The Presence of Cu Facilitates Adsorption of Tetracycline (TC) onto Water Hyacinth Roots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Plant Collection, Acclimation and Preparation
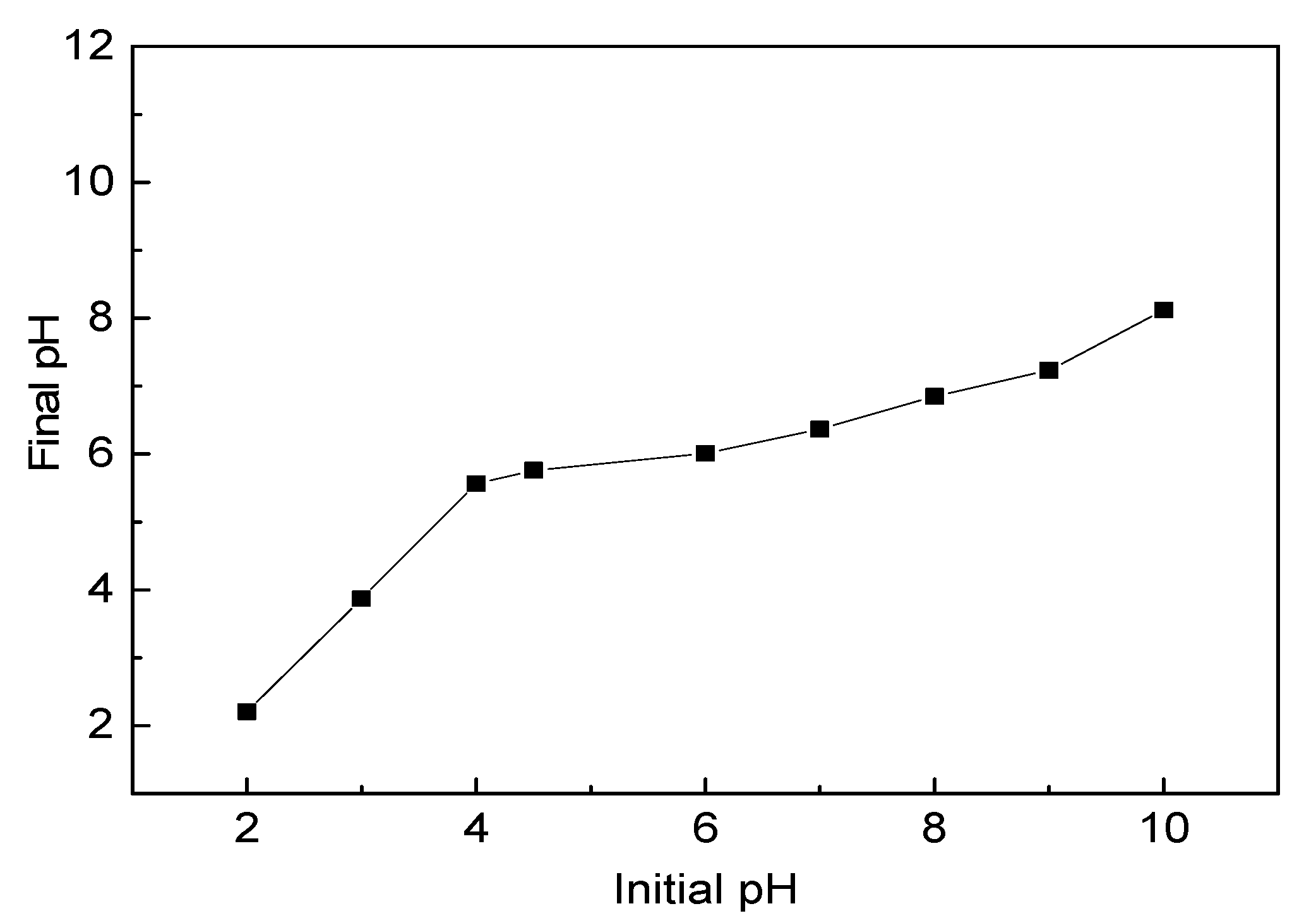
2.3. The Point of Zero Charge (pHPZC) of Water Hyacinth Roots
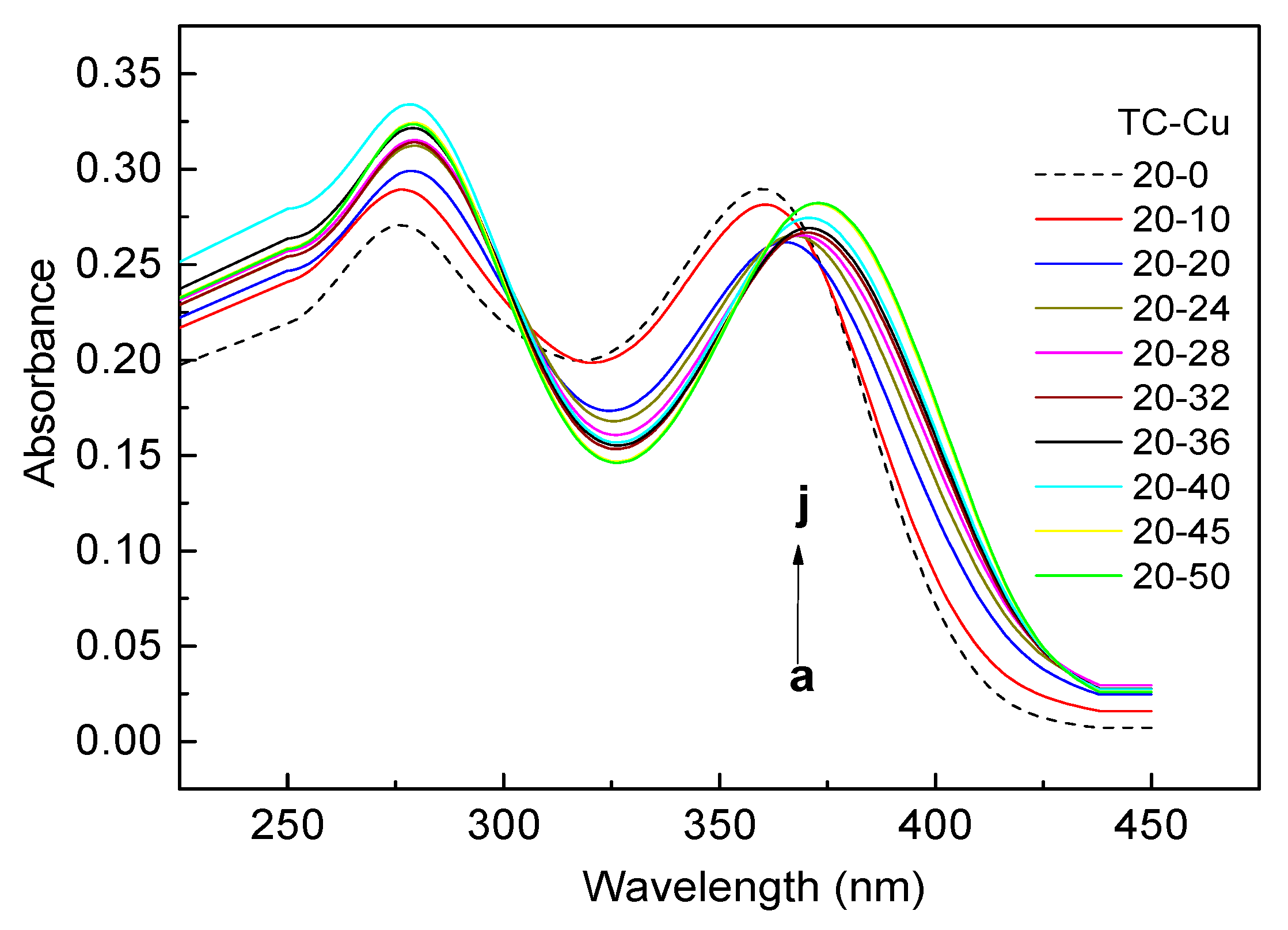
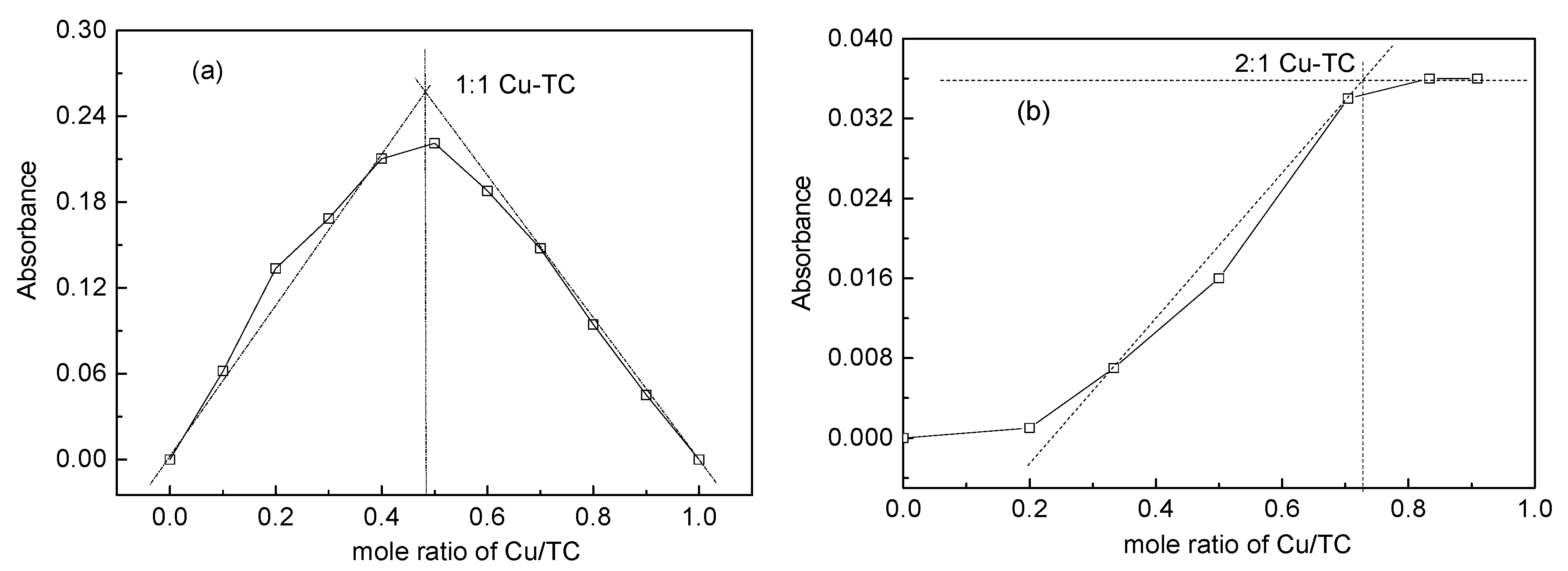
2.4. The Complexation of TC with Cu(II)
2.5. Batch Adsorption Experiment.
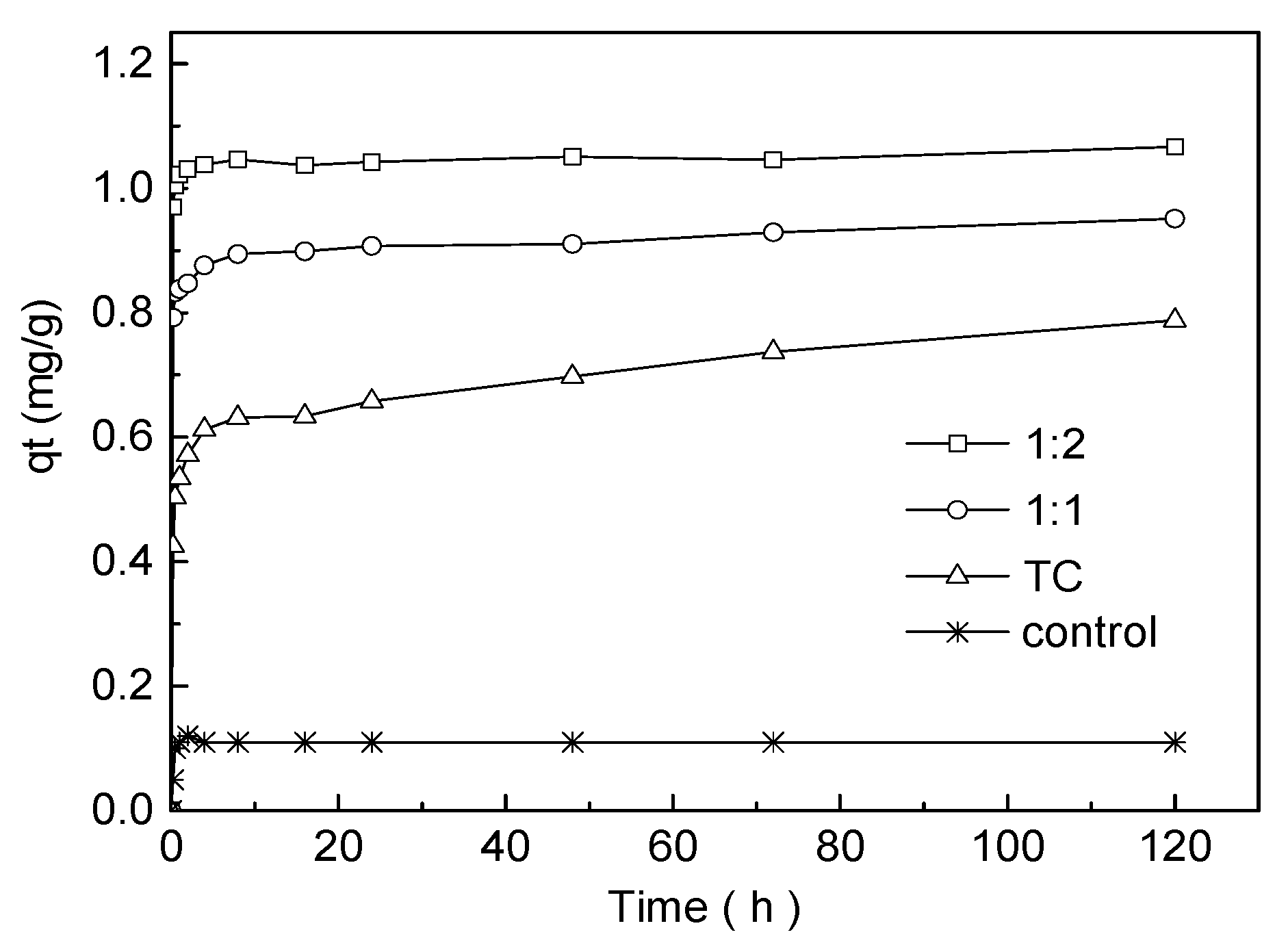
2.5.1. Adsorption Kinetics of TC in the Presence/Absence of Cu
2.5.2. Adsorption Isotherm of TC in the Presence/Absence of Cu
2.5.3. Adsorption of TC as Affected by pH and Cu(II)
2.6. Analysis of TC
2.7. Data Analysis
3. Results and Discussion
3.1. The Point of Zero Charge (pHPZC) of Water Hyacinth Roots
3.2. Complexes of TC with Cu(II)
3.3. Adsorption Kinetics Experiment
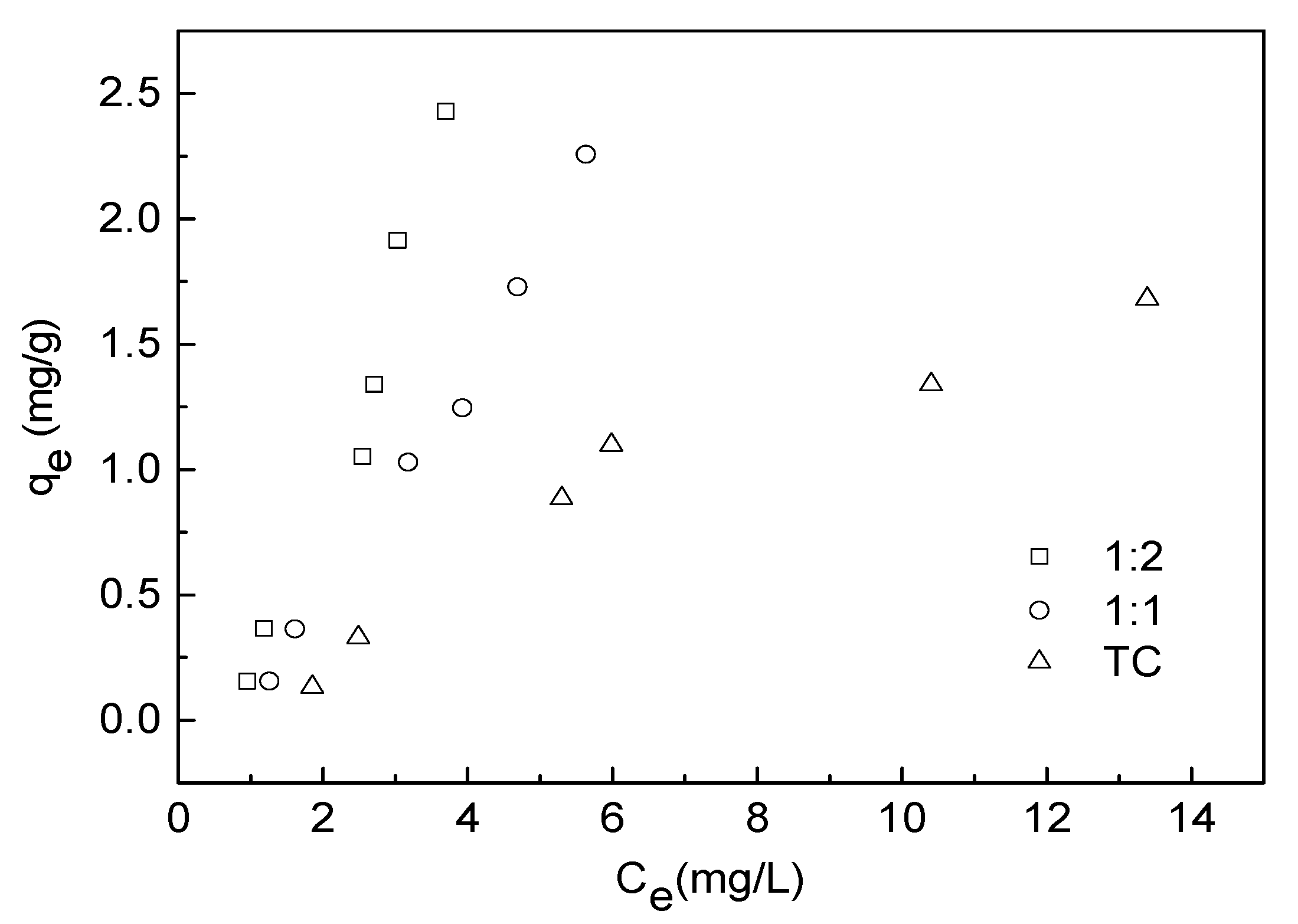
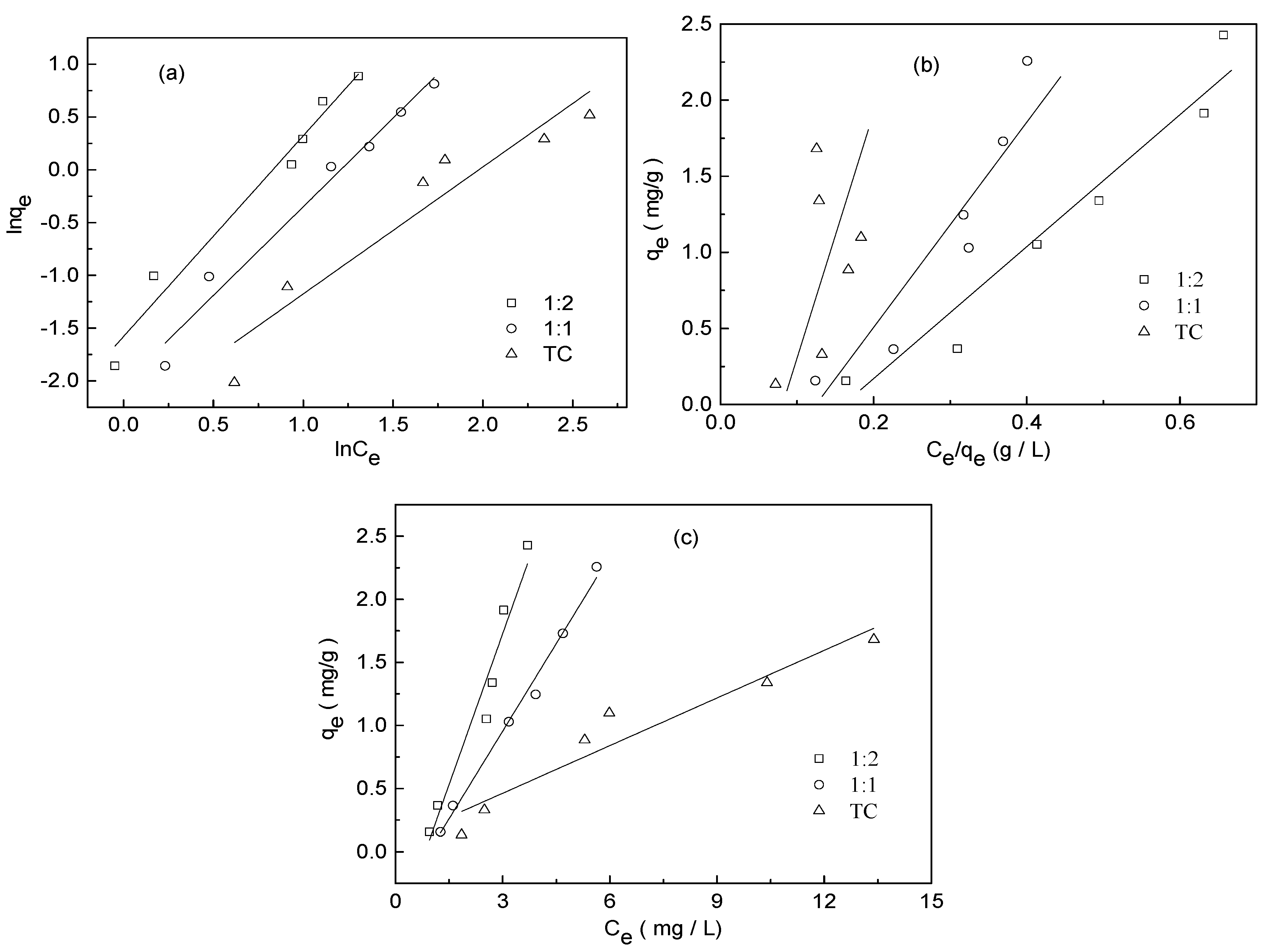
3.4. Adsorption Isotherm Experiment
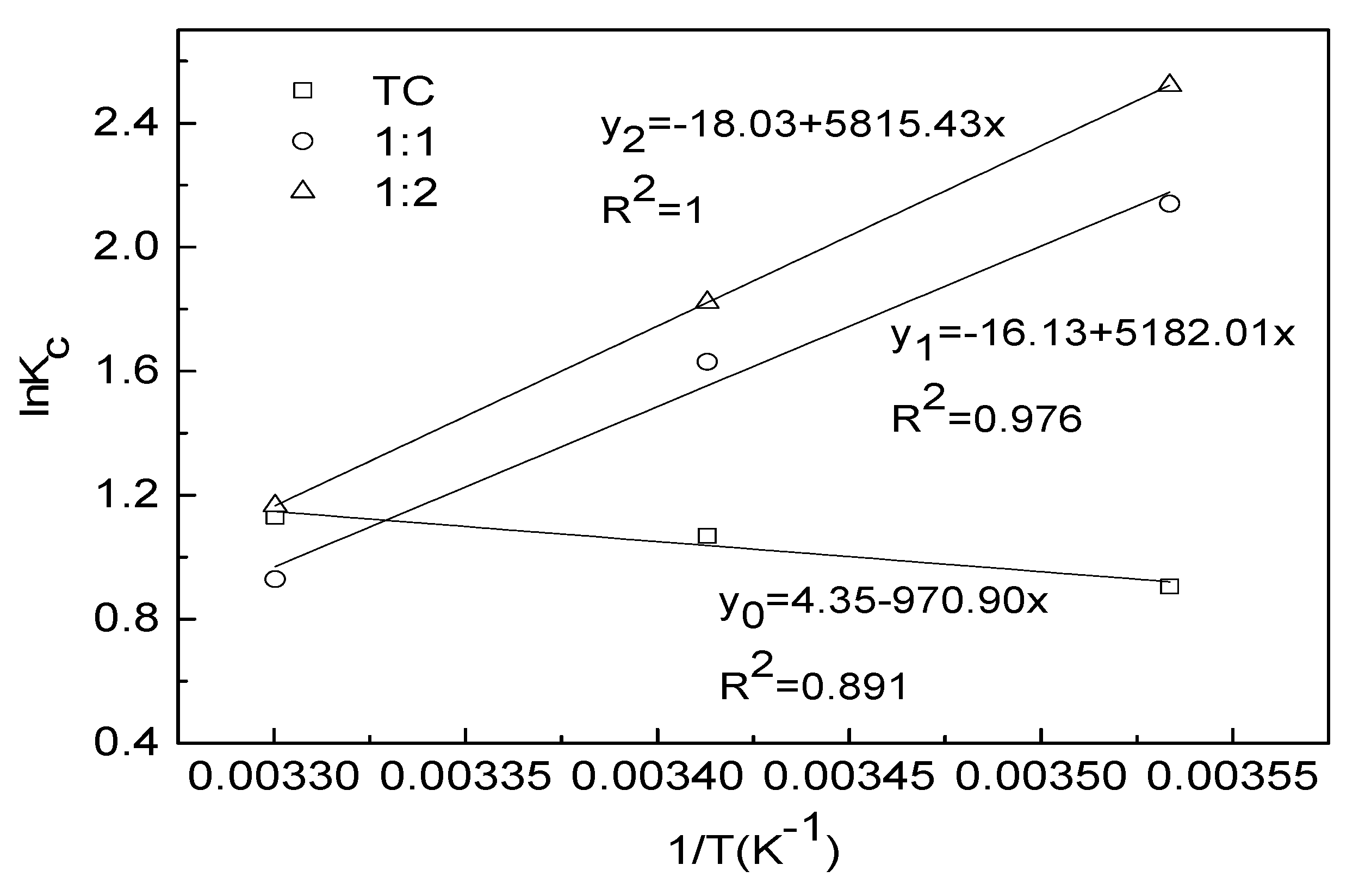
3.5. Adsorption Thermodynamic Parameters of TC in Different Complexation
3.6. Sorption of TC onto Water Hyacinth as Affected by pH and Cu(II)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarmah, A.K.; Meyer, M.T.; Boxall, A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (vas) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Thielebruhn, S.; Beck, I.C. Effects of sulfonamide and tetracycline antibiotics on soil microbial activity and microbial biomass. Chemosphere 2005, 59, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.; Badia-Fabregat, M.; Vicent, T.; Caminal, G.; Rodríguez-Mozaz, S.; Balcázar, J.L.; Barceló, D. Fungal treatment for the removal of antibiotics and antibiotic resistance genes in veterinary hospital wastewater. Chemosphere 2016, 152, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, H.J.; Zheng, Y.M.; Qu, J.H.; Chen, J.P. Systematic study of synergistic and antagonistic effects on adsorption of tetracycline and copper onto a chitosan. J. Colloid Interface Sci. 2010, 344, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Gao, Y.; Luo, J.; Yan, S.; Rengel, Z.; Zhang, Z. Interaction of veterinary antibiotic tetracyclines and copper on their fates in water and water hyacinth (eichhornia crassipes). J. Hazard. Mater. 2014, 280, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Duan, L.; Zhu, D.Q.; Chen, W. Effects of Cu(II) and Ni(II) ions on adsorption of tetracycline to functionalized carbon nanotubes. J. Zhejiang Univ. A 2014, 15, 653–661. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, K.; Gao, B.; Zhang, G.; Liu, X.; Zhao, Y. Adsorption of tetracycline on soil and sediment: Effects of pH and the presence of Cu(II). J. Hazard. Mater. 2011, 190, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, D.; Zhang, G.; Frigon, M.; Meng, X.; Li, K. Adsorption mechanisms and the effect of oxytetracycline on activated sludge. Bioresour. Technol. 2014, 151, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Mahmoud, A.E.D.; Fawzy, M.; Radwan, A. Artificial intelligence modeling of Cadmium(II) biosorption using rice straw. Appl. Water Sci. 2017, 7, 823–831. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Zhou, Q.X.; Ying, W.; Qiang, Y.; Xie, X.J. Effects of soil/solution ratios and cation types on adsorption and desorption of tetracycline in soils. Soil Sci. Soc. Am. J. 2010, 74, 1553–1561. [Google Scholar] [CrossRef]
- Wang, Y.J.; Jia, D.A.; Sun, R.J.; Zhu, H.W.; Zhou, D.M. Adsorption and cosorption of tetracycline and Copper(II) on montmorillonite as affected by solution pH. Environ. Sci. Technol. 2008, 42, 3254–3259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, Y.; Guo, Y.; Gu, X.; Wang, X.; Zhang, Y. Interactions of tetracycline with Cd(II), Cu(II) and Pb(II) and their cosorption behavior in soils. Environ. Pollut. 2013, 180, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Huang, C.H. Transformation of tetracyclines mediated by Mn(II) and Cu(II) ions in the presence of Oxygen. Environ. Sci. Technol. 2009, 43, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.D.; Zhu, Y.G.; Fu, B.J.; Marschner, P.; He, J.Z. The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community. Environ. Pollut. 2006, 143, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolyakov, B.S. Uptake of Zn, Cu, Pb, and Cd by water hyacinth in the initial stage of water system remediation. Appl. Geochem. 2012, 27, 1214–1219. [Google Scholar] [CrossRef]
- Rodríguez, M.; Brisson, J.; Rueda, G.; Rodríguez, M.S. Water quality improvement of a reservoir invaded by an exotic macrophyte. Invasive Plant Sci. Manag. 2012, 5, 290–299. [Google Scholar] [CrossRef]
- Zhang, Z.; Rengel, Z.; Meney, K. Cadmium accumulation and translocation in four emergent wetland species. Water Air Soil Pollut. 2010, 212, 239–249. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Sun, P.Z.; Li, D.S.; Huang, C.H. Transformation of tetracycline antibiotics and Fe(II)/(III) species induced by their complexation. Environ. Sci. Technol. 2016, 50, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, M. Oecd: Organization for economic co-operation and development. J. Jpn. Soc. Water Environ. 2013, 14, 437–443. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M. Bio-based Methods for Wastewater Treatment: Green Sorbents. In Phytoremediation, 1st ed.; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Basel, Switzerland, 2016; Volume 1, pp. 209–238. [Google Scholar]
- Vasiliu, S.; Bunia, I.; Racovita, S.; Neagu, V. Adsorption of cefotaxime sodium salt on polymer coated ion exchange resin microparticles: Kinetics, equilibrium and thermodynamic studies. Carbohydr. Polym. 2011, 85, 376–387. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M.; Radwan, A. Optimization of Cadmium (CD2+) removal from aqueous solutions by novel biosorbent. Int. J. Phytoremediation 2016, 18, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Samad, J.E.; Hashim, S.; Ma, S.; Regalbuto, J.R. Determining surface composition of mixed oxides with pH. J. Colloid Interface Sci. 2014, 436, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.P.; Guerra, W.; Silveira, J.N.; Ferreira, A.M.D.C.; Bortolotto, T.; Fischer, F.L.; Terenzi, H.; Neves, A.; Pereira-Maia, E.C. Two new ternary complexes of copper(II) with tetracycline or doxycycline and 1,10-phenanthroline and their potential as antitumoral: Cytotoxicity and DNA cleavage. Inorg. Chem. 2011, 50, 6414–6424. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Liu, F.Q.; Xu, C.; Chen, T.P.; Li, A.M. An integrative technique based on synergistic coremoval and sequential recovery of copper and tetracycline with dual-functional chelating resin: Roles of amine and carboxyl groups. ACS Appl. Mater. Interfaces 2013, 5, 11808. [Google Scholar] [CrossRef] [PubMed]
- Wang, A. Complexation characteristics of ciprofloxacin with Zn(II) and determination of ciprofloxacin. Heilongjiang Med. J. 2008, 2, 3. [Google Scholar]
- Wen, M.Q.; Gao, Y.T.; Luo, Y.G.; Wang, H.H. Absorption spectrum studies on interaction of tetracyline (tc)-Cu(II) complex with DNA. Photograph. Sci. Photochem. 2005, 23, 71–78. [Google Scholar]
- Zhao, Y.; Tong, F.; Gu, X.; Cheng, G.; Wang, X.; Zhang, Y. Insights into tetracycline adsorption onto goethite: Experiments and modeling. Sci. Total Environ. 2014, 470, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Wang, F.; Bian, Y.; Jin, X.; Song, Y.; Kengara, F.O.; Xu, R.; Jiang, X. Effects of pH and Metal Ions on Oxytetracycline Sorption to Maize-Straw-Derived Biochar. Bioresour. Technol. 2013, 136, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.A.; Zhou, D.M.; Wang, Y.J.; Zhu, H.W.; Chen, J.L. Adsorption and cosorption of Cu(II) and tetracycline on two soils with different characteristics. Geoderma 2008, 146, 224–230. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. Cheminform 2006, 136, 681–689. [Google Scholar]
- Bao, Y.; Zhou, Q.; Wang, Y. Adsorption characteristics of tetracycline by two soils: Assessing role of soil organic matter. Soil Res. 2009, 47, 286–295. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Wu, L.; Lan, H.; Qu, J. Preparation of amino-Fe(III) functionalized mesoporous silica for synergistic adsorption of tetracycline and copper. Chemosphere 2015, 138, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Wu, K. The effects of copper coexistence on the adsorption of tetracycline onto loess soil. J. Water Sustain. 2015, 1, 13–20. [Google Scholar]
- Figueroa, R.A.; Leonard, A.; Mackay, A.A. Modeling tetracycline antibiotic sorption to clays. Environ. Sci. Technol. 2004, 38, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, T.K.; Craig, D.A.; Michae, T.M.; Dana, W.K. Effects of sorbate speciation on sorption of selected sulfonamides in three loamy soils. J. Agric. Food Chem. 2007, 55, 1370–1376. [Google Scholar]
- Anirudhan, T.S.; Radhakrishnan, P.G. Kinetics, thermodynamics and surface heterogeneity assessment of Uranium (VI) adsorption onto Cation exchange resin derived from a lignocellulosic residue. Appl. Surf. Sci. 2009, 255, 4983–4991. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Q.X.; Ai-Min, L.I.; Liu, F.Q.; Shao, L.; Chen, J.L. Adsorption and thermodynamic properties of sorbic acid on the polymeric adsorption resins. Chin. J. Appl. Chem. 2003, 20, 1139–1142. [Google Scholar]
- Putra, E.K.; Pranowo, R.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009, 43, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Huwe, B. Effect of pH and soil structure on transport of sulfonamide antibiotics in agricultural soils. Environ. Pollut. 2016, 213, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, F.; Duan, L.; Yang, H.; Gao, J. Tetracycline adsorption onto rice husk ash, an agricultural waste: Its kinetic and thermodynamic studies. J. Mol. Liq. 2016, 222, 487–494. [Google Scholar] [CrossRef]
- Chen, G.C.; Shan, X.Q.; Wang, Y.S.; Pei, Z.G.; Shen, X.E.; Wen, B.; Owens, G. Effects of Copper, Lead, and Cadmium on the sorption and desorption of atrazine onto and from Carbon nanotubes. Environ. Sci. Technol. 2008, 42, 8297–8302. [Google Scholar] [CrossRef] [PubMed]


| Condition | qe,exp (mg g−1) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg g−1) | k1 (h−1) | R2 | qe,cal (mg g−1) | k2 (mg g−1 h−1) | R2 | ||
| TC | 0.78745 | 0.25855 | 0.02367 | 0.844 | 0.77737 | 0.69354 | 0.996 |
| 1:1 | 0.95123 | 0.10802 | 0.02379 | 0.768 | 0.94619 | 2.03175 | 0.999 |
| 1:2 | 1.06662 | 0.04234 | 0.01476 | 0.346 | 1.06207 | 4.41873 | 0.999 |
| Adsorbate | Freundlich Model | Langmuir Model | Linear Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Kf ((mg g−1) (mg−1 L)1/n) | n | R2 | qmax (mg/g) | b | R2 | A | B | R2 | |
| TC | 0.0926 | 0.8313 | 0.892 | 0.0548 | −0.1573 | 0.510 | 0.0838 | 0.1260 | 0.903 |
| 1:1 TC−Cu | 0.1314 | 0.5955 | 0.973 | −0.1421 | −0.2190 | 0.776 | −0.4349 | 0.4629 | 0.989 |
| 1:2 TC−Cu | 0.2062 | 0.5261 | 0.970 | −0.3784 | −0.2717 | 0.927 | −0.6676 | 0.7976 | 0.941 |
| Condition | ΔH0/KJ·mol−1 | ΔS0/J·(mol·K)−1 | ΔG0/KJ·mol−1 | ||
|---|---|---|---|---|---|
| 283 K | 293 K | 303 K | |||
| TC | 8.072 | 36.18 | −2.130 | −2.604 | −2.848 |
| 1:1 | −43.08 | −134.13 | −5.035 | −3.970 | −2.340 |
| 1:2 | −48.35 | −149.88 | −5.933 | −4.439 | −2.935 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Tang, B.; Zhang, Q.; Liu, L.; Fan, R.; Zhang, Z. The Presence of Cu Facilitates Adsorption of Tetracycline (TC) onto Water Hyacinth Roots. Int. J. Environ. Res. Public Health 2018, 15, 1982. https://doi.org/10.3390/ijerph15091982
Lu X, Tang B, Zhang Q, Liu L, Fan R, Zhang Z. The Presence of Cu Facilitates Adsorption of Tetracycline (TC) onto Water Hyacinth Roots. International Journal of Environmental Research and Public Health. 2018; 15(9):1982. https://doi.org/10.3390/ijerph15091982
Chicago/Turabian StyleLu, Xin, Beibei Tang, Qi Zhang, Lizhu Liu, Ruqin Fan, and Zhenhua Zhang. 2018. "The Presence of Cu Facilitates Adsorption of Tetracycline (TC) onto Water Hyacinth Roots" International Journal of Environmental Research and Public Health 15, no. 9: 1982. https://doi.org/10.3390/ijerph15091982





