Cardiorespiratory Interaction and Autonomic Sleep Quality Improve during Sleep in Beds Made from Pinus cembra (Stone Pine) Solid Wood
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Ethics
2.3. Experimental Design
2.4. Material
2.5. Physiological Variables
2.6. Psychometric Variables
2.7. Statistical Evaluation
3. Results
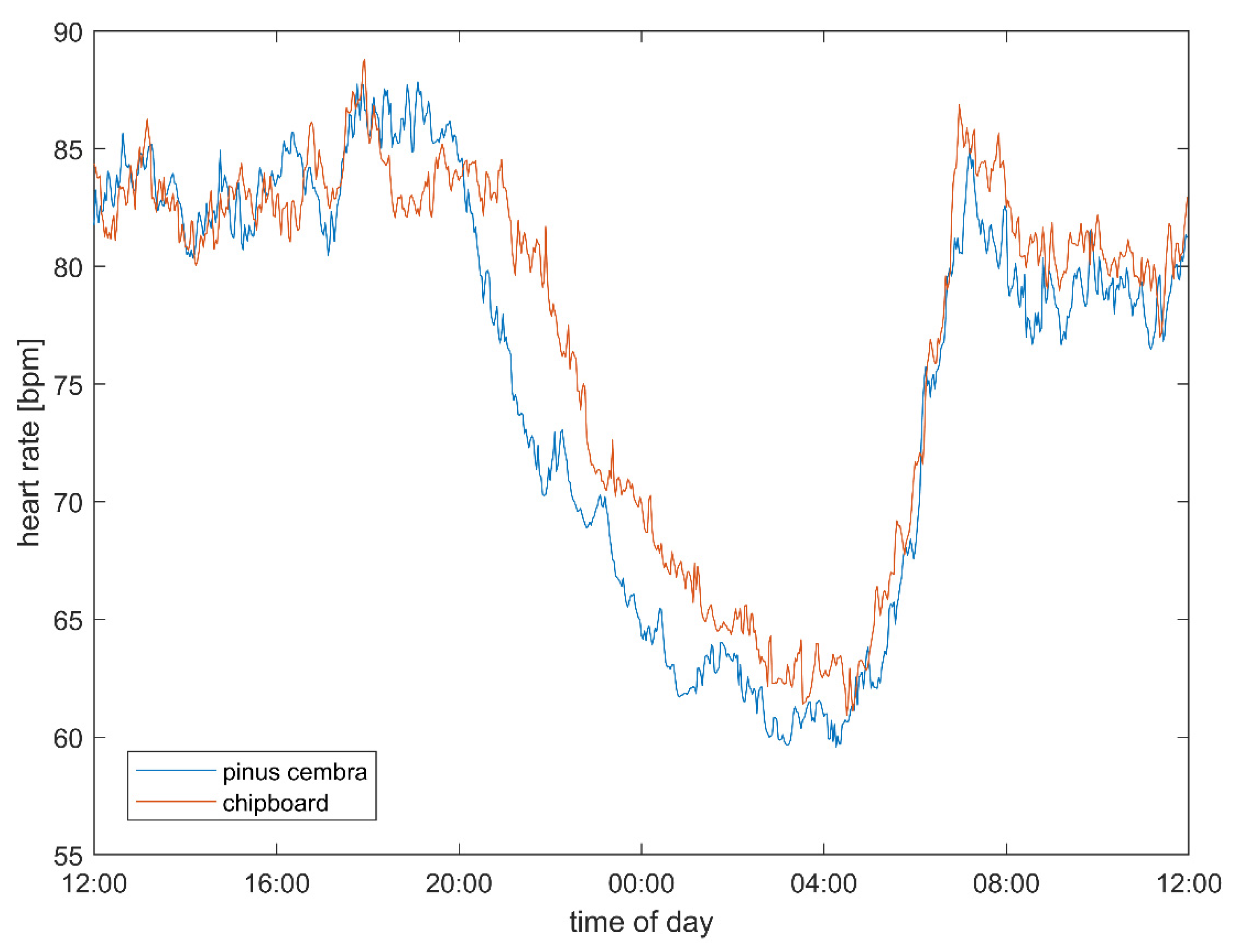
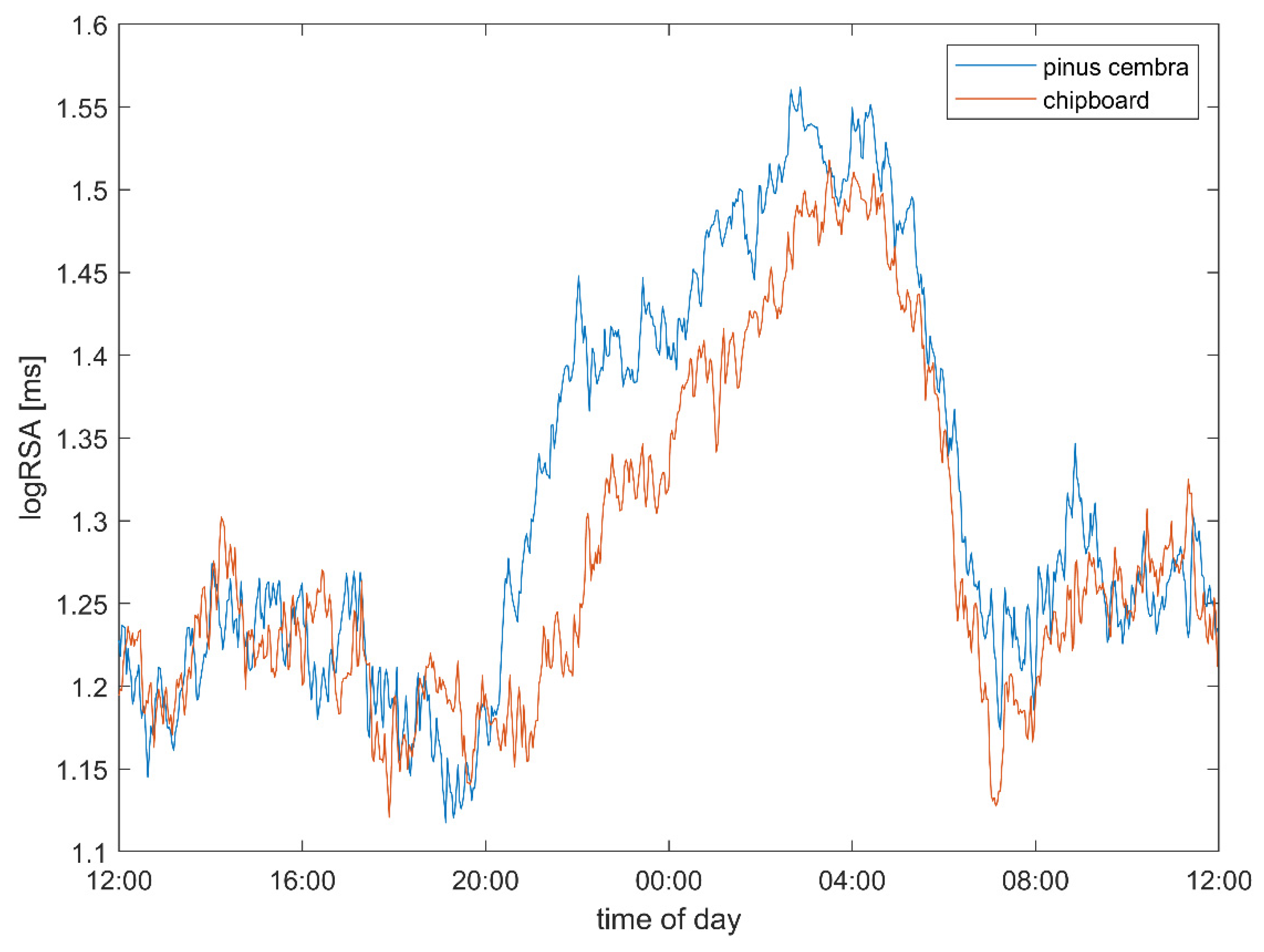
3.1. Heart Rate and Vagal Activity
3.2. Psychometric Results
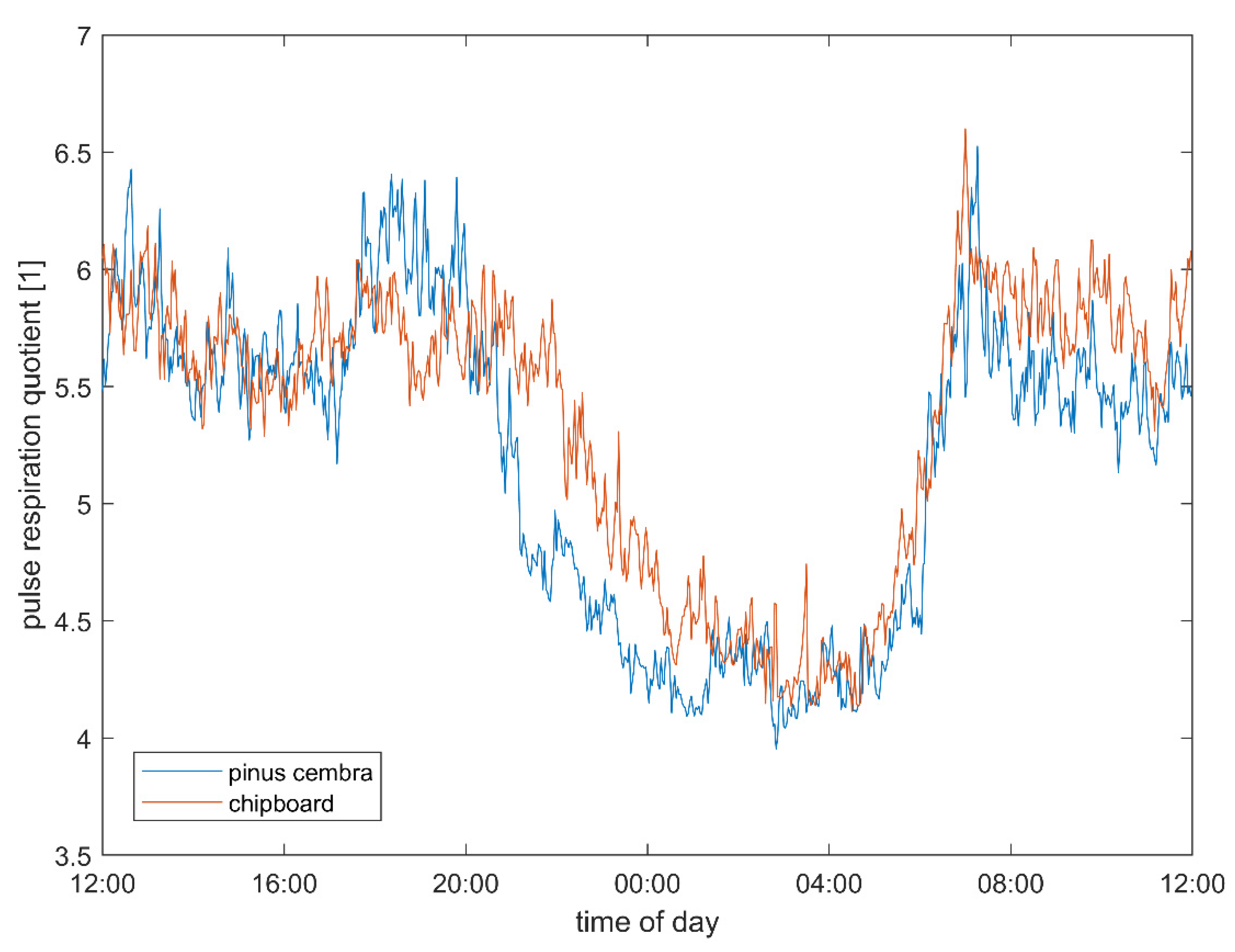
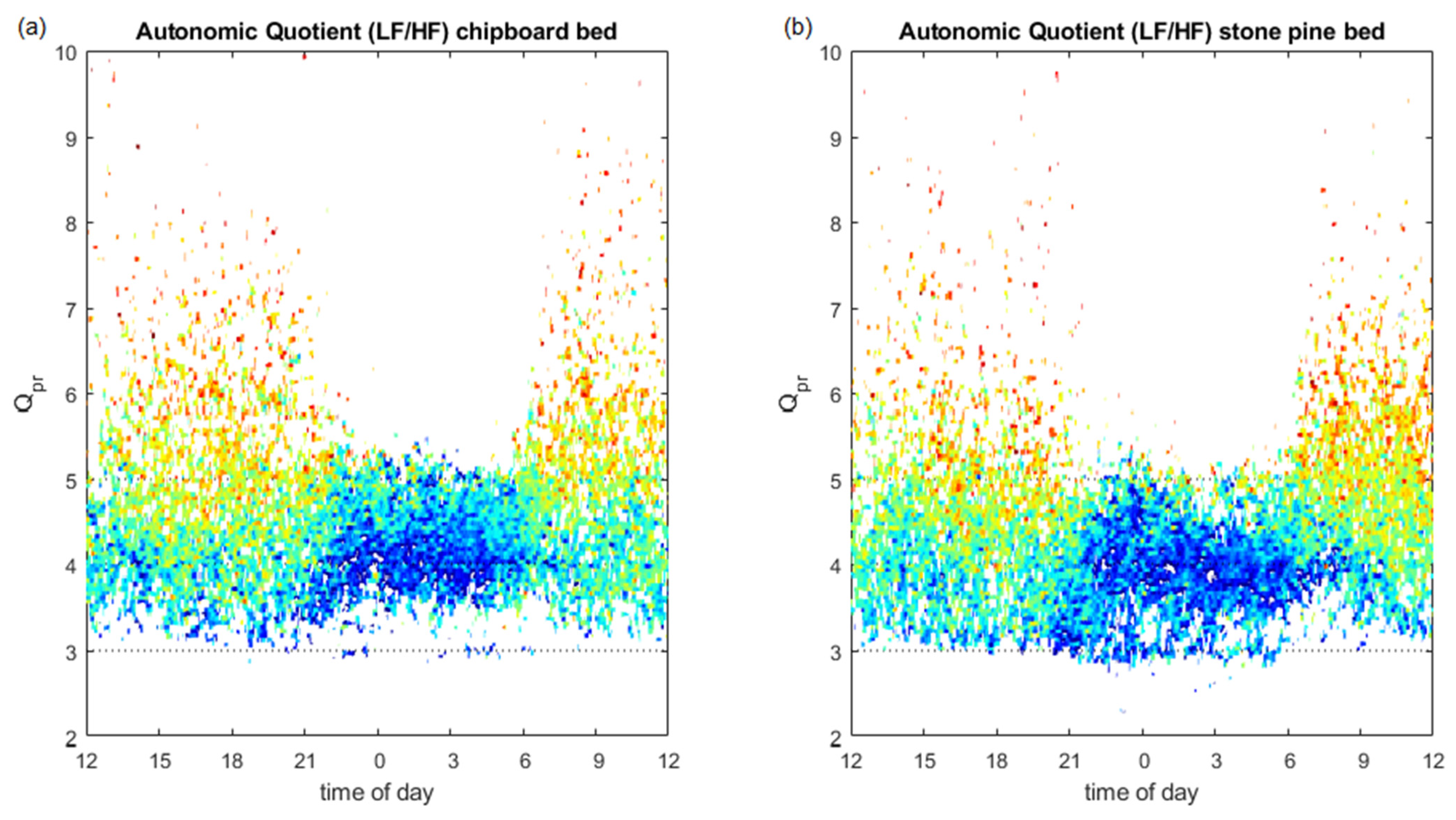
3.3. Pulse–Respiration Quotient
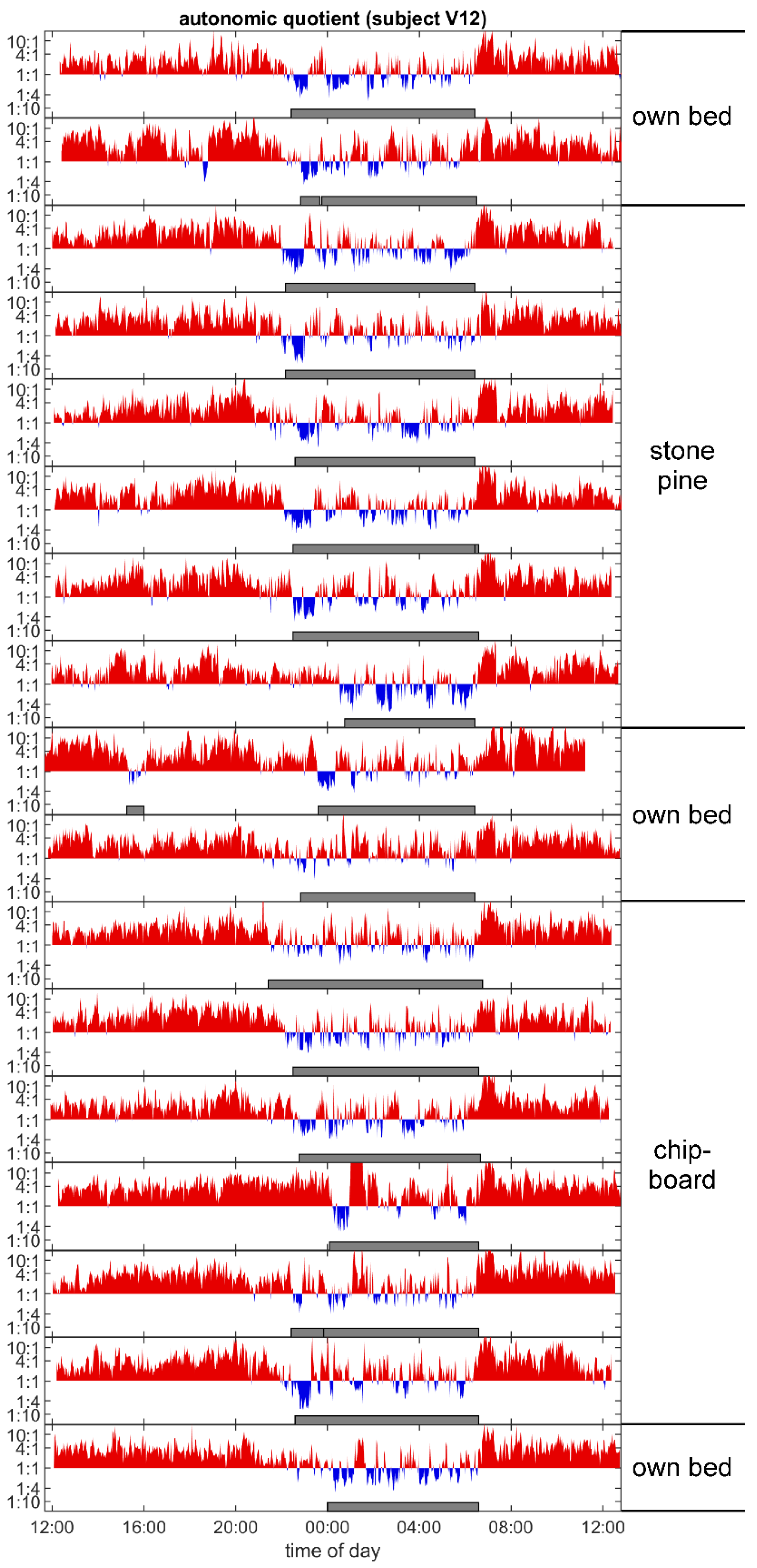
3.4. Autonomic Quotient Interaction
3.5. Sleep Architecture
4. Discussion
4.1. Experimental Design
4.2. Heart Rate and Autonomic Parameters
4.3. Possible Health Consequences
4.4. Pulse–Respiration Quotient
4.5. Relation of Autonomic Quotient to Daytime and the Pulse–Respiration Quotient
4.6. Autonomic Sleep Architecture
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiménez, P.; Dunkl, A.; Eibel, K.; Denk, E.; Grote, V.; Kelz, C.; Moser, M. Wood or Laminate?—Psychological Research of Customer Expectations. Forests 2016, 7, 275. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, P.; Dunkl, A.; Eibel, K.; Denk, E.; Grote, V.; Kelz, C.; Moser, M. Evaluating Psychological Aspects of Wood and Laminate Products in Indoor Settings with Pictures. For. Prod. J. 2015, 65, 263–271. [Google Scholar] [CrossRef]
- Aviat, F.; Gerhards, C.; Rodriguez-Jerez, J.J.; Michel, V.; Bayon, I.L.; Ismail, R.; Federighi, M. Microbial Safety of Wood in Contact with Food: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 491–505. [Google Scholar] [CrossRef]
- Da Costa, A.R.; Kothari, A.; Bannister, G.C.; Blom, A.W. Investigating bacterial growth in surgical theatres: Establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann. R. Coll Surg. Engl. 2008, 90, 417–419. [Google Scholar] [CrossRef] [Green Version]
- Milling, A.; Kehr, R.D.; Wulf, A.; Smalla, K. Survival of bacteria on wood and plastic particles: Dependence on wood species and environmental conditions. Holzforschung 2005, 59, 72–81. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef]
- Pailhories, H.; Munir, M.T.; Aviat, F.; Federighi, M.; Belloncle, C.; Eveillard, M. Oak in Hospitals, the Worst Enemy of Staphylococcus aureus? Infect. Control Hosp. Epidemiol. 2017, 38, 382–384. [Google Scholar] [CrossRef] [Green Version]
- Nyrud, A.Q. Benefits from wood interior in a hospital room: A preference study. Archit. Sci. Rev. 2014, 57, 125–131. [Google Scholar] [CrossRef]
- Bringslimark, T.; Nyrud, A. Patient rooms with different degrees of wood: A preference study conducted among hospital staff. In Proceedings of the 11th World Conference on Timber Engineering 2010, Trentino, Italy, 20–24 June 2010; Volume 3, pp. 2645–2648. [Google Scholar]
- Andersen, L.; Corazon, S.S.; Stigsdotter, U.K. Nature Exposure and Its Effects on Immune System Functioning: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1416. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation by a-pinene on autonomic nervous activity. J. Wood Sci. 2016, 62, 568–572. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological Effects of Touching Wood. Int. J. Environ. Res. Public Health 2017, 14, 801. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological Effects of Touching the Wood of Hinoki Cypress (Chamaecyparis obtusa) with the Soles of the Feet. Int. J. Environ. Res. Public Health 2018, 15, 2135. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, E.; Fukagawa, M.; Okamoto, T.; Ohnuki, K.; Shimizu, K.; Kondo, R. (-)-Bornyl acetate induces autonomic relaxation and reduces arousal level after visual display terminal work without any influences of task performance in low-dose condition. Biomed. Res. 2011, 32, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Song, C.; Ikei, H.; Kagawa, T.; Miyazaki, Y. Analysis of Individual Variations in Autonomic Responses to Urban and Forest Environments. Evid. Based Complement. Altern. Med. 2015, 2015, 671094. [Google Scholar] [CrossRef]
- Lambertz, M.; Langhorst, P. Simultaneous changes of rhythmic organization in brainstem neurons, respiration, cardiovascular system and EEG between 0.05 Hz and 0.5 Hz. J. Auton. Nerv. Syst. 1998, 68, 58–77. [Google Scholar] [CrossRef]
- Moser, M.; Lehofer, M.; Hildebrandt, G.; Voica, M.; Egner, S.; Kenner, T. Phase-and Frequency Coordination of Cardiac and Respiratory Function. Biol. Rhythm. Res. 1995, 26, 100–111. [Google Scholar] [CrossRef]
- Moser, M.; Fruhwirth, M.; Messerschmidt, D.; Goswami, N.; Dorfer, L.; Bahr, F.; Opitz, G. Investigation of a Micro-test for Circulatory Autonomic Nervous System Responses. Front. Physiol. 2017, 8, 448. [Google Scholar] [CrossRef] [Green Version]
- Lehofer, M.; Moser, M.; Hoehn-Saric, R.; McLeod, D.; Liebmann, P.; Drnovsek, B.; Egner, S.; Hildebrandt, G.; Zapotoczky, H.G. Major depression and cardiac autonomic control. Biol. Psychiatry 1997, 42, 914–919. [Google Scholar] [CrossRef]
- Honzikova, N.; Krticka, A.; Novakova, Z.; Zavodna, E. A dampening effect of pulse interval variability on blood pressure variations with respect to primary variability in blood pressure during exercise. Physiol. Res. 2003, 52, 299–309. [Google Scholar] [PubMed]
- Kralemann, B.; Fruhwirth, M.; Pikovsky, A.; Rosenblum, M.; Kenner, T.; Schaefer, J.; Moser, M. In vivo cardiac phase response curve elucidates human respiratory heart rate variability. Nat. Commun. 2013, 4, 2418. [Google Scholar] [CrossRef] [Green Version]
- Moser, M.; Lehofer, M.; Sedminek, A.; Lux, M.; Zapotoczky, H.G.; Kenner, T.; Noordergraaf, A. Heart rate variability as a prognostic tool in cardiology. A contribution to the problem from a theoretical point of view. Circulation 1994, 90, 1078–1082. [Google Scholar] [CrossRef] [Green Version]
- Scholkmann, F.; Wolf, U. The Pulse-Respiration Quotient: A Powerful but Untapped Parameter for Modern Studies About Human Physiology and Pathophysiology. Front. Physiol. 2019, 10, 371. [Google Scholar] [CrossRef] [Green Version]
- Moser, M.; Penter, R.; Fruehwirth, M.; Kenner, T. Why life oscillates—Biological rhythms and health. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; Volume 2006, pp. 424–428. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Whitehurst, L.N.; Cellini, N.; McDevitt, E.A.; Duggan, K.A.; Mednick, S.C. Autonomic activity during sleep predicts memory consolidation in humans. Proc. Natl. Acad. Sci. USA 2016, 113, 7272–7277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penzel, T.; Wessel, N.; Riedl, M.; Kantelhardt, J.W.; Rostig, S.; Glos, M.; Suhrbier, A.; Malberg, H.; Fietze, I. Cardiovascular and respiratory dynamics during normal and pathological sleep. Chaos 2007, 17, 015116. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Venditti Jr, F.J.; Manders, E.S.; Evans, J.C.; Larson, M.G.; Feldman, C.L.; Levy, D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 1994, 90, 878–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Hobi, V. Basler Befindlichkeits-Skala; Beltz Test GmbH: Weinheim, Germany, 1985. [Google Scholar]
- Grote, V. Sleep Recreation and Heart Rate Variability as Indicators for Well-Being and Health. Ph.D. Thesis, Faculty of Natural Sciences at the University of Graz, Graz, Austria, 2009. [Google Scholar] [CrossRef]
- Kallus, K.W. Recovery-Stress Questionaire; Swets Test Services: Frankfurt, Germany, 1995. (In German) [Google Scholar]
- Kallus, W.; Kellmann, M.E. The Recovery-Stress Questionnaires: User Manual; Pearson Assessment & Information GmbH: Frankfurt am Main, Germany, 2016. [Google Scholar]
- Conway, M.W. Monday morning blues. Occup. Health Saf. 2001, 70, 56. [Google Scholar]
- Task Force of the ESC and NASPE. Heart Rate Variability. Standards of Measurement, Physiological Interpretation and Clinical Use (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology). Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Gallasch, E.; Moser, M.; Kozlovskaya, I.B.; Kenner, T.; Noordergraaf, A. Effects of an eight-day space flight on microvibration and physiological tremor. Am. J. Physiol. 1997, 273, R86–R92. [Google Scholar] [CrossRef]
- Gallasch, E.; Rafolt, D.; Moser, M.; Hindinger, J.; Eder, H.; Wiesspeiner, G.; Kenner, T. Instrumentation for assessment of tremor, skin vibrations, and cardiovascular variables in MIR space missions. IEEE Trans. Biomed. Eng. 1996, 43, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Topcu, C.; Fruhwirth, M.; Moser, M.; Rosenblum, M.; Pikovsky, A. Disentangling respiratory sinus arrhythmia in heart rate variability records. Physiol. Meas. 2018, 39, 054002. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, M.; Fruhwirth, M.; Moser, M.; Pikovsky, A. Dynamical disentanglement in an analysis of oscillatory systems: An application to respiratory sinus arrhythmia. Philos. Trans. A Math. Phys. Eng. Sci. 2019, 377, 20190045. [Google Scholar] [CrossRef] [Green Version]
- Grote, V.; Levnajic, Z.; Puff, H.; Ohland, T.; Goswami, N.; Fruhwirth, M.; Moser, M. Dynamics of Vagal Activity Due to Surgery and Subsequent Rehabilitation. Front. Neurosci. 2019, 13, 1116. [Google Scholar] [CrossRef]
- Cysarz, D.; Zerm, R.; Bettermann, H.; Fruhwirth, M.; Moser, M.; Kroz, M. Comparison of respiratory rates derived from heart rate variability, ECG amplitude, and nasal/oral airflow. Ann. Biomed. Eng. 2008, 36, 2085–2094. [Google Scholar] [CrossRef]
- von Bonin, D.; Grote, V.; Buri, C.; Cysarz, D.; Heusser, P.; Moser, M.; Wolf, U.; Laederach, K. Adaption of cardio-respiratory balance during day-rest compared to deep sleep--an indicator for quality of life? Psychiatry Res. 2014, 219, 638–644. [Google Scholar] [CrossRef]
- Moody, G.; Mark, R.; Bump, M.; Weinstein, J.; Berman, A.; Mietus, J.; Goldberger, A. Clinical Validation of the ECG-Derived Respiration (EDR) Technique. Comput. Cardiol. 1986, 13, 507–510. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavior Science, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Huisman, E.R.C.M.; Morales, E.; van Hoof, J.; Kort, H.S.M. Healing environment: A review of the impact of physical environmental factors on users. Build. Environ. 2012, 58, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, F.; Wang, J.; Doyle, J.; Hancock, P.; Mak, C.M.; Liu, S. How indoor environmental quality affects occupants’ cognitive functions: A systematic review. Build. Environ. 2021, 193, 107647. [Google Scholar] [CrossRef]
- Yang, D.; Mak, C.M. Relationships between indoor environmental quality and environmental factors in university classrooms. Build. Environ. 2020, 186, 107331. [Google Scholar] [CrossRef]
- Barrett, P.; Davies, F.; Zhang, Y.; Barrett, L. The impact of classroom design on pupils’ learning: Final results of a holistic, multi-level analysis. Build. Environ. 2015, 89, 118–133. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, G. Balneology and biological rhythm. Dtsch Med. Wochenschr. 1954, 79, 1404–1405. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effect of olfactory stimulation by Hinoki cypress (Chamaecyparis obtusa) leaf oil. J. Physiol. Anthr. 2015, 34, 44. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological Effects of Nature Therapy: A Review of the Research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Human physiological responses to wooden indoor environment. Physiol. Behav. 2017, 174, 27–34. [Google Scholar] [CrossRef]
- Burnard, M.D.; Kutnar, A. Wood and human stress in the built indoor environment: A review. Wood Sci. Technol. 2015, 49, 969–986. [Google Scholar] [CrossRef]
- Nozaki, K.; Miao, E.A. A licence to kill during inflammation. Nature 2019, 570, 316–317. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Libert, C. Inflammation: A nervous connection. Nature 2003, 421, 328–329. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Olofsson, P.S.; Rosas-Ballina, M.; Levine, Y.A.; Tracey, K.J. Rethinking inflammation: Neural circuits in the regulation of immunity. Immunol. Rev. 2012, 248, 188–204. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, P.S.; Katz, D.A.; Rosas-Ballina, M.; Levine, Y.A.; Ochani, M.; Valdes-Ferrer, S.I.; Pavlov, V.A.; Tracey, K.J.; Chavan, S.S. alpha7 nicotinic acetylcholine receptor (alpha7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol. Med. 2012, 18, 539–543. [Google Scholar] [CrossRef]
- Kobayashi, H.; Song, C.; Ikei, H.; Park, B.J.; Lee, J.; Kagawa, T.; Miyazaki, Y. Forest Walking Affects Autonomic Nervous Activity: A Population-Based Study. Front. Public Health 2018, 6, 278. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhaes, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.; Scifo, R.; Gnoula, C.; Morel, L.; Lobaccaro, J.M.; Simpore, J. Anticancer activity of essential oils and their chemical components—A review. Am. J. Cancer Res. 2014, 4, 591–607. [Google Scholar] [PubMed]
- Myers, C.E.; Trepel, J.; Sausville, E.; Samid, D.; Miller, A.; Curt, G. Monoterpenes, Sesquiterpenes and Diterpenes as Cancer Therapy. U.S. Patent No. 5602184, 11 February 1997. [Google Scholar]
- Gascon, M.; Triguero-Mas, M.; Martinez, D.; Dadvand, P.; Rojas-Rueda, D.; Plasencia, A.; Nieuwenhuijsen, M.J. Residential green spaces and mortality: A systematic review. Environ. Int. 2016, 86, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwenhuijsen, M.; Khreis, H. Green space is important for health. Lancet 2017, 389, 700. [Google Scholar] [CrossRef] [Green Version]
- Astell-Burt, T.; Feng, X. Association of Urban Green Space With Mental Health and General Health Among Adults in Australia. JAMA Netw. Open 2019, 2, e198209. [Google Scholar] [CrossRef] [Green Version]
- Sivarajah, S.; Smith, S.M.; Thomas, S.C. Tree cover and species composition effects on academic performance of primary school students. PLoS ONE 2018, 13, e0193254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, B.S.; Malecki, K.M.; Peppard, P.E.; Beyer, K.M.M. Exposure to neighborhood green space and sleep: Evidence from the Survey of the Health of Wisconsin. Sleep Health 2018, 4, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Popham, F. Effect of exposure to natural environment on health inequalities: An observational population study. Lancet 2008, 372, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Archangelidi, O.; Pujades-Rodriguez, M.; Timmis, A.; Jouven, X.; Denaxas, S.; Hemingway, H. Clinically recorded heart rate and incidence of 12 coronary, cardiac, cerebrovascular and peripheral arterial diseases in 233,970 men and women: A linked electronic health record study. Eur. J. Prev. Cardiol. 2018, 25, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Jabre, P.; Roger, V.L.; Weston, S.A.; Adnet, F.; Jiang, R.; Vivien, B.; Empana, J.P.; Jouven, X. Resting heart rate in first year survivors of myocardial infarction and long-term mortality: A community study. Mayo Clin. Proc. 2014, 89, 1655–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouven, X.; Escolano, S.; Celermajer, D.; Empana, J.P.; Bingham, A.; Hermine, O.; Desnos, M.; Perier, M.C.; Marijon, E.; Ducimetiere, P. Heart rate and risk of cancer death in healthy men. PLoS ONE 2011, 6, e21310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legeai, C.; Jouven, X.; Tafflet, M.; Dartigues, J.F.; Helmer, C.; Ritchie, K.; Amouyel, P.; Tzourio, C.; Ducimetiere, P.; Empana, J.P. Resting heart rate, mortality and future coronary heart disease in the elderly: The 3C Study. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Gallasch, E.; Rafolt, D.; Jernej, G.; Kemp, C.; Moser-Kneffel, E.; Kenner, T.; Baevskij, R.; Funtova, I. Cardiovascular monitoring in microgravity: The experiments PULSTRANS and SLEEP. In Health from Space Research; Medicine, A.S.F.A., Ed.; Springer: Wien, Austria; New York, NY, USA, 1992; pp. 167–198. [Google Scholar]
- Lehofer, M.; Moser, M.; Hoehn-Saric, R.; Hildebrandt, G.; Drnovsek, B.; Niederl, T.; Zapotoczky, H.G. Diminished pulse-respiration-coupling in depressed patients. Biol. Psychiatry 1996, 39, 526. [Google Scholar] [CrossRef]
- Moser, M.; Fruhwirth, M.; Penter, R.; Winker, R. Why life oscillates--from a topographical towards a functional chronobiology. Cancer Causes Control 2006, 17, 591–599. [Google Scholar] [CrossRef]
- Kleitman, N. Basic rest-activity cycle—22 years later. Sleep 1982, 5, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, M.; Fruhwirth, M.; Kenner, T. The symphony of life. Importance, interaction, and visualization of biological rhythms. IEEE Eng. Med. Biol. Mag. 2008, 27, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Schulz, G.; Lambertz, M.; Schulz, B.; Langhorst, P.; Krienke, B. Reticular formation of the lower brainstem. A common system for cardio-respiratory and somatomotor functions. Cross-correlation analysis of discharge patterns of neighbouring neurones. J. Auton. Nerv. Syst. 1985, 12, 35–62. [Google Scholar] [CrossRef]


| Heart Rate Variability (HRV) (n = 15) * | P. Cembra (24 h; 6×) | Chipboard (24 h; 6×) | Sleep (Mean; 6×) | Wake (Mean; 6×) | Bed Effect (2 × 2 ANOVA) (P. Cembra vs. Chipboard) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | Mean | SD | Mean | SD | P. Cembra | Chip-Board | P. Cembra | Chip-Board | F | P | Part. Eta2 | |||
| Heart rate (HR) | bpm | 74.09 | ± | 7.42 | 76.61 | ± | 7.39 | 60.91 | 63.76 | 79.97 | 82.18 | 9.56 | 0.008 ** | 0.41 |
| Standard deviation of RR (SDNN) | ms | 73.50 | ± | 22.04 | 70.23 | ± | 20.03 | 77.12 | 71.91 | 71.81 | 69.01 | 3.01 | 0.105 | 0.18 |
| Vagal tone (logRSArr) | log(ms) | 1.31 | ± | 0.18 | 1.27 | ± | 0.16 | 1.50 | 1.45 | 1.23 | 1.20 | 4.32 | 0.057 * | 0.24 |
| Total variability power (lnTOTrr) | ln(ms2) | 8.14 | ± | 0.58 | 8.07 | ± | 0.53 | 8.14 | 8.01 | 8.15 | 8.08 | 2.65 | 0.126 | 0.16 |
| Low frequency power (lnLFrr) | ln(ms2) | 6.77 | ± | 0.68 | 6.72 | ± | 0.62 | 6.72 | 6.59 | 6.81 | 6.77 | 1.75 | 0.207 | 0.11 |
| High frequency power (lnHFrr) | ms2 | 5.87 | ± | 0.81 | 5.78 | ± | 0.75 | 6.51 | 6.34 | 5.60 | 5.54 | 2.10 | 0.169 | 0.13 |
| Very low frequency power (lnVLFrr) | ms2 | 7.39 | ± | 0.51 | 7.30 | ± | 0.47 | 7.14 | 7.01 | 7.50 | 7.41 | 3.18 | 0.096 | 0.19 |
| Ratio LF/HF | [ ] | 0.89 | ± | 0.47 | 0.93 | ± | 0.47 | 0.21 | 0.25 | 1.21 | 1.23 | 0.46 | 0.509 | 0.03 |
| Pulse-respiration quotient (Qpr) | bpc | 5.03 | ± | 0.89 | 5.29 | ± | 0.91 | 4.09 | 4.27 | 5.45 | 5.72 | 5.30 | 0.037 * | 0.28 |
| Respiratory rate (ATMFrsa) | fpm | 15.60 | ± | 2.05 | 15.42 | ± | 2.01 | 15.10 | 15.13 | 15.84 | 15.56 | 0.45 | 0.514 | 0.03 |
| Heart Rate Variability (HRV) (n = 15; Sleep Measurements = 180 with Each 6 Epochs) * | P. Cembra (Sleep Mean 30 min Epochs) | P. Cembra (Core Sleep; 3 h) | Chipboard (Sleep Mean 30 min Epochs) | Chipboard (Core Sleep; 3 h) | Mean Difference (P. Cembra - Chipboard) | Bed Effect (2[×6] ANOVA) (P. Cembra vs. Chipboard) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| unit | 1 | 2 | 3 | 4 | 5 | 6 | Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | Mean | SD | Mean | SE | F | P | Part. Eta2 | |
| Heart rate (HR) | bpm | 64.04 | 62.74 | 63.30 | 63.04 | 61.77 | 61.47 | 62.73 | 8.47 | 67.01 | 66.00 | 67.11 | 65.52 | 64.43 | 64.07 | 65.69 | 7.28 | −2.96 | 1.03 | 8.33 | 0.012* | 0.37 |
| Standard deviation of RR (SDNN) | ms | 74.87 | 68.25 | 71.51 | 68.12 | 71.71 | 72.38 | 71.14 | 25.83 | 62.57 | 57.86 | 62.82 | 60.75 | 67.03 | 77.22 | 64.71 | 26.20 | 6.43 | 3.67 | 3.07 | 0.102 | 0.18 |
| Vagal tone (logRSArr) | log(ms) | 1.51 | 1.53 | 1.48 | 1.47 | 1.46 | 1.46 | 1.49 | 0.24 | 1.43 | 1.43 | 1.37 | 1.39 | 1.45 | 1.43 | 1.42 | 0.21 | 0.07 | 0.03 | 5.03 | 0.042* | 0.26 |
| Total variability power (lnTOTrr) | ln(ms2) | 8.73 | 8.53 | 8.59 | 8.59 | 8.65 | 8.64 | 8.62 | 0.75 | 8.40 | 8.18 | 8.44 | 8.34 | 8.50 | 8.77 | 8.44 | 0.70 | 0.18 | 0.10 | 3.18 | 0.096 | 0.19 |
| Low frequency power (lnLFrr) | ln(ms2) | 7.32 | 7.13 | 7.18 | 7.20 | 7.26 | 7.24 | 7.22 | 0.89 | 7.01 | 6.77 | 7.14 | 7.00 | 7.13 | 7.33 | 7.06 | 0.84 | 0.16 | 0.11 | 2.19 | 0.161 | 0.14 |
| High frequency power (lnHFrr) | ms2 | 7.24 | 7.18 | 7.02 | 6.97 | 6.99 | 6.95 | 7.06 | 1.01 | 6.93 | 6.81 | 6.66 | 6.73 | 6.91 | 6.94 | 6.83 | 0.88 | 0.23 | 0.13 | 3.14 | 0.098 | 0.18 |
| Very low frequency power (lnVLFrr) | ms2 | 7.62 | 7.30 | 7.49 | 7.54 | 7.59 | 7.66 | 7.53 | 0.68 | 7.23 | 6.93 | 7.45 | 7.26 | 7.40 | 7.85 | 7.35 | 0.67 | 0.18 | 0.09 | 3.80 | 0.072 | 0.21 |
| Ratio LF/HF | [ ] | 0.08 | −0.05 | 0.17 | 0.23 | 0.27 | 0.30 | 0.17 | 0.75 | 0.07 | −0.04 | 0.48 | 0.27 | 0.22 | 0.39 | 0.23 | 0.73 | −0.07 | 0.06 | 1.17 | 0.298 | 0.08 |
| Pulse-respiration quotient (Qpr) | bpc | 4.16 | 4.04 | 4.08 | 4.06 | 4.09 | 4.03 | 4.08 | 0.50 | 4.32 | 4.21 | 4.33 | 4.25 | 4.18 | 4.21 | 4.25 | 0.40 | −0.17 | 0.07 | 6.13 | 0.027* | 0.30 |
| Respiratory rate (ATMFrsa) | fpm | 15.76 | 15.83 | 15.77 | 15.69 | 15.26 | 15.48 | 15.63 | 1.86 | 15.78 | 15.93 | 15.77 | 15.57 | 15.69 | 15.40 | 15.69 | 1.70 | −0.06 | 0.14 | 0.16 | 0.694 | 0.01 |
| Scale (n = 15; Surveys = [30 to] 180) * | [Unit]; Frequency | P. Cembra (3 Weeks) | Chipboard (3 Weeks) | Mean Difference P. Cembra - Chipboard | Bed Effect (2 Groups Design) (P. Cembra vs. Chipboard) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SE | F | P | Part. Eta2 | ||||
| PSQI (n = 12) | [ ]; 2× | 4.25 | ± | 2.34 | 3.83 | ± | 2.32 | 0.42 | 0.63 | 0.43 | 0.524 | 0.04 |
| Sleep Recovery Scale (SRSHRI, main factor; n = 14) | [z], 12× (6 times each) | 0.06 | ± | 0.54 | −0.07 | ± | 0.63 | 0.13 | 0.23 | 0.33 | 0.575 | 0.03 |
| sleep characteristics (SRS, Factor [F]1) | 0.05 | ± | 0.54 | 0.00 | ± | 0.63 | 0.04 | 0.17 | 0.07 | 0.795 | 0.01 | |
| sleep quality (F2) | 0.01 | ± | 0.62 | −0.04 | ± | 0.64 | 0.04 | 0.22 | 0.04 | 0.850 | 0.00 | |
| sleep time (F3) | 0.08 | ± | 0.69 | −0.11 | ± | 0.61 | 0.19 | 0.24 | 0.63 | 0.440 | 0.05 | |
| Well-being/mood (BBS, total; n = 14) | [z], 12× (6 times each) | 0.16 | ± | 0.74 | −0.18 | ± | 0.78 | 0.34 | 0.13 | 7.01 | 0.020 * | 0.35 |
| intrapsychic stability | 0.16 | ± | 0.51 | −0.25 | ± | 0.89 | 0.40 | 0.16 | 6.64 | 0.023 * | 0.34 | |
| vitality | 0.12 | ± | 0.70 | −0.10 | ± | 0.78 | 0.23 | 0.16 | 1.96 | 0.185 | 0.13 | |
| social extraversion | 0.19 | ± | 0.86 | −0.19 | ± | 0.65 | 0.38 | 0.18 | 4.63 | 0.051 * | 0.26 | |
| vigility | 0.06 | ± | 0.78 | −0.09 | ± | 0.80 | 0.15 | 0.09 | 2.70 | 0.125 | 0.17 | |
| Recovery-Stress Q. (RSTQ-Basic-24, multivariat, n = 15) | [z], 12× (6 times each) | 0.20 | 0.826 | 0.03 | ||||||||
| stress scales | −0.04 | ± | 0.84 | −0.04 | ± | 0.83 | 0.01 | 0.08 | 0.01 | 0.946 | 0.00 | |
| recovery scales | −0.02 | ± | 0.90 | 0.08 | ± | 0.69 | −0.10 | 0.16 | 0.39 | 0.540 | 0.03 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grote, V.; Frühwirth, M.; Lackner, H.K.; Goswami, N.; Köstenberger, M.; Likar, R.; Moser, M. Cardiorespiratory Interaction and Autonomic Sleep Quality Improve during Sleep in Beds Made from Pinus cembra (Stone Pine) Solid Wood. Int. J. Environ. Res. Public Health 2021, 18, 9749. https://doi.org/10.3390/ijerph18189749
Grote V, Frühwirth M, Lackner HK, Goswami N, Köstenberger M, Likar R, Moser M. Cardiorespiratory Interaction and Autonomic Sleep Quality Improve during Sleep in Beds Made from Pinus cembra (Stone Pine) Solid Wood. International Journal of Environmental Research and Public Health. 2021; 18(18):9749. https://doi.org/10.3390/ijerph18189749
Chicago/Turabian StyleGrote, Vincent, Matthias Frühwirth, Helmut K. Lackner, Nandu Goswami, Markus Köstenberger, Rudolf Likar, and Maximilian Moser. 2021. "Cardiorespiratory Interaction and Autonomic Sleep Quality Improve during Sleep in Beds Made from Pinus cembra (Stone Pine) Solid Wood" International Journal of Environmental Research and Public Health 18, no. 18: 9749. https://doi.org/10.3390/ijerph18189749







