Med. 1997, 18, 251–254. [Google Scholar] [CrossRef]
- Diaz-Castro, J.; Moreno-Fernandez, J.; Chirosa, I.; Chirosa, L.J.; Guisado, R.; Ochoa, J.J. Beneficial Effect of Ubiquinol on Hematological and Inflammatory Signaling during Exercise. Nutrients 2020, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Armanfar, M.; Jafari, A.; Dehghan, G.R.; Abdizadeh, L. Effect of coenzyme Q10 supplementation on exercise-induced response of inflammatory indicators and blood lactate in male runners. MJIRI Med. J. Islam. Repub. Iran 2015, 29, 202. [Google Scholar]
- Mehrabani, S.; Askari, G.; Miraghajani, M.; Tavakoly, R.; Arab, A. Effect of coenzyme Q10 supplementation on fatigue: A systematic review of interventional studies. Complement. Ther. Med. 2019, 43, 181–187. [Google Scholar] [CrossRef]
- Haas, R.S. The evidence basis for coenzyme Q therapy in oxidative phosphorylation disease. Mitochondrion 2007, 7, S136–S145. [Google Scholar] [CrossRef]
- Tibullo, D.; Volti, G.L.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef]
- Isenmann, E.; Trittel, L.; Diel, P. The effects of alpha lipoic acid on muscle strength recovery after a single and a short-term chronic supplementation—A study in healthy well-trained individuals after intensive resistance and endurance training. J. Int. Soc. Sports Nutr. 2020, 17, 61. [Google Scholar] [CrossRef]
- Morawin, B.; Turowski, D.; Naczk, M.; Siatkowski, I.; Zembron-Lacny, A. The Combination of α-lipoic Acid Intake with Eccentric Exercise Modulates Erythropoietin Release. Biol. Sport 2014, 31, 179–185. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, S.; Yoon, D.; Kim, J.; Sung, D.J. Role of creatine supplementation in exercise-induced muscle damage: A mini review. J. Exerc. Rehabil. 2015, 11, 244–250. [Google Scholar] [CrossRef]
- Van Loon, L.J.C.; Murphy, R.; Oosterlaar, A.M.; Cameron-Smith, D.; Hargreaves, M.; Wagenmakers, A.J.; Snow, R. Creatine supplementation increases glycogen storage but not GLUT-4 expression in human skeletal muscle. Clin. Sci. 2004, 106, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Tanaka, M.; Nozaki, S.; Mizuma, H.; Ataka, S.; Tahara, T.; Sugino, T.; Shirai, T.; Kajimoto, Y.; Kuratsune, H.; et al. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition 2008, 24, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Adaptations to endurance training depend on exercise-induced oxidative stress: Exploiting redox interindividual variability. Acta Physiol. 2017, 222, e12898. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Carretero, A.; Millan-Domingo, F.; Garcia-Dominguez, E.; Correas, A.G.; Olaso-Gonzalez, G.; Viña, J. Redox-related biomarkers in physical exercise. Redox Biol. 2021, 42, 101956. [Google Scholar] [CrossRef]
- Fuhrman, J.; Ferreri, D.M. Fueling the Vegetarian (Vegan) Athlete. Curr. Sports Med. Rep. 2010, 9, 233–241. [Google Scholar] [CrossRef]
- Venderley, A.M.; Campbell, W.W. Vegetarian Diets. Sports Med. 2006, 36, 293–305. [Google Scholar] [CrossRef]
- Hargreaves, S.M.; Raposo, A.; Saraiva, A.; Zandonadi, R.P. Vegetarian Diet: An Overview through the Perspective of Quality of Life Domains. Int. J. Environ. Res. Public Health 2021, 18, 4067. [Google Scholar] [CrossRef]
- Marsh, K.; Zeuschner, C.; Saunders, A. Health Implications of a Vegetarian Diet: A review. Am. J. Lifestyle Med. 2011, 6, 250–267. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of Nutritional Quality of the Vegan, Vegetarian, Semi-Vegetarian, Pesco-Vegetarian and Omnivorous Diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Kaviani, M.; Shaw, K.; Chilibeck, P.D. Benefits of Creatine Supplementation for Vegetarians Compared to Omnivorous Athletes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 3041. [Google Scholar] [CrossRef]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports Nutr. 2012, 9, 33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).