Abstract
The last few decades have seen rapid industrialization and urban development in many regions globally; with associated pollution by potentially toxic elements; which have become a threat to human health and the food chain. This is particularly prevalent in a number of regions in China that host multiple mineral resources and are important agricultural locations. Solutions to protect contamination of the food chain are more effective and sustainable if locally sourced materials are available; and in this context; we review the potential of local (sepiolite) mineral deposits to treat water contamination in the Hunan Municipality; central south China; widely recognized for significant environmental pollution issues (particularly by Hg; Cd; Pb; and Cr) and the high agricultural productivity of the region. Sepiolite is an abundant fibrous clay mineral with modest to good adsorption properties and extensive industrial process applications. It shows reasonable performance as an adsorbent for element removal. In addition; a number of surface modification strategies are available that improve this capability. We review these studies; focused on sorption reaction mechanisms and regeneration potential; with a view to present options for a localized and effective economic strategy for future application.
1. Introduction
With the international scale of rapid industrialization and urbanization, many developing and emerging economies have exploited local natural resources. These activities are energy-intensive, associated with significant interventions in the natural ecosystem including the water balance, which leads to emissions of pollutants to water, soil, and air. Metal pollution is one of the most serious and frequently encountered problems. For example, the River Ganga in India, which is considered sacred by Indian society, has serious pollution from Mn, Cr, Pb, Cd, and other potentially toxic elements (PTEs) [1]. Pollutants, such as Cd, Cu and Pb, in the Red River in Vietnam, are also much higher than the local discharge standards, and the maximum enrichment factor for Cd is 19.3 [2]. China’s rapid industrialization has also led to a severe deterioration in water quality in the country’s lakes and rivers. More than 80 percent of Chinese rivers and lakes, including seven key river systems, are contaminated with different types and to different degrees of PTEs with As, Cd, Hg, and Pb being the most frequently detected in these rivers [3,4,5]. Soils in China also suffer from high degrees of contamination. A report of the national survey of soil contamination of China, which was published in 2014, showed that the exceedance of environmental standards for Cd, Hg, As, Cu, Pb, Cr, Zn, and Ni in soil samples reached 7.0%, 1.6%, 2.7%, 2.1%, 1.5%, 1.1%, 0.9%, and 4.8%, respectively [6].
The persistence of PTE pollution has the potential to impact on both the human and wider ecological environment because of their long residence time and the potentially toxic impact through biological amplification [7,8]. PTE enrichment in the human body through the food chain eventually destroys the normal function of proteins and enzymes in the body, and high concentrations can form more toxic compounds, which do great harm to organisms [9,10]. Acute toxicity mainly affects the normal function of a particular organ and can damage or destroy the reproductive organs with intergenerational influence on child health [4,11].
Many techniques including adsorption, precipitation, biological treatment, ion exchange, and membrane separation are used to deal directly with PTE pollution [12,13,14]. Among them, adsorption, which refers to the adsorption of metals by means of intermolecular force or electron transfer and electron pair bonding [15], is widely used in pollution control because it is cheap and easy to apply and operate and systems are often reusable. Adsorption processes often refer generically to removal of target pollutants, which may also include other mechanisms such as ion exchange (the exchange of aqueous pollutant ions with available surface ions on the solid phase) and precipitation (where solution conditions exceed solubility conditions for specific species). Many kinds of adsorbent materials have been applied for the removal of soluble pollutant metals such as activated carbon, and modified complex materials such as metal ferrite doped carbon [16], and metal organic framework systems [17]. Capacities can be quite high, for example, 200–300 mg/g for Hg on modified carbon and similar range for, for example, U and Th on metal organic framework materials. However, applications are often limited due to its relatively high synthesis costs. Naturally derived clay minerals like kaolin, zeolite, sepiolite, bentonite, and perlite have also been utilized as alternative low-cost adsorbents for remediation of metal polluted environments. Whilst adsorption capacities for, for example, sepiolite may be an order of magnitude lower than synthetic systems [18] (there are many examples of studies of these materials for the treatment of metals by adsorption that show useful level of performance [19,20]. The focus for the future development of adsorption based systems should be on identifying adsorbent materials that are cheap and effective adsorbents in the context of the treatment scenarios (for example, at point source or to deal with diffuse pollution), to improve the effectiveness of these treatments and to ensure that no secondary pollution is produced [21,22,23,24]. Secondary tasks include the need for good solid/water separation and the regeneration of adsorbent. In this review, we focus on the potential of one mineral system in the context of a local demand for treatment, potentially supported by locally derived materials, which fits with the circular economy principles of resource use and efficiency. There are a number of serious regional pollution problems, within the Central Southern Chinese province of Hunan. It is rich in mineral resources, which have been extensively exploited over recent decades, compromising its significant contribution to food production from a strong agricultural sector. In addition to extensive base metal deposits, one of the regions’ other significant mineral resources is sepiolite, a clay mineral with already widespread industrial and manufacturing applications, located in Xiangtan City, Hunan Province. We focus our review to consider work describing mechanisms for the removal of locally relevant metal pollutants, the modification and regeneration steps from the view of economic efficiency and resource sustainability. We identified a number of published studies of this topic, presented in Chinese literature and academic repositories. Our review, therefore, also provides wider international access to relevant data, which will be of benefit to similar locations worldwide.
2. Metal Pollution in Hunan, China
It is well known that there are abundant reserves of non-ferrous metals in Hunan Province, and most ores for mining, mineral processing, and smelting of non-ferrous and rare earth elements are located in the Xiangjiang Valley. The Xiangjiang River, which cuts across Hunan Province, is a main water resource for drinking water, process water, and the irrigation of crops. Because of prolonged mining and smelting activities for non-ferrous metals, wastewater has been discharged to the surrounding environment and the Xiangjiang Valley is the most infamous polluted area in central China [25]. Water, soils, and crops in Xiangjiang River basin are heavily contaminated by Cd, Hg, Pb, as well as As [26,27]. The “12th Five-Year” Plan for Comprehensive Prevention and Control of Heavy Metal Pollution indicates that the five major PTE pollutants in China are identified as Pb, Hg, Cd, Cr, and As [28]. In 2015, the state statistics for “the discharge of major pollutants in regional wastewater”, Hg, Cd, and As in Hunan province account for 20.3%, 37.9%, and 32.6% of the total emissions, respectively [29]. The “11th Five-Year Plan” Xiangjiang River basin water pollution prevention and control plan of Hunan province reported that the pollution in Xiangjiang is predominantly caused by Hg, Cd, Pb, As, Zn, and others [30]. The monitoring data for the main pollution indicators in the Xiangjiang are shown in Table 1 with Figure 1 summarizing the geographical distribution of these sources/effects.

Table 1.
A summary of monitoring data for pollution indicators in Xiangjiang River.
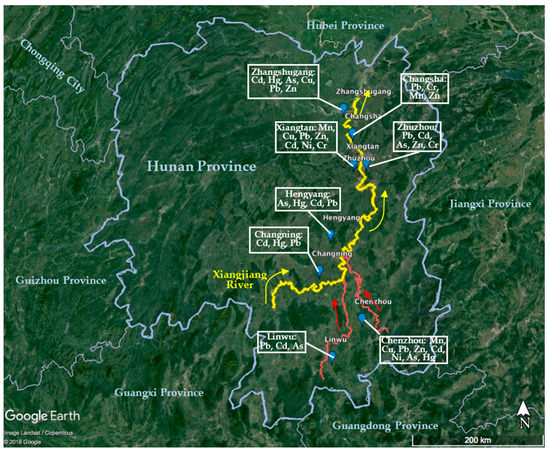
Figure 1.
Geographical distribution of the main PTE pollution sources in Xiangjiang Valley. Data from references in Table 1.
3. Natural Sepiolite
The mineral sepiolite was discovered by German scholar Woemer in 1789, and the original name of sepiolite Meerschaum means “foam of the sea” in German. In 1847, it was officially renamed sepiolite. It is a clay mineral with light color and low density, and has the chemical formula Mg8(OH2)4[Si6O15]2(OH)4·8H2O [39]. In 1947, sepiolite was first discovered in Jiangxi, China by Chinese geologists Zhang Renjun. By genetic classification, we can divide the sepiolite into two types: sedimentary and hydrothermal sepiolite. The world has proven reserves of around 80 million tons, the main production of raw sepiolite is from deposits in Spain, followed by China, the United States, and Turkey [40]. China has about 30% of the world’s sepiolite reserves. Among Chinese sepiolite reserves, 70% of sepiolite comes from Hunan Province. The city of Xiangtan, in Hunan province, hosts more than 20 million tonnes of sepiolite reserves.
Sepiolite is a clay mineral with a hydrous magnesium silicate, it is a member of the orthorhombic crystal system. It presents a structure of needle-like particles and has talc-like layers that consist of two layers of tetrahedral silica and a central octahedral magnesium layer [41]. As a result of its particular crystal structure, sepiolite has great sorptive, rheological, and catalytic properties, and it is also widely used in a variety of industrial and commercial applications.
The structure of sepiolite is a fibrous needle form, with a hollow channel in the direction of the fiber, which gives special rheological properties. The flow properties of sepiolite means that it is used in drilling muds as a thickener and suspension agent [42,43]. The acidity and alkalinity of sepiolite itself makes its catalytic activity more versatile and widespread. As a negative charge carrier, sepiolite can be utilized to remove pollutant cations [44]. It has a large specific surface area, which can reach 800–900 m2/g, and with its porous properties, provides its good access to adsorption sites. This performance can play a part in applications for bleaching, cleaning agent, and other sorption functions. We focus here on those applications relevant to metal adsorption in the context of local environmental contamination.
4. Modification of Sepiolite
Despite the wide application of sepiolite in a variety of industrial processes when compared with other sorbent systems, it has relatively low surface acidity, narrow channels, low surface area, and poor thermal stability. This limits some applications of natural sepiolite [44]. The adsorption performance of the “modified” sepiolite can be much better than that of natural sepiolite, and studies have shown that the specific surface area can be increased significantly from 29–87 m2/g [45]. The adsorption and removal capability of magnetic modified sepiolite for the heavy metal Cr (VI) is 10 times that of natural sepiolite [46] and of similar magnitude in the case of Hg2+ for surfactant modified sepiolite [18], but is still 0.5 to 0.3 of modified carbon and synthetic metal organic systems highlighted in the introduction above. When comparing natural sepiolite with a number of modified sepiolite systems to remove Pb2+, it was found that the order of adsorption capacity is as follows: H2O2 modified sepiolite > KNO3 modified sepiolite > natural sepiolite. When the initial Pb2+ concentration is 2.5 mg/L, the adsorption capacity of H2O2 modified sepiolite is twice as much as natural sepiolite [47]. According to other studies, the adsorption of Cr (VI) by activated sepiolite follows the following order: acid activated-mercapto silane organic modified sepiolite > sulphur silane modified sepiolite > acid activated modified sepiolite > natural sepiolite [48]. For the adsorption of Pb2+ thermally modified sepiolite > natural sepiolite > and for the adsorption of Cd2+ thermally modified sepiolite > natural sepiolite [49]. This provides a range of strategies for modification and sources of reactants to enable optimization of technological approach.
4.1. Acid Treatment
In acid treatment the reaction with carbonate in the sepiolite dissolves these impurities and clears any surface obstruction. The H+ from the acid will replace the Ca2+, Mg2+, Na+, and K+ in the sepiolite interlayer, and improves the access to the surface and cavities in the sepiolite and increases the surface area and microporosity to provide improved adsorption performance [48,50,51].
4.2. Thermal Treatment
Thermal modification of sepiolite is the process of calcining natural sepiolite at different temperatures. At the different temperatures, the associated water in hygroscopic, zeolitic, and coordinated and structural octahedral hydroxyls groups will be removed to reduce the water film resistance, increasing the porosity and in doing so, improving the adsorption performance of sepiolite [52,53,54].
In calcination temperatures around 500 °C, the adsorbed water disappears from the sepiolite structure and apertures expand, with magnesium loss from the mineral structure. This increases the metal ion space, which is beneficial for the removal of Cr3+, at the same time. The removal ability of heavy metals from wastewater was improved because of the change of internal cavity structure of the thermally treated sepiolite [55].
4.3. Magnetic Modification
After treatment of heavy metal contaminated wastewater by sepiolite, it is difficult to achieve good separation of mineral/water mixtures, which leads to the difficulty of secondary reuse of sepiolite. The magnetization of sepiolite provides an effective way to facilitate separation and allows further treatment and/or reuse. In addition to using iron-based systems, the Fe3+ present in the modified sepiolite has oxidative properties, and the dissolution of Fe3+ adds acidity, which is beneficial to the removal of heavy metal ions [46,56,57,58]. In the process of adsorption, the phase structure of magnetic sepiolite did not show any obvious change. The adsorption mechanism is an ion exchange process between heavy metal ions and zeolite in the magnetised sepiolite crystal and coupled adsorption occurs between the heavy metal ions and the hydroxyl groups (Fe–OH and Si–OH) on the surface of magnetised sepiolite [59].
4.4. Organic Modification
Organic modification uses a range of molecules such as surfactants, polymerised organic matter, or microorganisms to load or graft copolymerization to the surface or in a cavity of the sepiolite and modify its structure and the properties of the material surface. This is a relatively new area of research for metal removal from wastewater [48,60,61,62,63].
4.5. Acid Thermal Treatment
Acid thermal modification combines acid treatment with thermal modification of sepiolite. This approach to removing metal ions from water is widely used, and the adsorption using this acid thermal modified sepiolite is much better than for natural sepiolite, or separate acid modified and thermal treatment sepiolite. The sepiolite was treated with hydrochloric acid solution, and then calcinated at 450 °C, and subsequently used to treat Zn2+, Pb2+, Cu2+, and Cd2+ in solution through an ion exchange and surface complex adsorption process [48].
5. Examples of the Application of Sepiolite to Potentially Toxic Element Removals from Aqueous Environmental Systems
It has been reported that sepiolite-based materials can be used to remove a wide range of pollutant elements from water and soils. We focus on reports relating to the key pollution metal elements in Hunan province (Hg, Cd, Pb, and Cr), where most of the research is still at the bench-scale phase.
5.1. The Removal Hg2+ from Wastewater
There are few studies on the removal of mercury ions using sepiolite and modified sepiolite. Those that are available show that modified sepiolite has a significantly improved effect on Hg2+ removal. As shown in Figure 2, the removal of Hg2+ was more than 90% after acid modification, acid thermal modification, and organic modification, compared with the natural sepiolite (about 50%).
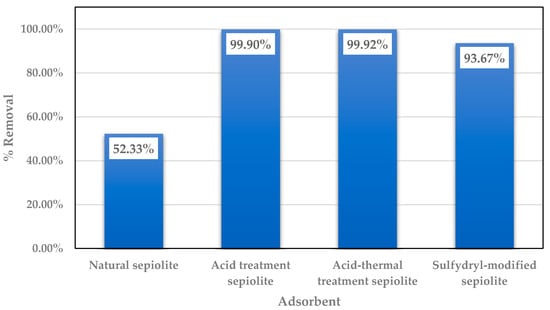
Figure 2.
The removal of Hg by sepiolite and various modified sepiolite products [64,65,66].
The mechanism of removing Hg2+ using sepiolite and different modified conditions is also different. The reaction mechanism for acid modified sepiolite is the dissolution of the impurities in the sepiolite by the acid, at the same time, the pore area is enlarged, and the acidic hydroxyl group is exposed to contact with the Hg ions [65]. In the case of acid thermal treatment, the mechanism of the reaction with adsorbing ions is mainly due to the combination the approach for both acid and thermal activation. The process of dissolving the impurities in the sepiolite results in improved thermal removal of the structural water in the sepiolite and contact resistance is reduced. The combined effect is better than that of pure acid modification or thermal modification [66]. The reaction of sulfhydryl modified sepiolite for Hg2+ conforms to the pseudo-second-order kinetics equation, and adsorption of Hg2+ onto sepiolite fits Langmuir and Freundlich isotherm models. The regression coefficient (R2 = 0.994) suggests that Hg2+ adsorption on sepiolite more closely followed the Langmuir model. The sulfhydryl modified sepiolite has a smoother surface, and its internal pores are enlarged, and the increased negative charge is conducive for reaction with metal ions, so as to more effectively remove the Hg2+ [64].
5.2. The Removal of Cd from Wastewater
Studies found that the adsorption reaction of sepiolite with Cd2+ conforms to a pseudo-second-order kinetics model, and its R2 is 0.999. It also satisfies the isothermal adsorption model of both Langmuir and Freundlich, and the degree of fit to the Langmuir isothermal model is high (R2 = 0.999). The saturated adsorption capacity of sepiolite for Cd2+ was 11.48 mg/g, and the saturated adsorption capacity of the acid modified sepiolite for Cd2+ was 13.62 mg/g [67,68]. The study on the adsorption of heavy metal Cd by sepiolite on acid and thermal treatment found that the adsorption of Cd2+ was increased by calcining the sepiolite, the main reason is that the CaO produced by the high temperature roasting makes the liquid alkaline, so that the Cd2+ was removed by precipitation reactions, in the process of acid treatment, the treatment effect of sulfuric acid is better than that of nitric acid and hydrochloric acid, which is due to the precipitation reaction between H2SO4 and Cd2+ [69]. The reaction mechanism of magnetic modified sepiolite to treat Cd2+ showed that the degree of fit to the Langmuir isothermal model was higher than Freundlich model, which indicates that the adsorption reaction of magnetic modified sepiolite and Cd2+ was based on single ion layer surface coverage [70]. Figure 3 shows that the adsorption capacity of Cd2+ was increased 3–17 times after modification, especially for the combined acid thermal modification.
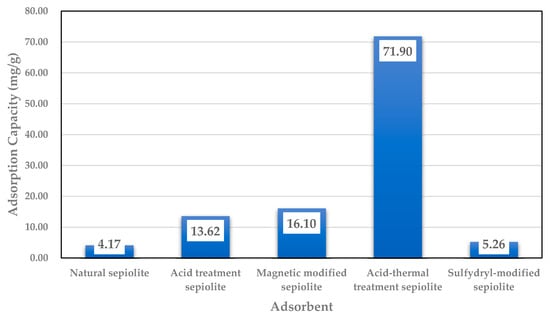
Figure 3.
Adsorption capacity of Cd by sepiolite from solutions [66,68,70,71].
5.3. The Removal of Pb from Wastewater
The adsorption of Pb2+ with sepiolite and modified sepiolite departs from the Langmuir isothermal model, as a result of the precipitation caused by the reaction. The reaction mechanism is not only due in part to the complexation at the ion exchange surface, but also that the Pb2+ will precipitate during the reaction process [68]. The reaction mechanism of sulfhydryl modified sepiolite and Pb2+ fits well with the Freundlich isothermal model, and it also conforms to the pseudo-second-order kinetics, for which the R2 = 0.9976, and its maximum adsorption capacity for Pb is 97 mg/g [63]. The adsorption of Pb by natural sepiolite and iron oxide-coated sepiolite was found that both fit well with the Freundlich and Langmuir isothermal models, with the degree of fit for the Langmuir model was the best (R2 = 0.990). It can be seen that the reaction mechanism of sulfhydryl modified sepiolite and natural sepiolite and iron oxide coated sepiolite differ. The introduction of sulfhydryl group and the stable coordination bond with the heavy metal ions in the sulfhydryl group have a good influence on adsorption, the adsorption effect of iron coating sepiolite on pollutant metals is higher than that for natural sepiolite, which may be due to replacement of the zeolite water in the sepiolite and increase in adsorption sites [72,73]. In addition, the effect of organic modification (dodecyl benzene sulfonic acid sodium and sodium chloride modified sepiolite) on the adsorption of Pb2+ is greater than that of unmodified sepiolite and its mechanism is related to the organic modification providing surface enrichment of macromolecule groups for the adsorption of metal ions. Adsorption isotherm has a good fit to the Langmuir model and pseudo-second-order kinetic equation, the quasi secondary maximum adsorption capacity is 226.8 mg/g [74]. The sepiolite has a good performance for the adsorption of Pb2+ after acid soaking and high temperature calcination, and its removal at 50 mg/L Pb2+ is 80.9%. The reaction has a best fit to the Freundlich adsorption isotherm [75].
5.4. The Application of Remove Cr in the Wastewater by Sepiolite
The mechanism for sepiolite adsorption of Cr (VI) occurs in two stages. Firstly, to remove part of the contamination by surface of physical adsorption, followed by the likely reduction of Cr (VI) to Cr (III) [76,77].
At pH = 2, it was found that amine functionalized natural and acid-activated sepiolites [78] had the best adsorption effect on Cr (VI), and the adsorption capacity was 37 mg/g and 60 mg/g, respectively. The system showed a good fit to the Freundlich isothermal model compared with Langmuir and D–R isothermal models. It shows that in the process of adsorption should be simultaneously to multiple sites. By analyzing the R2 value for the pseudo-second-order kinetics, the cause of limited adsorption efficiency in chemical adsorption process can be analyzed. In the study of the adsorption treatment of Cr (VI) with sepiolite-supported nanoscale zero-based iron (S-NZVI), the maximum adsorption capacity of S-NZVI for Cr (VI) was 43.86 mg/g, with its response fitting well to the Freundlich isothermal model, the R2 is greater than for Langmuir isothermal model, which suggested that that is due to the S-NZVI surface heterogeneity the effects on the Cr (VI) removal, the linear relationship between the removal of Cr (VI) and the input of S-NZVI fits to pseudo-first-order kinetics [77]. In the study of the adsorption of Cr on magnetic modified sepiolite, it was found that the reaction is also strongly related to the Freundlich isothermal model. This shows that the magnetic surface of modified sepiolite exhibits heterogeneity, with adsorption between monolayer and multilayer adsorption mechanisms. The reaction has a good fit to pseudo-second-order dynamics (R2 > 0.99) and the maximum adsorption capacity was 3.6 mg/g. Compared with natural sepiolite, the removal of Cr (VI) by modified sepiolite is much higher than for natural sepiolite [46].
Other studies of natural sepiolite adsorption of Cr3+, Cd2+, and Mn2+ showed the best adsorption effect for Cr3+ was on natural sepiolite, with good fit to the Langmuir isothermal model. The adsorption process is not only the ion exchange, but also the formation of complex and surface adsorption [79].
6. Regeneration of Sepiolite
The process of regeneration of modified sepiolite treated with wastewater is to restore most of the adsorption capacity of sepiolite, so that the material can be reused, reducing operational costs and preserving resources, which fitswith green production process philosophy. At present, there are few reports on the adsorption and regeneration of sepiolite, and it is of great significance to find an economical and efficient method for the treatment of waste water [80,81].
The method of sepiolite regeneration includes: acid regeneration, alkaline regeneration, and salt regeneration. Jia et al. used salt regeneration to regenerate the sepiolite that had adsorbed Zn2+, it shows that the capacity adsorption of regenerated sepiolite is still high although some minor reduction occurs [82]. The adsorption ability of sepiolite pre- and post-regeneration is shown in Table 2.

Table 2.
The adsorption ability of sepiolite before and after regeneration.
From Table 2, we can see that the regenerated sepiolite maintains good adsorption properties for metals ions. Li et al. [87] used hydrochloric acid, sodium chloride, and sodium hydroxide in three ways to regenerate the sepiolite. It was found that NaOH had the best treatment effect on the regeneration of sepiolite, and the treatment effect was similar with that of water and sodium chloride, with the use of hydrochloric acid being the poorest. Li et al. [88] used two different kinds of acid, salt and alkaline, to study sepiolite regeneration, and showed that the NaOH and nitric acid had the best effect on sepiolite regeneration, but hydrochloric acid and NaOH treatments were not ideal for sepiolite re-use. Yan showed several different methods for the regeneration of sepiolite: two acid regeneration (HNO3, HCl), alkaline regeneration, and salt regeneration. It shows that the removal of metal ions was reduced by 19.72% when the regenerating solution is HNO3, the removal of metal ions was reduced by 16.28% when the regenerating solution is HCl, for NaCl, it was reduced by 5.32% and after five steps, for NaOH, the removal of metal ions was reduced by 6.24% [89]. The results of these trials show that regeneration process is metal specific, which must be considered when developing protocols for full trials and field applications.
7. Conclusions
Pollution from mining and industry continues to be serious environmental problem in Xiangjiang Valley, which is the key rice production area in Hunan province. We need to identify approaches to deal with the protection of the food chain over widespread areas. Adsorption is still an effective technology for removing metals from water, but requires careful consideration to deal with multiple metals, sources, and regional scale contamination. In this review, sepiolite based materials were selected as target adsorbents for heavy metals removal because of their local abundance and potential cost effective application. The characteristics and function of natural and modified sepiolites are reviewed and compared. The sorption performance of modified sepiolite obtained from acid, magnetic, organic, and thermal treatment is significantly improved over natural materials and can be re-used through regeneration. These methods include acid, alkaline, and salt regeneration. The sorption performance of sepiolite after regeneration is greater than 70% of its original performance.
We identified that the relatively low efficiency for heavy metal removal by natural sepiolite was because of the scarcity of exchange sites for contaminant metal ions and capacities are much lower than other synthetic sorbent materials. However, the modification of sepiolite is viable and provides potentially useful adsorption capacity. However, the process of modification also increases the cost of any potential application. In our review, few reports have considered the cost–benefit for modification of sepiolite and application of modified sepiolite on removing metals. Most studies still focus on the mechanism of modification and regeneration of sepiolite. In order to fully establish the potential of sepiolite, as a low-cost and effective adsorbent, further field scale research involving a product life cycle approach is required. This will identify the full potential of local resources to treat local pollution and meet with recent national (China) international (EU–China) agreements on resource conservation and environmental protection.
Author Contributions
Drafting the manuscript, Z.W. & L.L.; Data collection, Z.W., L.L., & N.S.; Critical review, Z.W., & A.H.; Writing–review & editing, A.H., Z.W., & B.R.; Funding Acquisition, A.H.; All authors have given approval of the version to be published.
Funding
This research was part funded by the Hunan Provincial Hundred Talents Program.
Acknowledgments
Zhenghua Wang was supported by the China Scholarship Council for one year study at the University of the West of Scotland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paul, D. Research on heavy metal pollution of river Ganga: A review. Ann. Agrar. Sci. 2017, 15, 278–286. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, W.; Li, Z.; Li, J.; Ge, C.; Liu, J.; Bai, X.; Feng, H.; Yu, L. Assessment of heavy metal pollution in Red River surface sediments, Vietnam. Mar. Pollut. Bull. 2016, 113, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xiao, D. Status and control technology of heavy metal pollution. Energy Energy Conserv. 2012, 2, 49–50. (In Chinese) [Google Scholar]
- Wang, H.; Fang, F.; Xie, H. Research situation and outlook on heavy metal pollution in water environment of China. Guangdong Trace Elements Sci. 2010, 1, 14–18. (In Chinese) [Google Scholar]
- Yue, X.; Liu, K.; Lin, X.; Zhou, Q.; Mao, G.; Zhou, B.; Zhao, J. Current status of heavy metal pollution in seven major water systems in China. Prev. Med. Trib. 2014, 3, 209–213. (In Chinese) [Google Scholar]
- Duan, Q.; Lee, J.; Liu, Y.; Chen, H.; Hu, H. Distribution of heavy metal pollution in surface soil samples in China: A graphical review. Bull. Environ. Contam. Toxicol. 2016, 97, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Morton-Bermea, O.; Hernández-Álvarez, E.; González-Hernández, G.; Romero, F.; Lozano, R.; Beramendi-Orosco, L.E. Assessment of heavy metal pollution in urban topsoils from the metropolitan area of Mexico City. J. Geochem. Explor. 2009, 101, 218–224. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Zhao, Z.; Cai, Y. Heavy metal contamination in surface sediments of representative reservoirs in the hilly area of southern China. Environ. Sci. Pollut. Res. 2017, 24, 26574–26585. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sinha, S.N. Isolation and characterization of a phosphate solubilizing heavy metal tolerant bacterium from River Ganga, West Bengal, India. Songklanakarin J. Sci. Technol. 2015, 37, 651–657. [Google Scholar]
- Rajbanshi, A. Study on heavy metal resistant bacteria in Guheswori Sewage Treatment Plant. Our Nat. 2008, 6, 52–57. [Google Scholar] [CrossRef]
- Wu, W.; Wu, P.; Yang, F.; Sun, D.L.; Zhang, D.X.; Zhou, Y.K. Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Sci. Total Environ. 2018, 630, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Li, L.; Qu, Z.; Xu, H.; Xu, J.; Yan, N. A novel method for the sequential removal and separation of multiple heavy metals from wastewater. J. Hazard. Mater. 2018, 342, 617–642. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, G.; Pan, B.; Zhang, W.; Lv, L. A new combined process for efficient removal of Cu (II) organic complexes from wastewater: Fe (III) displacement/UV degradation/alkaline precipitation. Water Res. 2015, 87, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Carro, L.; Barriada, J.L.; Herrero, R.; Vicente, M.E.S.D. Interaction of heavy metals with Ca-pretreated Sargassum muticum algal biomass: Characterization as a cation exchange process. Chem. Eng. J. 2015, 264, 181–187. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Tang, J.; Wang, B.; Huang, F. Advance of the treatment of heavy metal wastewater by adsorption. Chem. Ind. Eng. Prog. 2013, 11, 2749–2756. [Google Scholar]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.; Alqadami, A.A.; Alsheri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ghfar, A.A. Novel metal-organic framework (MOF) based composite material for the sequestration of U(VI) and Th (IV) metal ions from aqueous environment. Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef] [PubMed]
- Vaizogullar, A.I.; Ugurlu, M.; Kula, I. Comparing adsorption activity of raw sepiolite and CTAB modified sepiolite: Kinetic and adsorption study for removal of Hg2+. Int. J. Environ. 2015, 4, 19–31. [Google Scholar] [CrossRef][Green Version]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Alaba, P.A.; Oladoja, N.A.; Sani, Y.M.; Ayodele, O.B.; Mohammed, I.Y.; Olupinla, S.F.; Daud, W.M.W. Insight into wastewater decontamination using polymeric adsorbents. J. Environ. Chem. Eng. 2018, 6, 1651–1672. [Google Scholar] [CrossRef]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef]
- Ko, D.; Mines, P.D.; Jakobsen, M.H.; Yavuz, C.T.; Hansen, H.C.B.; Andersen, H.R. Disulfide polymer grafted porous carbon composites for heavy metal removal from stormwater runoff. Chem. Eng. J. 2018, 348, 685–692. [Google Scholar] [CrossRef]
- Jlassi, K.; Abidi, R.; Benna, M.; Chehimi, M.M.; Kasak, P.; Krupa, I. Bentonite-decorated calix 4 arene: A new, promising hybrid material for heavy-metal removal. Appl. Clay Sci. 2018, 161, 15–22. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Wang, Z.H.; Hursthouse, A.; Ren, B.Z. Gemini surfactant-modified activated carbon for remediation of hexavalent chromium from water. Water 2018, 10. [Google Scholar] [CrossRef]
- Xie, X.; Wang, F.; Wang, G.; Mei, R.; Wang, C. Study on heavy metal pollution in surface water in China. Environ. Sci. Manag. 2017, 2, 31–34. (In Chinese) [Google Scholar]
- Cao, C.; Li, Y. Heavy metal pollution in Hunan is shocking. Environ. Educ. 2014, 12, 27–30. (In Chinese) [Google Scholar]
- Tian, B. Interpretation of “Hunan experiment” for heavy metal removal. People’s Friend 2012, 8, 15–16. (In Chinese) [Google Scholar]
- Liu, J.; Chen, M. Construction of heavy metal monitoring technical system during twelfth five-year plan in China. Environ. Sci. Manag. 2014, 39, 125–128. (In Chinese) [Google Scholar]
- Qiao, R. Study on the Legal Problems of Water Treatment in Xiangjiang River Basin. Master’s Thesis, University of Geosciences, Beijing, China, 2017. (In Chinese). [Google Scholar]
- Lei, M.; Qin, P.; Tie, B. The Present Situation and Analysis of Heavy Metal Pollution in Hunan Xiangjiang River Basin. In Proceedings of the Third National Symposium on Agricultural Environmental Science, Tianjing, China, 23–26 October 2009. (In Chinese). [Google Scholar]
- Wang, Q.; Wang, S.; Liu, M. Study evaluation on pollution of Xiang River Valley in Hunan Province. China Water Wastewater 2004, 8, 104–106. (In Chinese) [Google Scholar]
- Lei, D. Analysison heavy metals pollution status in Hunan Province and its remediation strategy. Hunan Nonferrous Met. 2012, 1, 57–60. (In Chinese) [Google Scholar]
- Liu, Y.; Gao, L.; Li, Z.; Liu, S.; Huang, K.; Li, J. Analysis on heavy metals pollution status and reasons in Xiangjiang River and discussion on its countermeasures. Environ. Prot. Sci. 2010, 36, 26–29. (In Chinese) [Google Scholar] [CrossRef]
- Wang, L.; Guo, Z.; Xiao, X.; Chen, T.; Liao, X.; Song, J.; Wu, B. Heavy metal pollution of soils and vegetables in the midstream and downstream of the Xiangjiang River, Hunan Province. J. Geogr. Sci. 2008, 18, 353–362. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Hu, J.; Peng, G.; Xie, H.; Li, Z.; Peng, G. Characteristics and potential ecological risk assessment of heavy metal pollution in the sediments of middle reaches of Xiangjiang River. J. Ecol. Rural Environ. 2017, 33, 135–141. (In Chinese) [Google Scholar]
- Zhang, K.; Yang, X.; Wu, Y.; Wu, B.; Kuang, X. Pollution characteristics and ecological risk assessment of heavy metals in surface sediments in Changsha-Zhuzhou-Xiangtan Reach, Xiang Jiang River, China. J. Agric. Resour. Environ. 2015, 32, 60–65. (In Chinese) [Google Scholar]
- Fang, X.; Tian, D.; Xie, R. Soil physical and chemical properties of the wasteland in Xiangtan manganese mine. Xiangtan Manganese Min. 2006, 5, 1494–1501. (In Chinese) [Google Scholar]
- Tian, Q.; Zhang, G.; Xie, Y.; Mo, Y. Distribution and ecological risk assessment of heavy metals in surface sediments from main tributary entrances of Dongting Lake. Asian J. Ecotoxicol. 2017, 12, 191–200. (In Chinese) [Google Scholar]
- Zhou, Z. Overview of the resources of sepiolite clay in China and abroad. Land Resour. Herald 1985, 1, 148–156. (In Chinese) [Google Scholar]
- Wang, C.; Wang, G.; Tao, T.; Zhu, M.; Qu, F. Research progress and application status of sepiolite functional green building materials. Bull. Chin. Ceram. Soc. 2017, 10, 3285–3291. (In Chinese) [Google Scholar]
- Zhuang, G.; Gao, J.; Chen, H.; Zhang, Z. A new one-step method for physical purification and organic modification of sepiolite. Appl. Clay Sci. 2018, 153, 1–8. [Google Scholar] [CrossRef]
- Carmona, J.A.; Ramírez, P.; Trujillo-Cayado, L.A.; Caro, A.; Muñoz, J. Rheological and microstructural properties of sepiolite gels. Influence of the addition of ionic surfactants. J. Ind. Eng. Chem. 2018, 59, 1–7. [Google Scholar] [CrossRef]
- Wang, C. Research on Low Density Cement Slurry System for Complex Well Cementing. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2004. (In Chinese). [Google Scholar]
- Liang, K. The Mineralogy Research of the Sepiolite and Its Application in Environment Conservation. Ph.D. Thesis, Central South University, Changsha, China, 2008. (In Chinese). [Google Scholar]
- Duan, E.; Han, J.; Song, Y.; Guan, Y.; Zhao, W.; Yang, B.; Guo, B. Adsorption of styrene on the hydrothermal-modified sepiolite. Mater. Lett. 2013, 111, 150–153. [Google Scholar] [CrossRef]
- Jia, M.; Dai, Y.; Du, T.; Liu, C. Preparation of magnetically modified sepiolite and adsorption of hexavalent chromium. Environ. Chem. 2011, 30, 1546–1552. (In Chinese) [Google Scholar]
- Liu, C.; Huang, Y.; Yu, F.; Sun, X.; Xu, F.; Wu, W.; Zhong, M. Sepiolite modification on Pb adsorption characteristics. Environ. Chem. 2013, 32, 2024–2029. (In Chinese) [Google Scholar]
- Li, C.; Yu, J. Modification and application of sepiolite in chromium-containing wastewater treatment. Plat. Finish. 2013, 35, 17–22. (In Chinese) [Google Scholar]
- Feng, Y. Study on the Adsorption of Lead and Cadmium by Sepiolite. Master’s Thesis, University of South, Hengyang, China, 2007. (In Chinese). [Google Scholar]
- Feng, Y.; He, S.; Gao, W.; Li, H. Adsorption of organic matter and heavy metals in wastewater on sepiolite. Water Purif. Technol. 2006, 25, 63–66. (In Chinese) [Google Scholar]
- Shi, T.; Jia, S.; Chen, Y.; Wen, Y.; Chen, J.; Huang, R.; Wang, Z.; Liu, Y. Removal of heavy metal ions from wastewater by modified natural ores as adsorbents: A review. Technol. Water Treat. 2009, 35, 18–23. (In Chinese) [Google Scholar]
- Zhang, Y.; Wang, L.; Wang, F.; Liang, J.; Ran, S.; Sun, J. Phase transformation and morphology evolution of sepiolite fibers during thermal treatment. Appl. Clay Sci. 2017, 143, 205–211. [Google Scholar] [CrossRef]
- Li, H. Study on kinetic and thermodynamics for heating modified sepiolite adsorbing acid blue 62 from aqueous solution. Pop. Sci. Technol. 2017, 19, 36–38. (In Chinese) [Google Scholar]
- Miura, A.; Nakazawa, K.; Takei, T.; Kumada, N.; Kinomura, N.; Ohki, R.; Koshiyama, H. Acid-, base-, and heat-induced degradation behavior of Chinese sepiolite. Ceram. Int. 2012, 38, 4677–4684. (In Chinese) [Google Scholar] [CrossRef]
- Valentin, J.L.; Lopez-Manchado, M.A.; Rodriguez, A.; Posadas, P.; Ibarra, L. Novel anhydrous unfolded structure by heating of acid pre-treated sepiolite. Appl. Clay Sci. 2007, 36, 245–255. [Google Scholar] [CrossRef]
- Chen, W.; Ma, L.; Liu, H. Adsorption capacity of magnetic modified sepiolite humic acid in source water. J. Hohai Univ. (Nat. Sci.) 2017, 45, 109–115. (In Chinese) [Google Scholar]
- Li, C.; Xia, Q.; Dai, B. Research on and modification and application of sepiolite in Lead and Zinc contained wastewater treatment. Plat. Finish. 2015, 37, 19–26. (In Chinese) [Google Scholar]
- Li, C.; Xia, Q.; Cao, Y.; Dai, B.; Song, F.; Liu, Z.; Ge, H. Treatment of nickel-containing wastewater with magnetic modified sepiolite. Electroplat. Finish. 2015, 1, 47–52. (In Chinese) [Google Scholar]
- Yu, T.; Dai, Y.; Wang, W.; Jia, M.; Li, F.; Gong, M. Adsorption characteristic and mechanism of heavy metals onto magnetically modified sepiolite. Environ. Chem. 2013, 32, 1566–1570. (In Chinese) [Google Scholar]
- Zhang, Q. Study on Adsorption Characteristic of Sepiolite. Master’s Thesis, Hebei University of Technology, Tianjin, China, 2002. [Google Scholar]
- Zhou, Q. Synthesis of Organo-functionalized Clay Minerals and Their Adsorption Performance for Heavy Metal Ions. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2016. [Google Scholar]
- Liang, X.; Xu, Y.; Sun, G.; Wang, L.; Sun, Y.; Yang, S.; Xu, Q. Preparation and characterization of mercapto functionalized sepiolite and their application for sorption of lead and cadmium. Chem. Eng. J. 2011, 174, 436–444. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Y.; Wang, L.; Sun, Y.; Lin, D.; Sun, Y.; Qin, X.; Wan, Q. Sorption of Pb2+ on mercapto functionalized sepiolite. Chemosphere 2013, 90, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, B.; Zhang, J.; Liu, J. Adsorption of Hg (II) in water by sulfydryl-modified sepiolite. Environ. Sci. 2016, 37, 2187–2194. (In Chinese) [Google Scholar]
- Jin, S.; Yang, W.; Tang, M. Study on surface modification and application of sepiolite. Non-Met. Mines 2001, 4, 23–24. (In Chinese) [Google Scholar]
- Luo, D.; Yi, P.; Chen, A.; Shi, H. Adsorption of modified unchanged meerschaum on Pb2+, Hg2+ and Cd2+ in wastewater. Technol. Water Treat. 2003, 29, 89–91. (In Chinese) [Google Scholar]
- Huang, J.; Wu, Z.; Chen, L.; Sun, Y. The sorption of Cd (II) and U (VI) on sepiolite: a combined experimental and modeling studies. J. Mol. Liquids 2015, 209, 706–712. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, X.; Sun, G.; Sun, Y.; Qin, X.; Wang, L. Surface chemical characteristics of sepiolites and their adsorption mechanisms of Pb2+, Cd2+ and Cu2+. J. Agro-Environ. Sci. 2009, 28, 2057–2063. [Google Scholar]
- Xu, Y.; Liang, X.; Sun, G.; Sun, Y.; Qin, X.; Wang, L.; Dai, X. Effects of acid and heating treatments on the structure of sepiolite and its adsorption of lead and cadmium. Environ. Sci. 2010, 31, 1560–1567. (In Chinese) [Google Scholar]
- Wang, W.; Dai, Y.; Jia, M.; Li, X.; Du, T. Surface point of zero charge of magnetic sepiolite and adsorption characteristics of cadmium. Environ. Chem. 2012, 31, 1691–1696. (In Chinese) [Google Scholar]
- Xie, J. Study on the Adsorption Characteristics of Sulfydryl-Modified Sepiolite to Hg (II) and Cd (II). Master’s Thesis, Southwest University, Chongqing, China, 2016. (In Chinese). [Google Scholar]
- Eren, E.; Gumus, H. Characterization of the structural properties and Pb (II) adsorption behavior of iron oxide coated sepiolite. Desalination 2011, 273, 276–284. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Suzuki, N.; Sato, K.; Fukata, N.; Murakami, M.; Shimizu, T. Active mercury (II) ion removal: Stoichiometrically controlled thiol-functionalized mesoporous silica by a mass production spray dry system. Bull. Chem. Soc. Jpn. 2009, 82, 1039–1043. [Google Scholar] [CrossRef]
- Chen, B. Adsorption of Pb (II) by Sodium Dodecyl Benzene Sulfonate Modified Sepiolite. Master’s Thesis, Hunan University, Changsha, China, 2013. (In Chinese). [Google Scholar]
- Liu, Y.; Jiang, M. Treatment of mult-metals wastewater using sepiolite modified with acid and heating. Guangzhou Chem. Ind. 2011, 21, 137–139. (In Chinese) [Google Scholar]
- Marjanović, V.; Lazarević, S.; Janković-Častvan, I.; Potkonjak, B.; Janaćković, Đ.; Petrović, R. Chromium (VI) removal from aqueous solutions using mercaptosilane functionalized sepiolites. Chem. Eng. J. 2010, 166, 198–206. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, V.; Lazarević, S.; Janković-Častvan, I.; Jokić, B.; Janaćković, D.; Petrović, R. Adsorption of chromium (VI) from aqueous solutions onto amine-functionalized natural and acid-activated sepiolites. Appl. Clay Sci. 2013, s80–81, 202–210. [Google Scholar] [CrossRef]
- Kocaoba, S. Adsorption of Cd (II), Cr (III) and Mn (II) on natural sepiolite. Desalination 2009, 244, 24–30. [Google Scholar] [CrossRef]
- Fang, J. Preparation of Co-Fe-Sepiolite Sorbent and Its Regenerated by High Temperature Gas Catalytic Oxidation Method. Master’s Thesis, Guangxi Normal University, Guilin, China, 2010. [Google Scholar]
- Feng, J. Study on the Modification of Sepiolite and Its Regeneration by Catalytic Oxidation Process Using High Temperature Gas Flow. Master’s Thesis, Guangxi Normal University, Guilin, China, 2014. [Google Scholar]
- Jia, N.; Wang, H.; Huo, J. The study on adsorption of Zn2+ by modified meerschaum. Chinamining Mag. 2006, 4, 70–72. (In Chinese) [Google Scholar]
- Liang, K.; Wang, D.; Long, L.; Xi, C. Study on the recovery of gallium using from acid lixivium of zinc modified meerschaum residues. Acta Mineral. Sin. 2006, 26, 277–280. (In Chinese) [Google Scholar]
- Zheng, Y.; Xie, Y.; Wang, A. Adsorption of Pb2+ onto chitosan-grafted-poly (acrylic acid)/sepiolite composite. Environ. Sci. 2009, 30, 2575–2579. (In Chinese) [Google Scholar]
- Gaber, S.; Haija, M.A.; Priyabrata, P.; Selvaraj, M.; Banat, F. Removal of iron from industrial lean methyldiethanolamine solvent by adsorption on sepiolite. Sep. Sci. Technol. 2018, 53, 404–416. [Google Scholar] [CrossRef]
- Yu, S.; Zhai, L.; Zhong, S.; Qiu, Y.; Cheng, L.; Ren, X. Synthesis and structural characterization of magnetite/sepiolite composite and its sorptive properties for Co (II) and Cd (II). J. Taiwan Inst. Chem. Eng. 2016, 59, 221–228. [Google Scholar] [CrossRef]
- Li, S.; Dai, Y.; Li, N.; Shi, L.; Tang, W. Preparation of iron modified sepiolite and its adsorption characteristics of antimony. Technol. Water Treat. 2009, 35, 49–52. (In Chinese) [Google Scholar]
- Li, C.; Yu, J. Modification and application of sepiolite inlead and zinc smelter wastewater treatment. Sci. Technol. Eng. 2013, 13, 9153–9157. (In Chinese) [Google Scholar]
- Yan, H. The Modification of Sepiolite and Its Application in Heavy Metal Wastewater Treatment. Master’s Thesis, Northwest University, Chongqing, China, 2013. (In Chinese). [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).