Metals in Occluded Water: A New Perspective for Pollution in Drinking Water Distribution Systems
Abstract
:1. Introduction
2. Materials and Methods
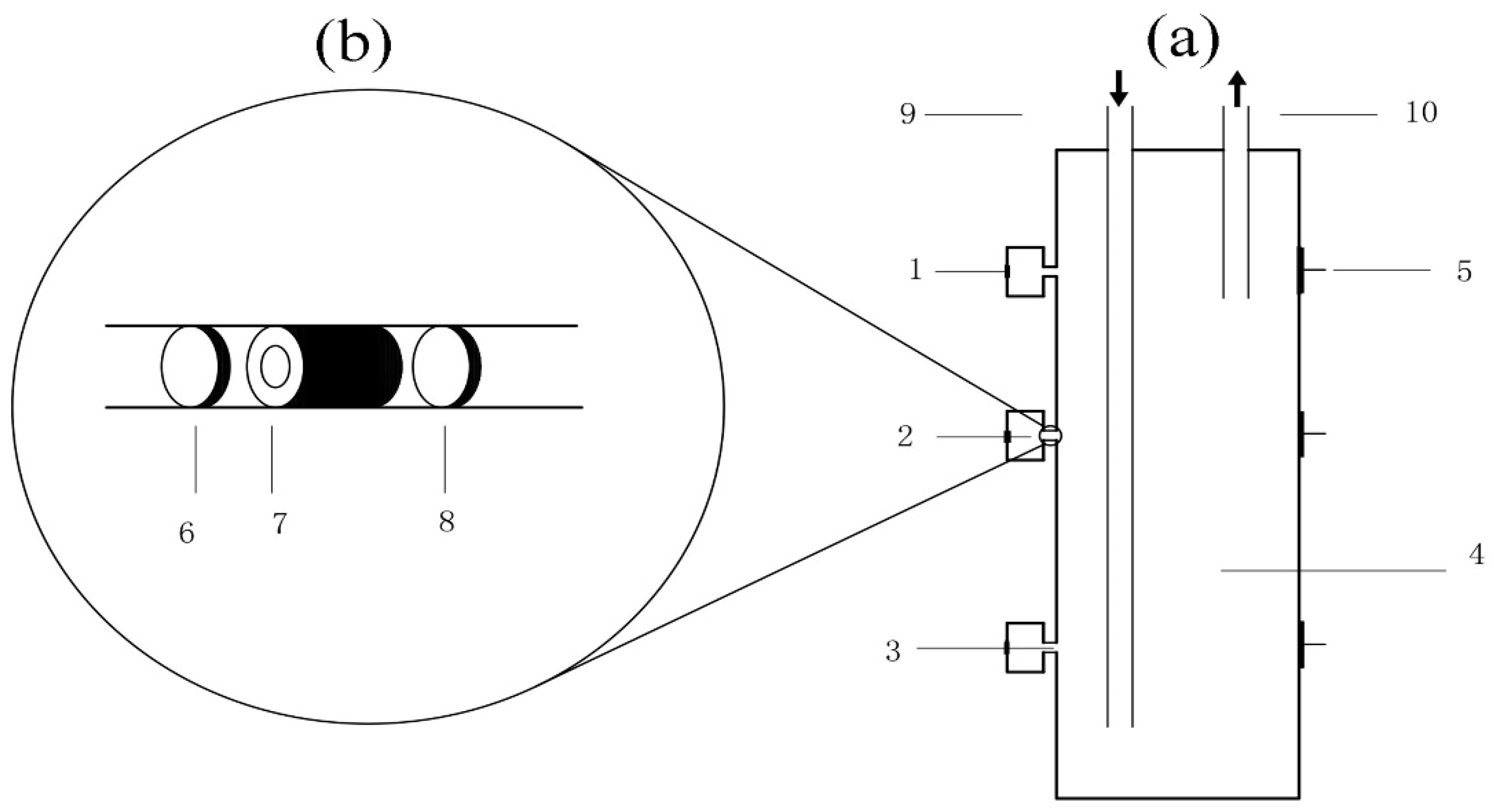
2.1. Experimental Set Up
2.2. Experimental Operation
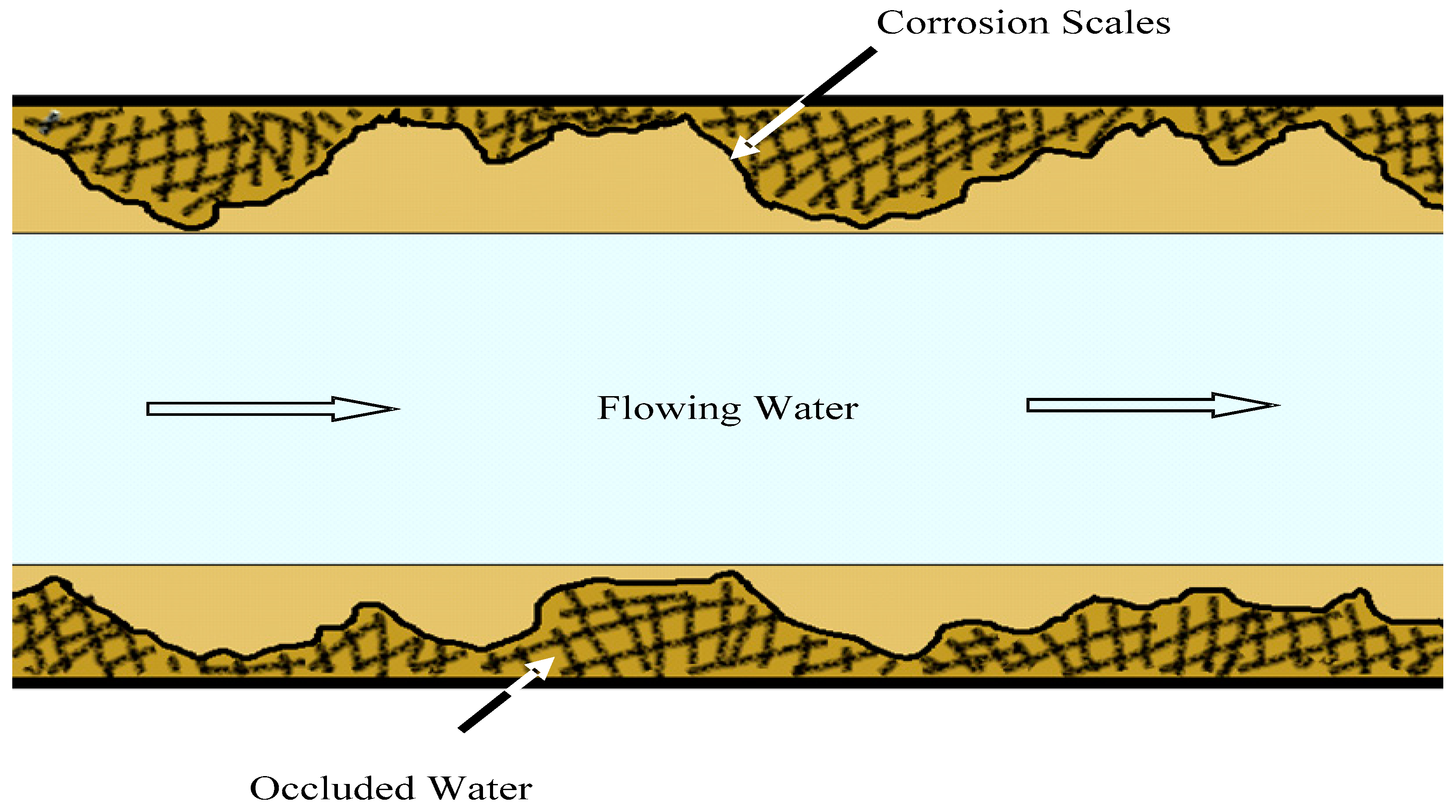
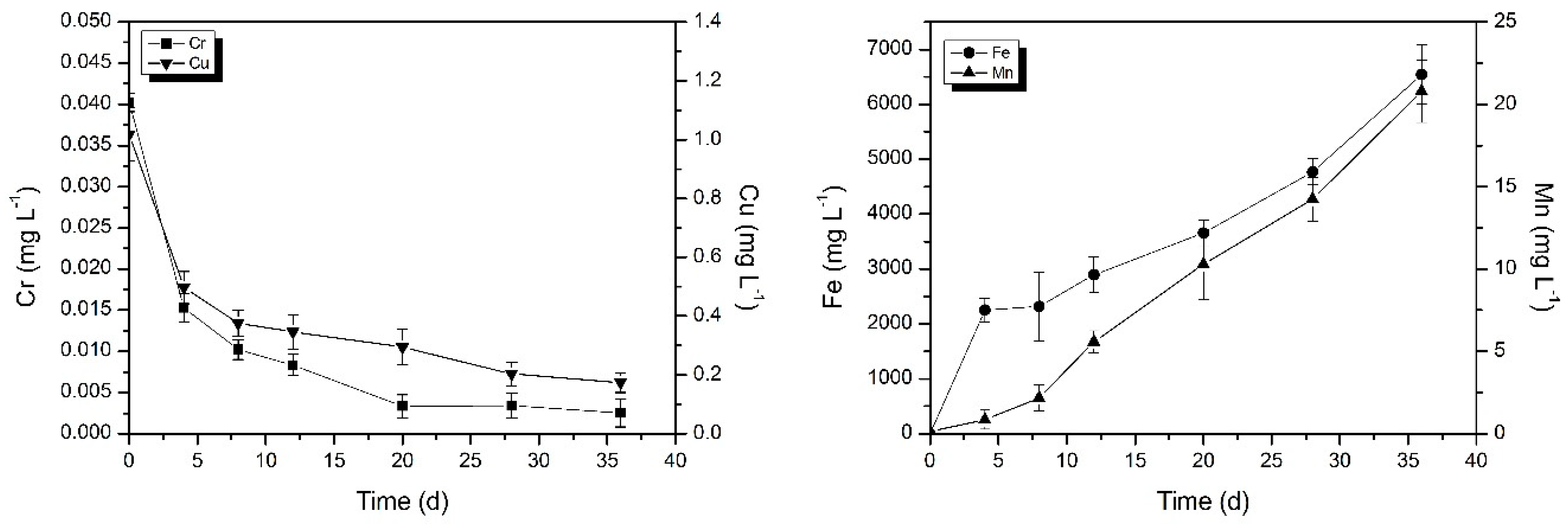
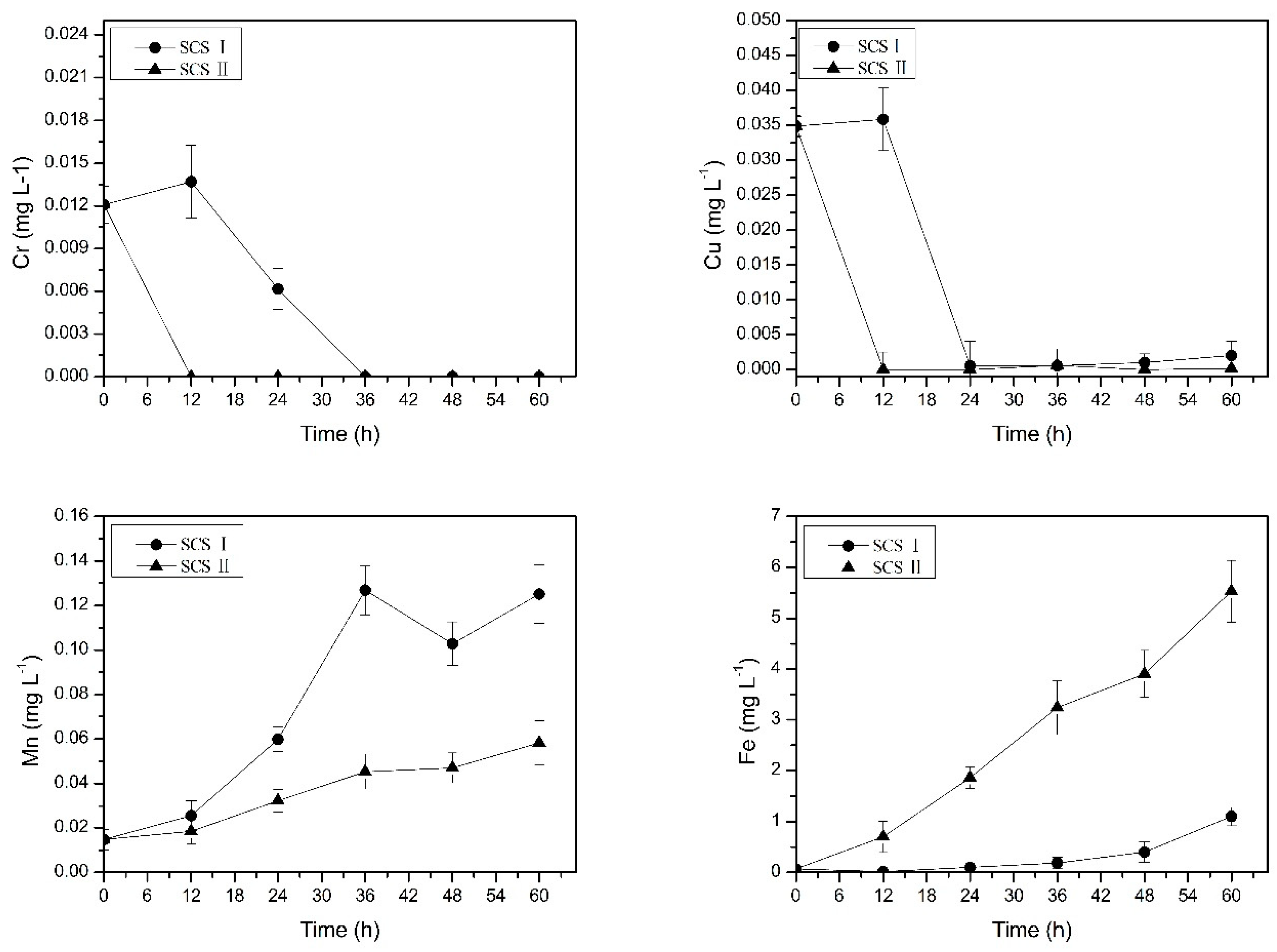
2.2.1. Phase I: The Change in Metal Concentration in Occluded Area
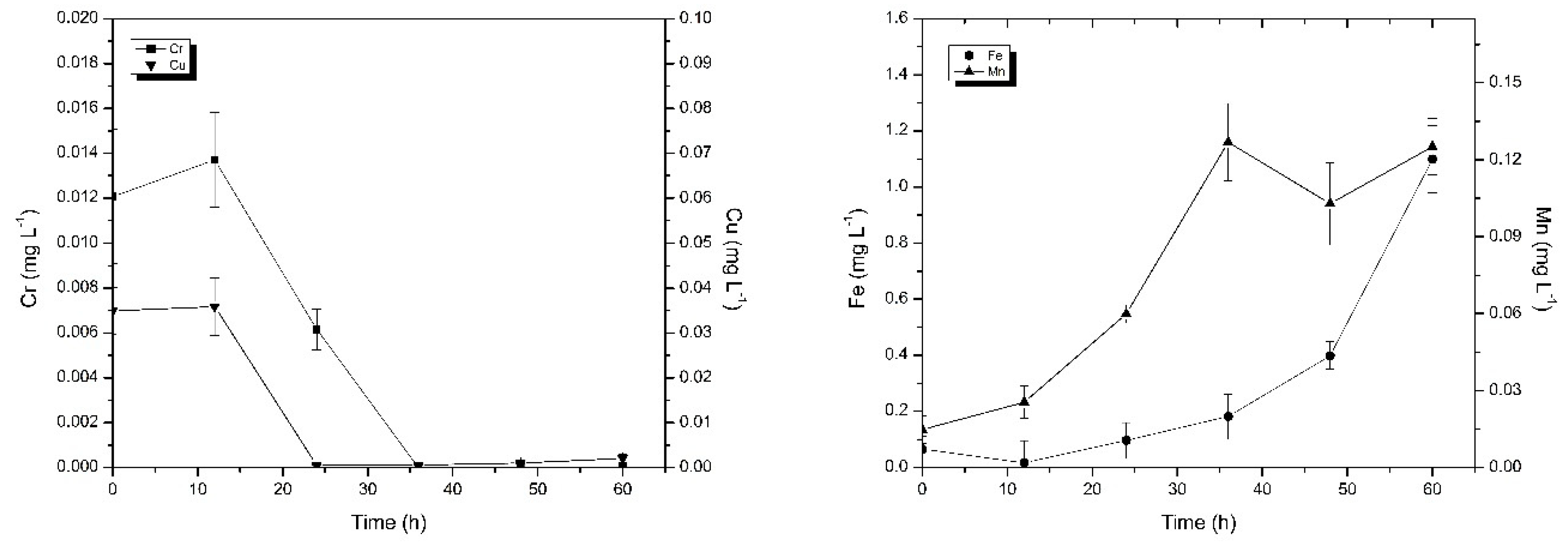
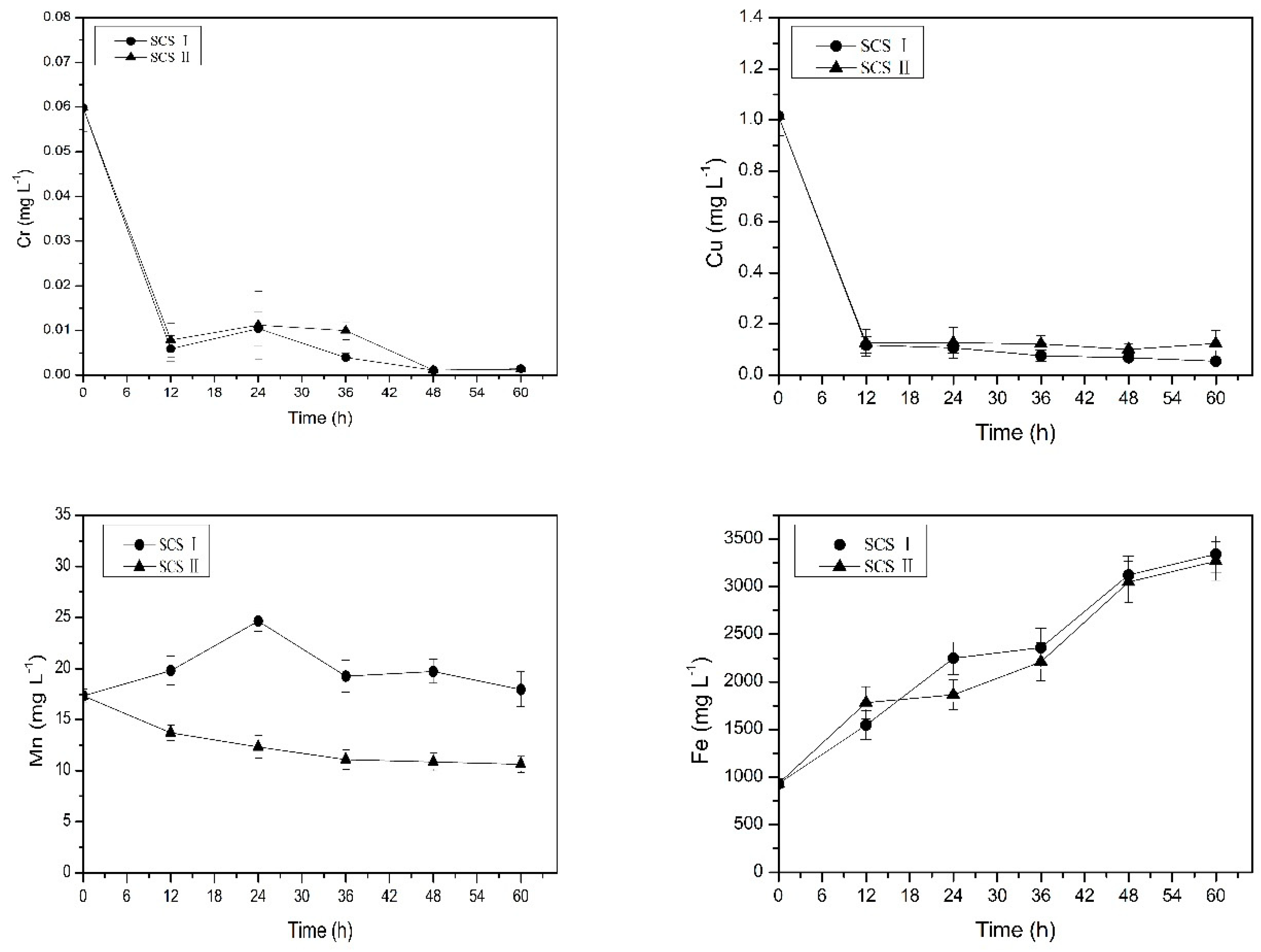
2.2.2. Phase II: The Source and Migration of Metals in the Occluded Water
2.3. Analysis Method
3. Results and Discussion
3.1. Study on the Change of Metal Concentration in Occluded Water
3.2. The Source and Migration of Metal in the Occluded Water
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, M.; Nystrom, V.; Pieper, K.; Parks, J.; Little, B.; Guilliams, R.; Esqueda, T.; Edwards, M. The relationship between discolored water from corrosion of old iron pipe and source water conditions. Environ. Eng. Sci. 2018, 35, 943–952. [Google Scholar] [CrossRef]
- Mussared, A.; Fabris, R.; Vreeburg, J.; Jelbart, J.; Drikas, M. The origin and risks associated with loose deposits in a drinking water distribution system. Water Sci. Technol. Water Supply 2019, 19, 291–302. [Google Scholar] [CrossRef]
- Zhang, Y.; Edwards, M. Accelerated chloramine decay and microbial growth by nitrification in premise plumbing. J. Am. Water Works Assoc. 2009, 101, 51–62. [Google Scholar]
- Park, J.; Kim, J.S.; Kim, S.; Shin, E.; Oh, K.H.; Kim, Y.; Kim, C.H.; Hwang, M.A.; Jin, C.M.; Na, K.; et al. A waterborne outbreak of multiple diarrhoeagenic Escherichia coli infections associated with drinking water at a school camp. Int. J. Infect. Dis. 2018, 66, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Maki, G.; McElmurry, S.; Kilgore, P.; Love, N.; Misikir, H.; Perri, M.; Zervos, M. Bacterial colonization of drinking water: Implications for an aging U.S. water infrastructure. Int. J. Infect. Dis. 2019, 79, 30–31. [Google Scholar] [CrossRef]
- Rubino, F.; Corona, Y.; Pérez, J.J.; Smith, C. Bacterial Contamination of Drinking Water in Guadalajara, Mexico. Int. J. Environ. Res. Public Health 2019, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Bodrud-Doza, M.; Islam, A.R.M.T.; Hossain, S.; Moniruzzaman, M.; Deb, N.; Bhuiyan, M.A.Q. Appraising spatial variations of As, Fe, Mn and NO3 contaminations associated health risks of drinking water from Surma basin, Bangladesh. Chemosphere 2019, 218, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, Q.; Song, L.; Shi, B. Risk assessment of heavy metals in pipe scales and loose deposits formed in drinking water distribution systems. Sci. Total Environ. 2019, 652, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Z.; Chen, Y.; Hai, Y. Characteristics of iron corrosion scales and water quality variations in drinking water distribution systems of different pipe materials. Water Res. 2016, 106, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ling, W.; Liu, S.; Zhao, J.; Liu, R.; Chen, Q.; Qiang, Z.; Qu, J. Development of systems for detection, early warning, and control of pipeline leakage in drinking water distribution: A case study. J. Environ. Sci. 2011, 23, 1816–1822. [Google Scholar] [CrossRef]
- Swietlik, J.; Raczyk-Stanislawiak, U.; Piszora, P.; Nawrocki, J. Corrosion in drinking water pipes: The importance of green rusts. Water Res. 2012, 46, 1–10. [Google Scholar] [CrossRef]
- Yafeng, L.I.; Jiang, B.; Zhao, H.; Tao, C. Experimental study on internal corrosion of grey cast iron pipeline in water distribution. J. Shenyang Jianzhu Univ. (Nat. Sci.) 2010, 26, 321–325. [Google Scholar]
- Sarin, P.; Snoeyink, V.L.; Lytle, D.A.; Kriven, W.M. Iron corrosion scales: Model for scale growth, iron release, and colored water formation. J. Environ. Eng. 2004, 130, 364–373. [Google Scholar]
- McNeill, L.S.; Edwards, M. Iron pipe corrosion in distribution systems. J. Am. Water Works Assoc. 2001, 93, 88–100. [Google Scholar]
- Baylis, J.R. Prevention of corrosion and “Red Water”. J. Am. Water Works Assoc. 1926, 15, 598–633. [Google Scholar] [CrossRef]
- Nawrocki, J.; Raczykstanisławiak, U.; Świetlik, J.; Olejnik, A.; Sroka, M.J. Corrosion in a distribution system: Steady water and its composition. Water Res. 2010, 44, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, O.H.; Button, K.S.; Vuorinen, A.; Carlson, L.; Mair, D.M.; Yut, L.A. Bacterial, chemical, and mineralogical characteristics of tubercles in distribution pipelines. J. Am. Water Works Assoc. 1980, 72, 626–635. [Google Scholar] [CrossRef]
- Tong, H.; Zhao, P.; Zhang, H.; Tian, Y.; Chen, X.; Zhao, W.; Li, M. Identification and characterization of steady and occluded water in drinking water distribution systems. Chemosphere 2015, 119, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Nie, H.; Wang, W.; Huang, Y.; Wang, J. Occurrence and Toxicological Risk Assessment of Polycyclic Aromatic Hydrocarbons and Heavy Metals in Drinking Water Resources of Southern China. Int. J. Environ. Res. Public Health 2018, 15, 1422. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Shah, M.T.; Khan, S. Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem. J. 2011, 98, 334–343. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Ji, E.Y.; Qiu, Y.B. Under-deposit occluded cell corrosion inhibition mechanism of N80 steel in NaCl solution added with sodium laurate. Corros. Prot. 2010, 31, 111–114. [Google Scholar]
- Zhu, Y.; Zhao, Y. Study on the influential factors of underscale local corrosion of N80 steel. J. Nanyang Inst. Technol. 2009, 6, 46–50. [Google Scholar]
- PRC. State Standard of the People’s Republic of China: Standards for Drinking Water Quality; GB5749-2006; Ministry of health of the People’s Republic of China: Beijing, China, 2006.
- Turdi, M.; Yang, L. Trace elements contamination and human health risk assessment in drinking water from the agricultural and pastoral areas of Bay County, Xinjiang, China. Int. J. Environ. Res. Public Health 2016, 13, 938. [Google Scholar]
- U.S. EPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, 2nd ed.EPA: Washington, DC, USA, 1996. [Google Scholar]
- Li, M.; Liu, Z.; Chen, Y. Physico-chemical characteristics of corrosion scales from different pipes in drinking water distribution systems. Water 2018, 10, 931. [Google Scholar]
- Lytle, D.A.; Magnuson, M.L.; Snoeyink, V.L. Effect of oxidants on the properties of Fe(III) particles and suspensions formed from the oxidation of Fe(II). J. Am. Water Works Assoc. 2004, 96, 112–124. [Google Scholar] [CrossRef]
- Friedman, M.J.; Hill, A.S.; Reiber, S.H.; Valentine, R.L.; Larsen, G.; Young, A.; Korshin, G.V.; Peng, C.Y. Assessment of Inorganics Accumulation in Drinking Water System Scales and Sediments; Water Research Foundation: Denver, CO, USA, 2010. [Google Scholar]
- Abraham, M.R.; Susan, T.B. Water contamination with heavy metals and trace elements from Kilembe copper mine and tailing sites inWestern Uganda; implications for domestic water quality. Chemosphere 2017, 169, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Lopez-Roldan, R.; Cortina, J.L. Presence of metals in drinking water distribution networks due to pipe material leaching: A review. Toxicol. Environ. Chem. 2013, 95, 870–889. [Google Scholar]
- Han, B.; Chen, R.; Shi, B.; Xu, W.; Zhuang, Y. Practical evaluation of inorganic contaminant presence in a drinking water distribution system after hydraulic disturbance. J. Water Supply Res. Technol. -Aqua 2018, 67, 12–21. [Google Scholar] [CrossRef]
- Fontana, M.G. Corrosion Engineering; Tata McGraw-Hill Education: New York, NY, USA, 2005; pp. 46–50. [Google Scholar]
- Li, G.; Ding, Y.; Xu, H.; Jin, J.; Shi, B. Characterization and release profile of (Mn, Al)-bearing deposits in drinking water distribution systems. Chemosphere 2018, 197, 73–80. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447. [Google Scholar] [PubMed]
- Yang, F.; Shi, B.; Gu, J.; Wang, D.; Yang, M. Morphological and physicochemical characteristics of iron corrosion scales formed under different water source histories in a drinking water distribution system. Water Res. 2012, 46, 5423–5433. [Google Scholar] [CrossRef]
| Element | Weight Percentage (%) | Atomic Percentage (%) | Strength |
|---|---|---|---|
| C | 3.58 | 14.63 | 10.20 |
| Na | 0.18 | 0.37 | 0.99 |
| Mg | 0.19 | 0.38 | 2.18 |
| Al | 0.16 | 0.28 | 2.57 |
| Si | 0.04 | 0.06 | 0.83 |
| Sr | 0.04 | 0.02 | 0.49 |
| Pb | 0.10 | 0.02 | 2.15 |
| Ca | 0.15 | 0.19 | 3.64 |
| Cr | 0.16 | 0.15 | 3.42 |
| Mn | 0.70 | 0.54 | 4.27 |
| Fe | 94.42 | 83.07 | 1017.64 |
| Cu | 0.30 | 0.26 | 4.07 |
| SW1 | SFW2 | SOW3 | |
|---|---|---|---|
| pH | 7.06 | 7.03 | 3.95 |
| DO (mg L−1) | 7.50 | 7.96 | <0.50 |
| Cond (us cm−1) | 544.00 | 549.70 | 4023.80 |
| Cl- (mg L−1) | 62.56 | 69.31 | 594.72 |
| SO42- (mg L−1) | 143.43 | 152.62 | 1481.13 |
| NO3- (mg L−1) | 13.23 | 10.18 | 4.98 |
| Fe (mg L−1) | 0.384 | 0.065 | 934.210 |
| Mn (mg L−1) | 0.130 | 0.014 | 17.310 |
| Cu (mg L−1) | 1.018 | 0.035 | 1.015 |
| Cr (mg L−1) | 0.040 | 0.012 | 0.059 |
| Ca (mg L−1) | 4.264 | 4.267 | 17.012 |
| Mg (mg L−1) | 20.453 | 22.122 | 16.751 |
| SW1: simulation water | |||
| SFW2: simulation flowing water | |||
| SOW3: simulation occluded water DO: dissolved oxygen | |||
| Element | Weight Percentage (%) | Atomic Percentage (%) | Strength |
|---|---|---|---|
| C | 10.09 | 33.13 | 14.42 |
| Na | 1.64 | 2.83 | 5.24 |
| Mg | 0.46 | 0.75 | 2.97 |
| Si | 3.41 | 4.79 | 38.26 |
| Sr | 1.30 | 0.59 | 7.33 |
| Pb | 1.66 | 0.31 | 12.27 |
| Ca | 0.84 | 0.84 | 7.92 |
| Cr | 0.30 | 0.21 | 2.07 |
| Mn | 0.16 | 0.10 | 0.85 |
| Fe | 78.98 | 55.74 | 347.50 |
| Cu | 1.16 | 0.71 | 3.02 |
| Mg (ug g−1) | Al (ug g−1) | Mn (ug g−1) | Cu (ug g−1) | Cr (ug g−1) | Ni (ug g−1) | Fe (ug g−1) | As (ug g−1) | Zn (ug g−1) | |
|---|---|---|---|---|---|---|---|---|---|
| Before | 9.521 | 38.100 | 32.054 | 8.296 | 3.696 | 2.769 | 85.401 | 12.925 | 20.028 |
| After | 5.909 | 57.474 | 39.857 | 11.433 | 9.361 | 3.815 | 69.634 | 16.509 | 34.086 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, H.; Li, Z.; Hu, X.; Xu, W.; Li, Z. Metals in Occluded Water: A New Perspective for Pollution in Drinking Water Distribution Systems. Int. J. Environ. Res. Public Health 2019, 16, 2849. https://doi.org/10.3390/ijerph16162849
Tong H, Li Z, Hu X, Xu W, Li Z. Metals in Occluded Water: A New Perspective for Pollution in Drinking Water Distribution Systems. International Journal of Environmental Research and Public Health. 2019; 16(16):2849. https://doi.org/10.3390/ijerph16162849
Chicago/Turabian StyleTong, Huiyan, Zhongyue Li, Xingshuai Hu, Weiping Xu, and Zhengkun Li. 2019. "Metals in Occluded Water: A New Perspective for Pollution in Drinking Water Distribution Systems" International Journal of Environmental Research and Public Health 16, no. 16: 2849. https://doi.org/10.3390/ijerph16162849





