Characterizing the Interaction between Antibiotics and Humic Acid by Fluorescence Quenching Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Chemicals
2.2. Preparation of Humic Acid
2.3. Experimental Methods
2.4. Data Analysis
3. Results and Discussion
3.1. Spectroscopic Characteristics of HA
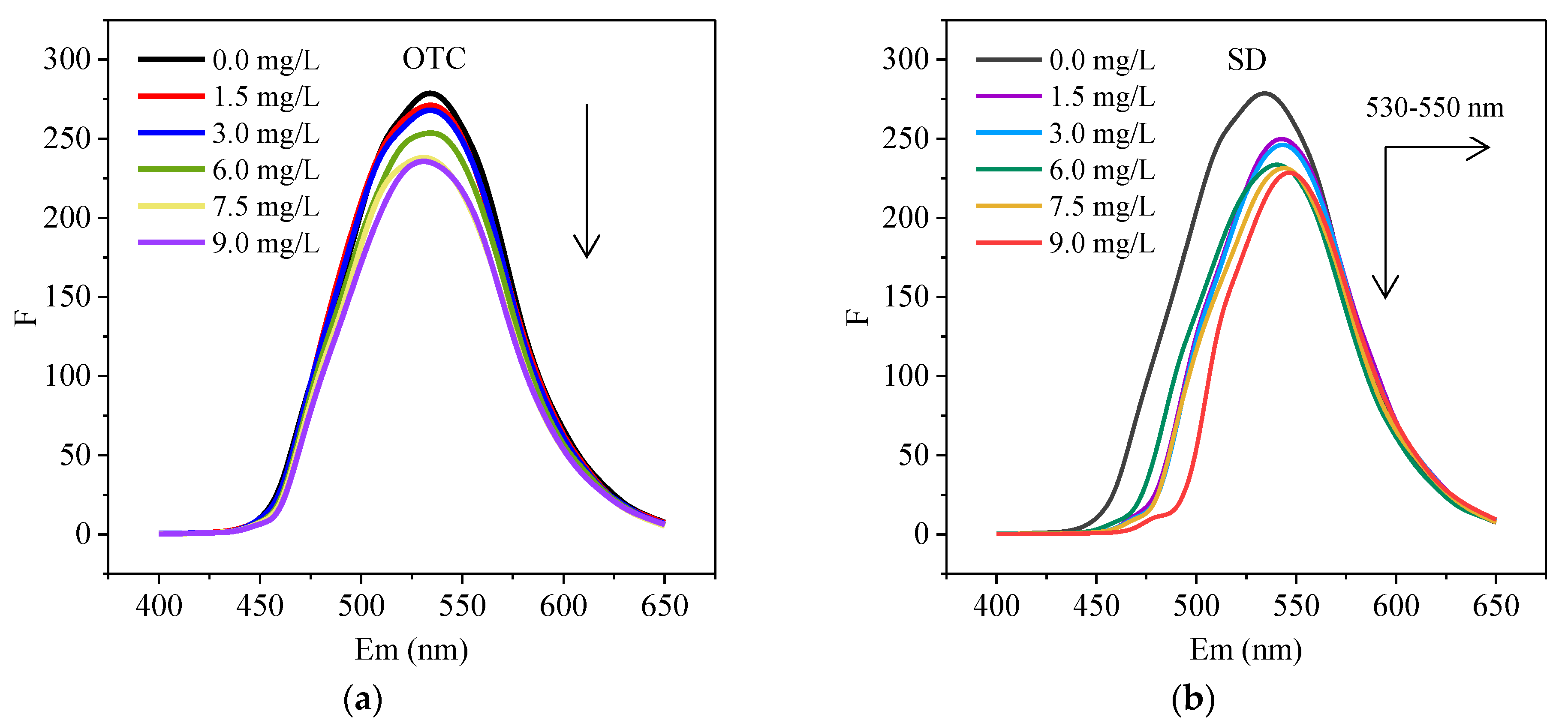
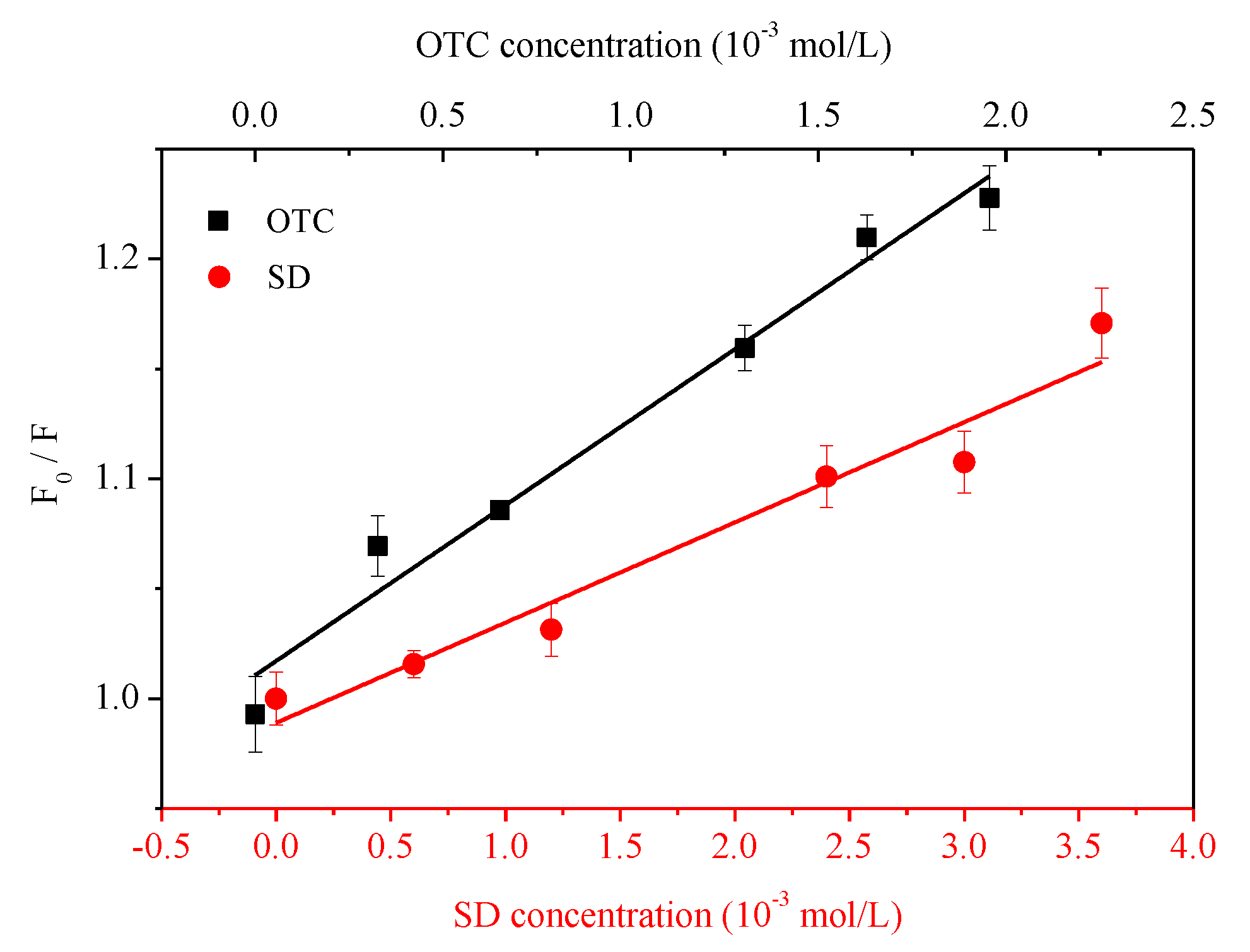
3.2. Quenching of HA Fluorescence by Antibiotics
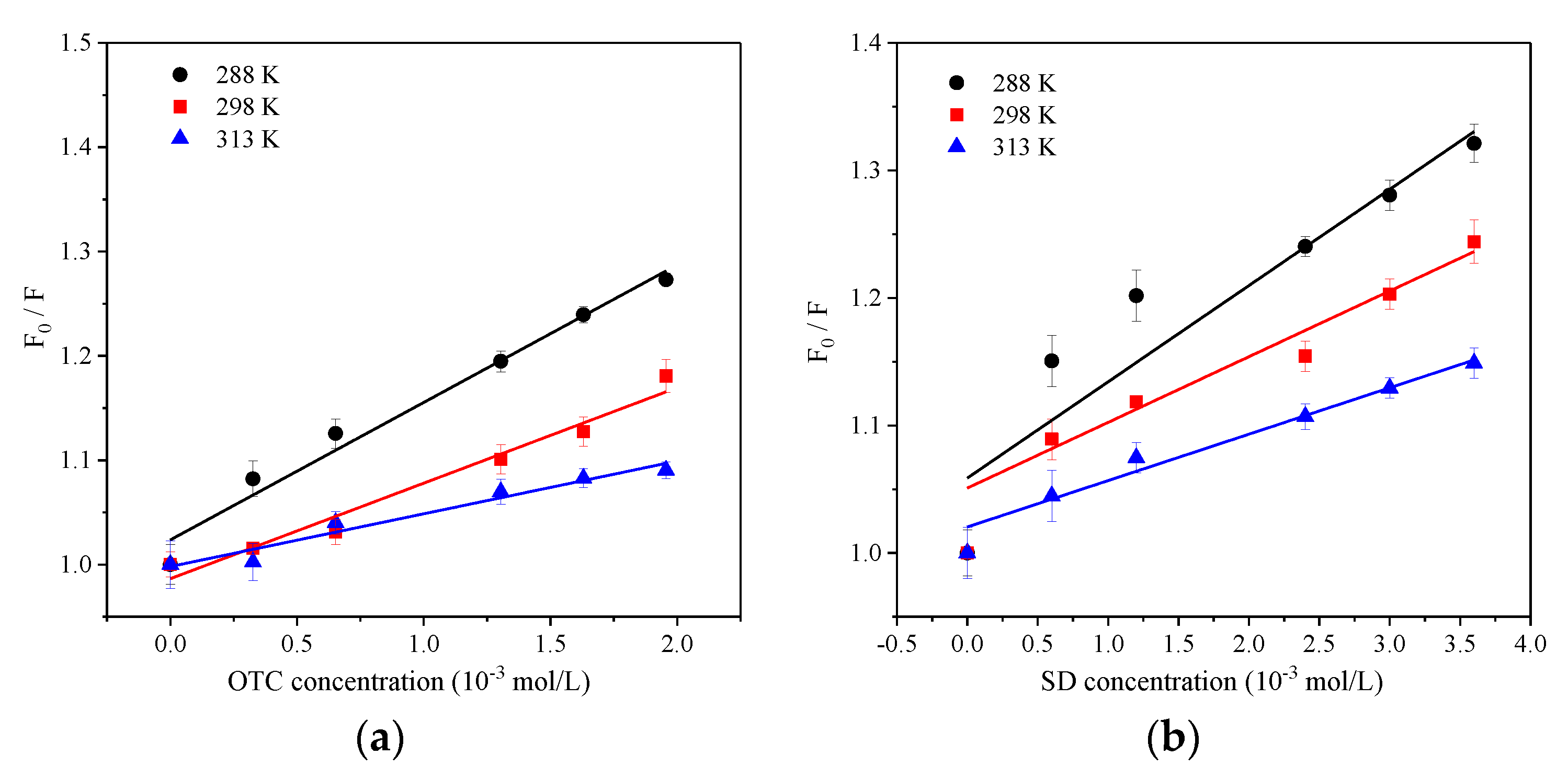
3.3. Temperature Effect
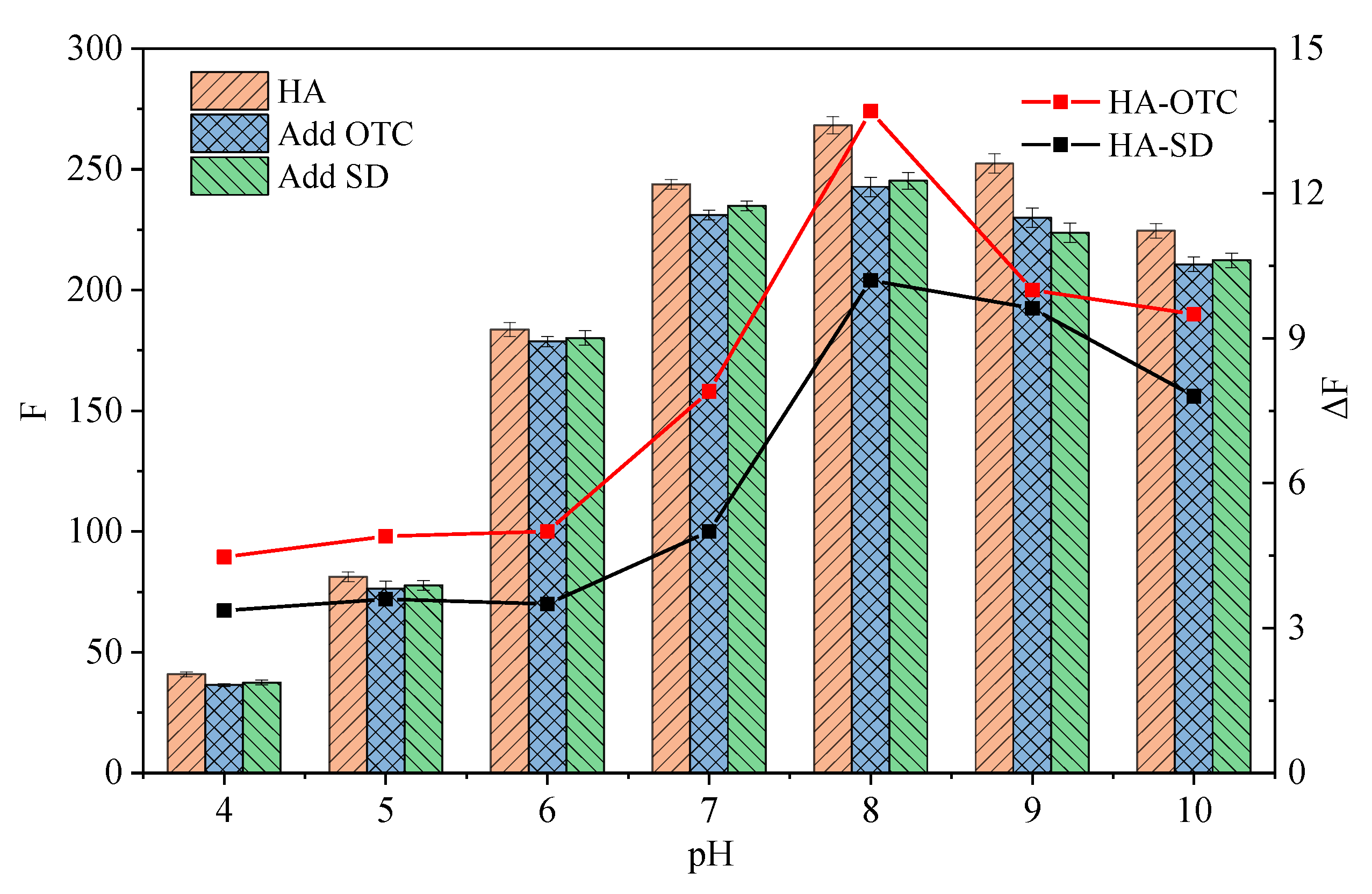
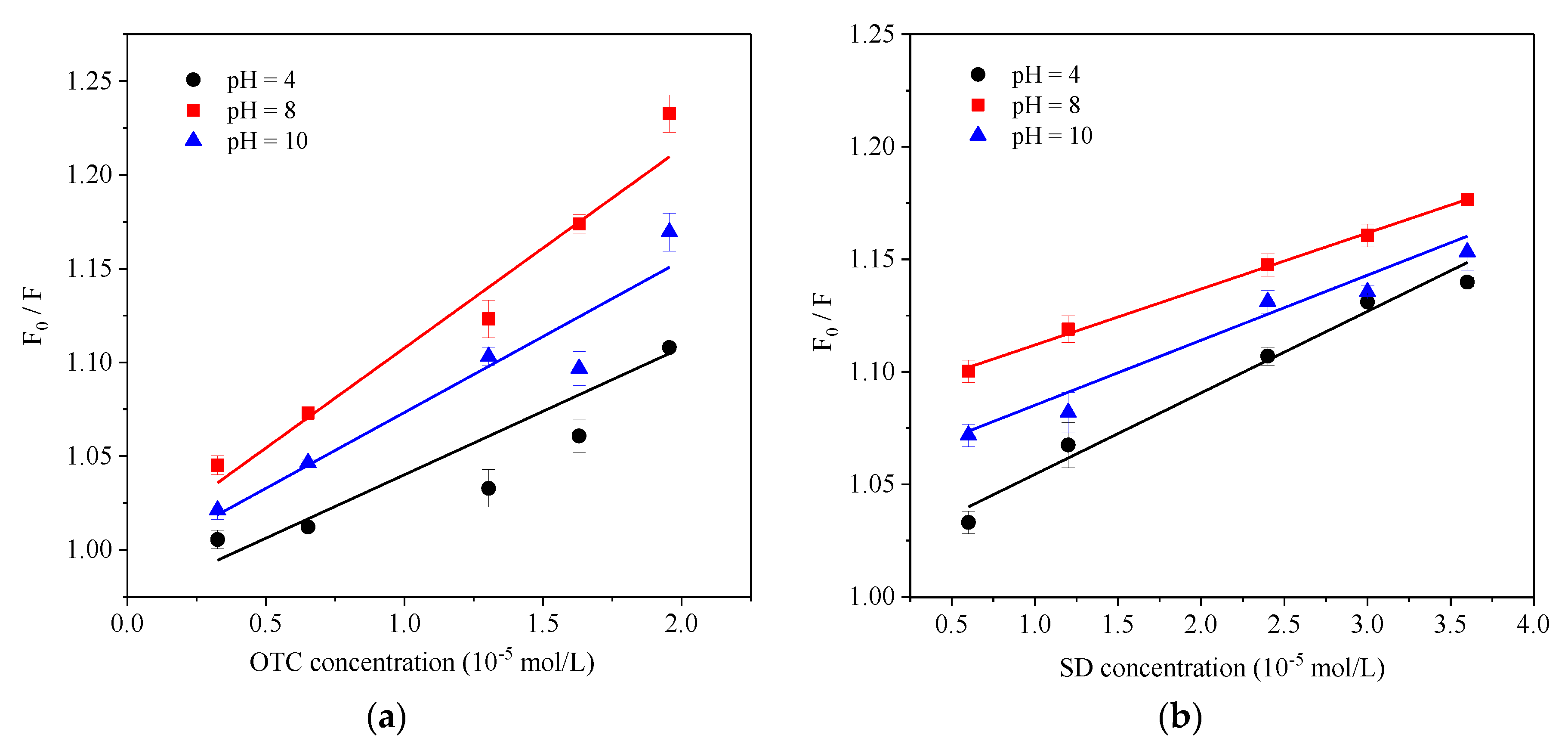
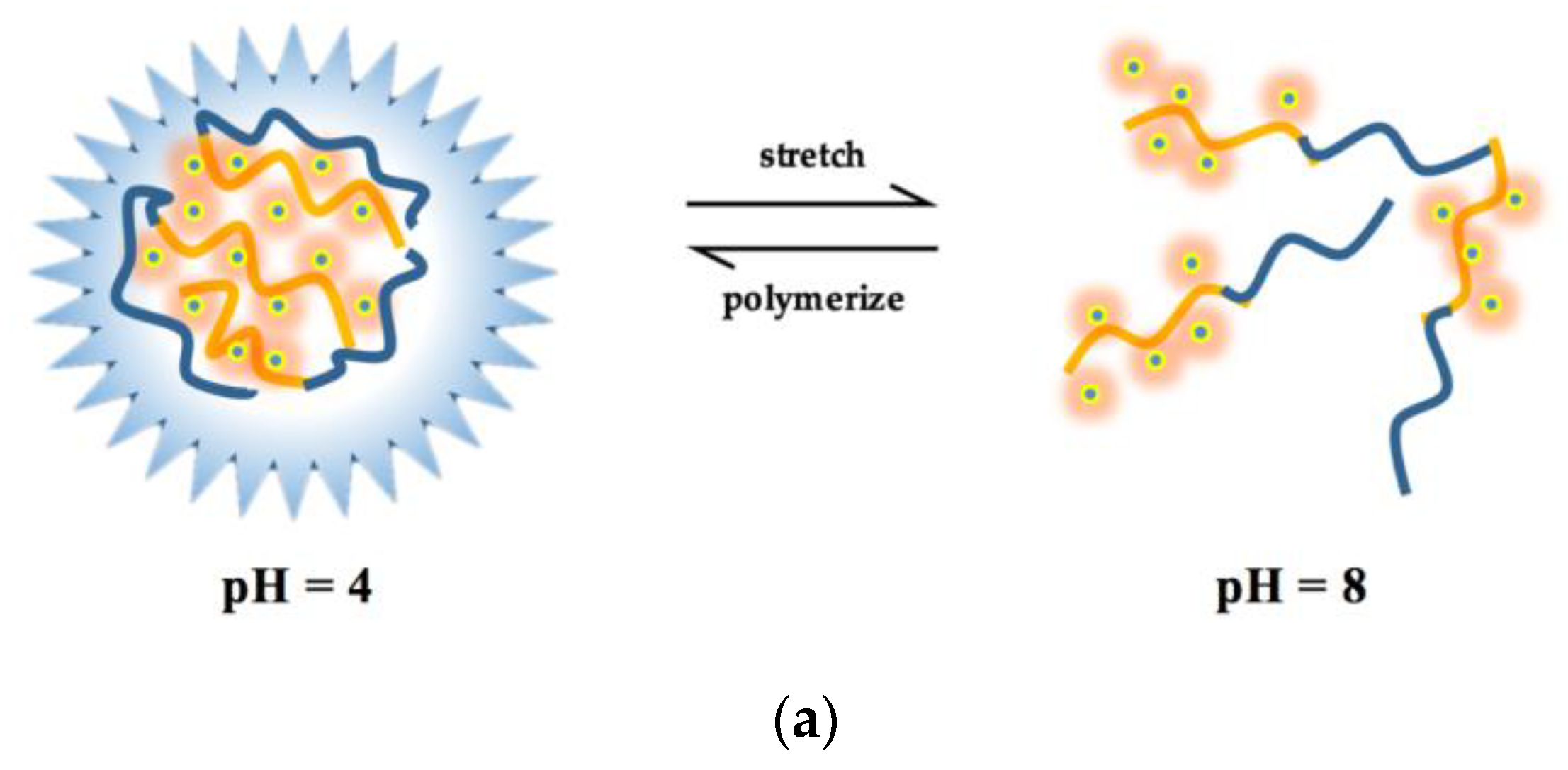
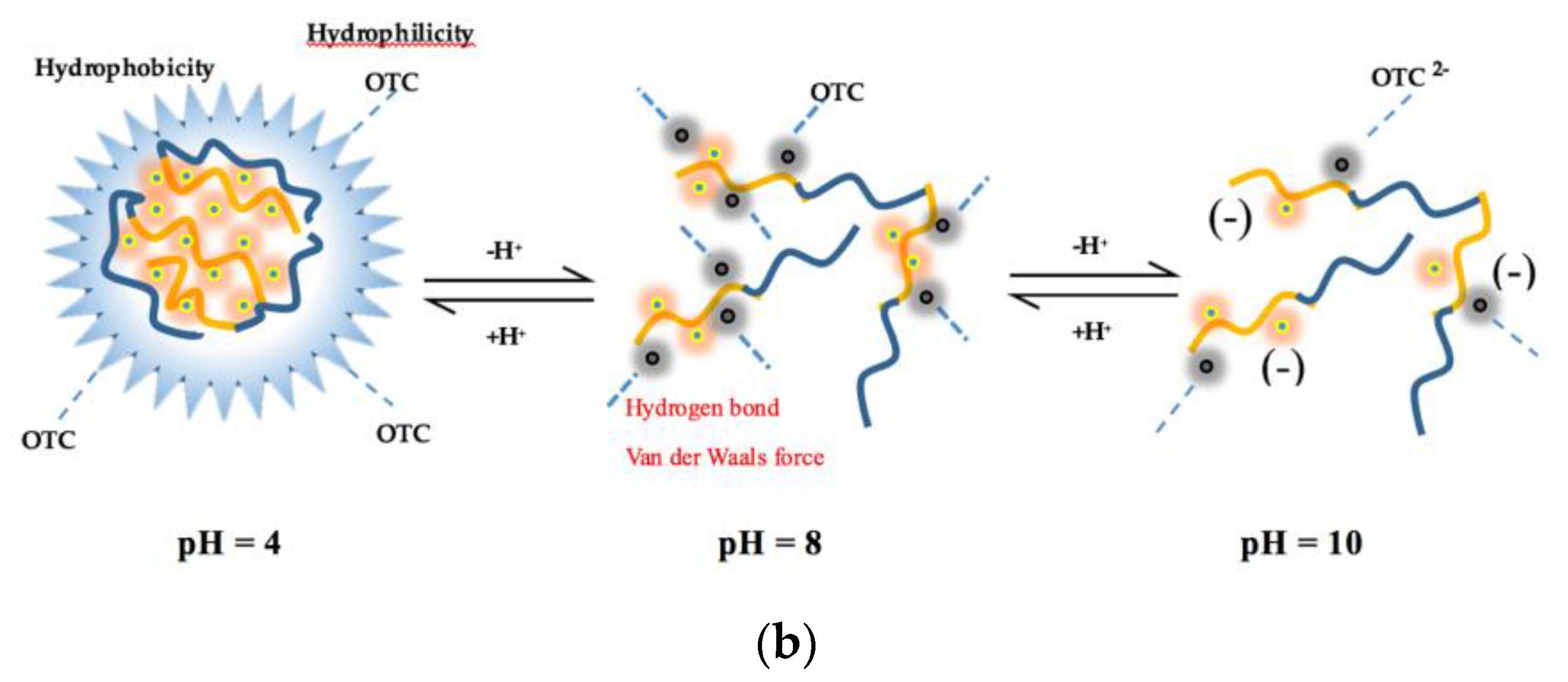
3.4. pH Effect
4. Conclusions
- (1)
- Both OTC and SD can quench HA. The quenching mechanism assigned to a single static quenching of the complex and formed conjugates at 1:1 proportions. The interaction forces were mainly hydrogen bonding or van der Waals forces; the value of KsvOTC and KbOTC were all greater than that of SD. That is, OTC had a stronger quenching effect on HA fluorescence, and the new formed complex was more stable.
- (2)
- The influence of environmental factors on the experiment showed that: with the increase of temperature, the Ksv and Kb value of two antibiotics quench HA gradually decreased, and the low temperature was beneficial to the interaction. The complex formed between OTC and HA was more stable than that of SD. Under neutral alkaline conditions, both OTC and SD had the strongest quenching effect on HA, and the formed complexes were the most stable. Both the value of KsvOTC and KdOTC were greater than SD in the experiment pH. The quenching effect of OTC and HA (Ksv) is significantly affected by pH, while less affected for SD.
Author Contributions
Funding
Conflicts of Interest
References
- Adams, J.L.; Tipping, E.; Thacker, S.A.; Quinton, J.N. An investigation of the distribution of phosphorus between free and mineral associated soil organic matter, using density fractionation. Plant Soil 2018, 427, 139–148. [Google Scholar] [CrossRef]
- Cotching, W.E. Organic matter in the agricultural soils of Tasmania, Australia—A review. Geoderma 2018, 312, 170–182. [Google Scholar] [CrossRef]
- Baldock, J.A.; Nelson, P.N. Soil Organic matter. Handb. Soil Sci. 2000, 194, B25–B84. [Google Scholar]
- Oleinikova, O.V.; Shirokova, L.S.; Drozdova, O.Y.; Lapitskiy, S.A.; Pokrovsky, O.S. Low biodegradability of dissolved organic matter and trace metals from subarctic waters. Sci. Total Environ. 2018, 618, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, K.M.G.; Li, W.; Wu, F.C.; Liu, C.Q.; Liao, H.Q.; Zeng, L.; Xiao, M. Environmental characteristics and changes of sediment pore water dissolved organic matter in four Chinese lakes. Environ. Sci. Pollut. Res. 2018, 25, 2783–2804. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.Y.; Wang, F.; Bian, Y.R.; Stedtfeld, R.D.; Liu, G.X.; Yu, J.P.; Jiang, X. Sorption of sulfamethazine to biochars as affected by dissolved organic matters of different origin. Bioresour. Technol. 2018, 248, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Sun, R.; Gao, X.; Xu, R.; Li, H. Low-molecular-weight organic acids enhance desorption of polycyclic aromatic hydrocarbons from soil. Eur. J. Soil Sci. 2015, 66, 339–347. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Jiang, Q.L.; Wang, R.Z.; Yuan, X.Y.; Yang, S.K.; Wang, W.K.; Zhao, Y.Q. Effects of Dissolved Organic Matter on Sorption of Oxytetracycline to Sediments. Geofluids 2018. [Google Scholar] [CrossRef]
- Mv, L.; Kögelknabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2010, 57, 426–445. [Google Scholar]
- Lehmann, J.; Solomon, D.; Kinyangi, J.; Dathe, L.; Wirick, S.; Jacobsen, C. Spatial complexity of soil organic matter forms at nanometre scales. Nat. Geosci. 2008, 1, 238–242. [Google Scholar] [CrossRef]
- Kleber, M.; Lehmann, J. Science, Language and Society: The Case of the “Humic Substances”. Available online: https://scisoc.confex.com/crops/2017am/webprogram/Paper106021.html (accessed on 20 June 2018).
- Gao, C.J.; Yang, S.K.; Wang, W.K.; Gao, L.C. Influence of Humic Acid Colloid on Adsorption of DDT in the Riverbed Sediments. Asian J. Chem. 2014, 26, 2637–2642. [Google Scholar] [CrossRef]
- Oh, S.; Shin, W.S.; Kim, H.T. Effects of pH, dissolved organic matter, and salinity on ibuprofen sorption on sediment. Environ. Sci. Pollut. Res. 2016, 23, 22882–22889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christl, I.; Milne, C.J.; Kinniburgh, D.G.; Kretzschmar, R. Relating ion binding by fulvic and humic acids to chemical composition and molecular size. 2. Metal binding. Environ. Sci. Technol. 2001, 35, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lam, K.C.; Dawson, R.W.; Liu, W.X.; Tao, S. The effect of pH, ion strength and reactant content on the complexation of Cu2+ by various natural organic ligands from water and soil in Hong Kong. Chemosphere 2003, 54, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Buffle, J.; Vuilleumier, J.J. Voltammetric study of humic and fulvic substances V. Interpretation of metal ion complexation measured by anodic stripping voltammetric methods. Sci. Total Environ. 1987, 60, 75–96. [Google Scholar] [CrossRef]
- Carballeira, J.L.; Antelo, J.M.; Arce, F. Analysis of the Cu2+-Soil Fulvic Acid Complexation by Anodic Stripping Voltammetry Using an Electrostatic Model. Environ. Sci. Technol. 2000, 34, 4969–4973. [Google Scholar] [CrossRef]
- Ramos, M.A.; Sarah, F.; Rocío, L.; M, A.J.; Florencio, A. Analysis of the effect of pH on Cu2+-fulvic acid complexation using a simple electostatic model. Environ. Sci. Technol. 2002, 36, 3109–3113. [Google Scholar] [CrossRef] [PubMed]
- Christl, I.; Kretzschmar, R. Relating ion binding by fulvic and humic acids to chemical composition and molecular size. 1. Proton binding. Environ. Sci. Technol. 2001, 35, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Schnitzer, M. Fluorescence excitation spectra on humic substances. Can. J. Soil Sci. 1980, 60, 373–379. [Google Scholar] [CrossRef]
- Saar, R.A.; Weber, J.H. Comparison of spectrofluorometry and ion-selective electrode potentiometry for determination of complexes between fulvic acid and heavy-metal ions. Anal. Chem. 2002, 52, 2095–2100. [Google Scholar] [CrossRef]
- Hsieh, P.C.; Hsu, S.H.; Lee, C.L.; Brimblecombe, P. pH dependence of binding benzo[h]quinoline and humic acid and effects on fluorescence quenching. Environ. Toxicol. Chem. 2010, 29, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Ghosh, S.; Xing, B. Nonideal binding between dissolved humic acids and polyaromatic hydrocarbons. Environ. Sci. Technol. 2007, 41, 6472–6478. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Xia, K.; Bhandari, A.; Das, K.; Pillar, G. Occurrence and fate of pharmaceuticals and personal care products (PPCPs) in biosolids. J. Environ. Qual. 2005, 34, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Gao, L.C.; Liu, Y.Y.; Zhao, Y.; Li, H.H.; Zhou, Y. The influence of humic acid colloid on adsorption behaviors of acetaminophen onto sediment. Fresenius Environ. Bull. 2015, 24, 4042–4049. [Google Scholar]
- Kulshrestha, P.; Giese, R.F.; Aga, D.S. Investigating the molecular interactions of oxytetracycline in clay and organic matter: Insights on factors affecting its mobility in soil. Environ. Sci. Technol. 2004, 38, 4097–4105. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.C.; Yu, G.H.; Yang, L.Y.; Jiang, H.L. Combination of two-dimensional correlation spectroscopy and parallel factor analysis to characterize the binding of heavy metals with DOM in lake sediments. J. Hazard. Mater. 2013, 263, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Marhuenda-Egea, F.C.; Martínez-Sabater, E.; Jordá, J.; Moral, R.; Bustamante, M.A.; Paredes, C.; Pérez-Murcia, M.D. Dissolved organic matter fractions formed during composting of winery and distillery residues: Evaluation of the process by fluorescence excitation-emission matrix. Chemosphere 2007, 68, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.J.; Ting, M.; Yun, P.; Yi, G.S.; Yin, L.S.; Xiang, W.G. Characterizing the Interaction Between Roxarsone and Humic Acid by Fluorescence Quenching Experiment. Environ. Sci. 2014, 35, 2620–2626. (In Chinese) [Google Scholar]
- Matthews, B.J.H.; Jones, A.C.; Theodorou, N.K.; Tudhope, A.W. Excitation-emission-matrix fluorescence spectroscopy applied to humic acid bands in coral reefs. Mar. Chem. 1996, 55, 317–332. [Google Scholar] [CrossRef]
- Sazanovich, I.V.; Petrov, E.P.; Chirvony, V.S. Interaction of cationic 5,10,15,20-tetrakis(4-N-methyl pyridyl) porphyrin with mono-and polynucleotides: A study by picosecond fluorescence spectroscopy. Opt. Spectrosc. 2006, 100, 209–218. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M. Interactions of hemin with bovine serum albumin and human hemoglobin: A fluorescence quenching study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 193, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Makarska-Bialokoz, M. Spectroscopic evidence of xanthine compounds fluorescence quenching effect on water-soluble porphyrins. J. Mol. Struct. 2015, 1081, 224–232. [Google Scholar] [CrossRef]
- Ping, F.Q.; Qiang, L.C.; Chang, W.F. Three-Dimensional Excitation Emission Matrix Fluorescence Spectroscopic Cha-racterization of the Complexation Between Mercury(II) and Dissolved Organic Matter. Environ. Sci. 2004, 25, 140–144. (In Chinese) [Google Scholar]
- Evans, D.; Dillon, P. Complexation between Hg(II) and Dissolved Organic Matter in Stream Waters: An Application of Fluorescence Spectroscopy. Biogeochemistry 2004, 71, 339–351. [Google Scholar]
- Hernández, D.; Plaza, C.; Senesi, N.; Polo, A. Detection of copper(II) and zinc(II) binding to humic acids from pig slurry and amended soils by fluorescence spectroscopy. Environ. Pollut. 2006, 143, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Yang, Y.; Zhang, D.; Wu, M.; Pan, B.; Xing, B. Identifying structural characteristics of humic acid to static and dynamic fluorescence quenching of phenanthrene, 9-phenanthrol, and naphthalene. Water Res. 2017, 122, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Quenching of Fluorescence; Springer: Boston, MA, USA, 2006; pp. 277–330. [Google Scholar]
- Jaycock, M.J.; Parfitt, G.D. Chemistry of Interfaces; Ellis Horwood limited: Chichester, UK, 1981. [Google Scholar]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.J.; Fitch, A.; Brar, M.S. Stability constants of Cu (II)-humate complexes: Comparison of select models. Soil Sci. 1993, 155, 77–91. [Google Scholar] [CrossRef]
- Means, J.C. Influence of salinity upon sediment-water partitioning of aromatic hydrocarbons. Mar. Chem. 1995, 51, 3–16. [Google Scholar] [CrossRef]
- Au, K.-K.; Penisson, A.C.; Yang, S.; O’Melia, C.R. Natural organic matter at oxide/water interfaces: Complexation and conformation. Geochim. Cosmochim. Acta 1999, 63, 2903–2917. [Google Scholar] [CrossRef]
- Kinniburgh, D.G.; Riemsdijk, W.H.V.; Koopal, L.K.; Borkovec, M.; Benedetti, M.F.; Avena, M.J. Ion binding to natural organic matter: Competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf. Physicochem. Eng. Asp. 1999, 151, 147–166. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhu, Y.X.; Lu, Z.B.; Peng, J.X.; Wang, P.; Cai, Z.Q.; Xiao, X.; Zhang, Y. Study on the Interaction Between Humic Acid and Pyrene by Polarity Probe. J. Xiamen Univ. (Nat. Sci.) 2007, 46, 59–62. (In Chinese) [Google Scholar]
- Hui, L.W.; Min, L.A.; Jun, W.X. Effects of pH values and ion strengthson bindings between humic acid and pyrene. Envrion. Chem. 2009, 28, 636–639. (In Chinese) [Google Scholar]
- Miano, T.M.; Senesi, N. Synchronous excitation fluorescence spectroscopy applied to soil humic substances chemistry. Sci. Total Environ. 1992, 117, 41–51. [Google Scholar] [CrossRef]
- Kukkonen, J.; Oikari, A. Bioavailability of organic pollutants in boreal waters with varying levels of dissolved organic material. Water Res. 1991, 25, 455–463. [Google Scholar] [CrossRef]
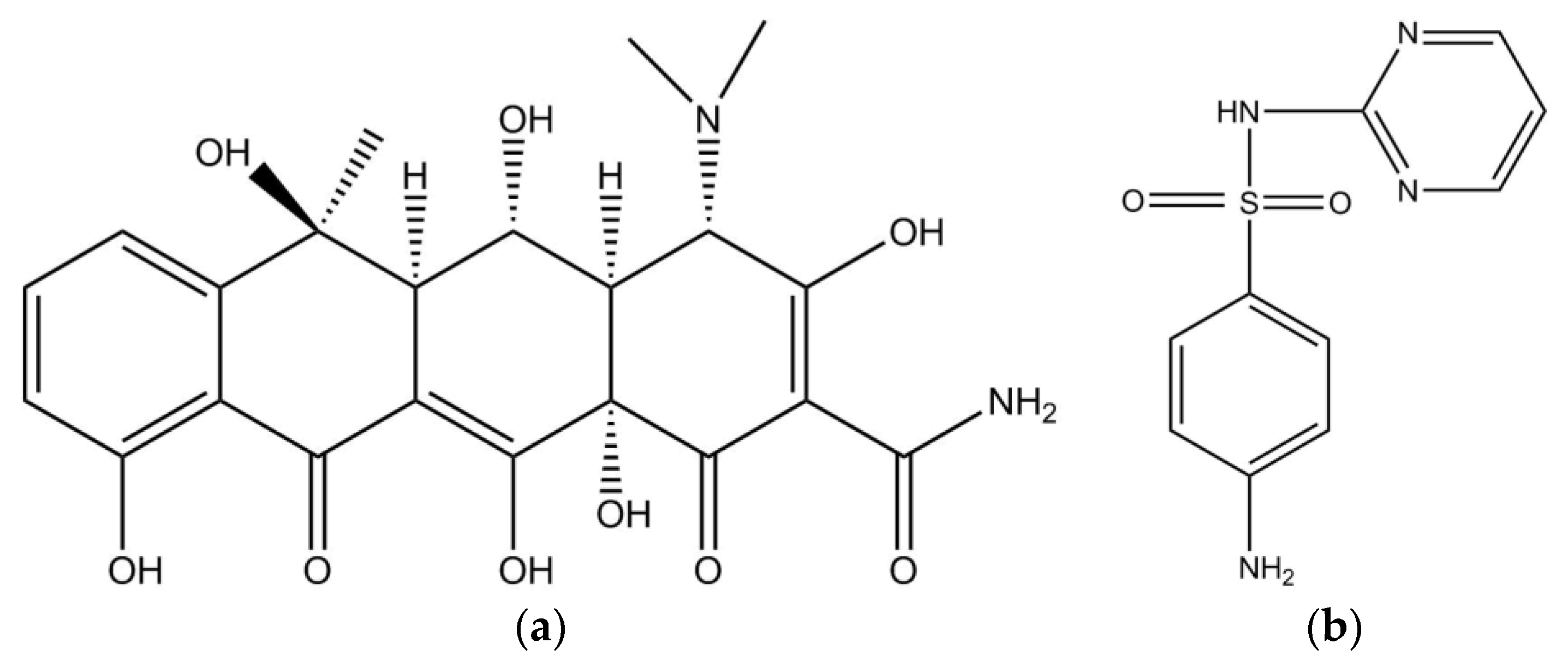
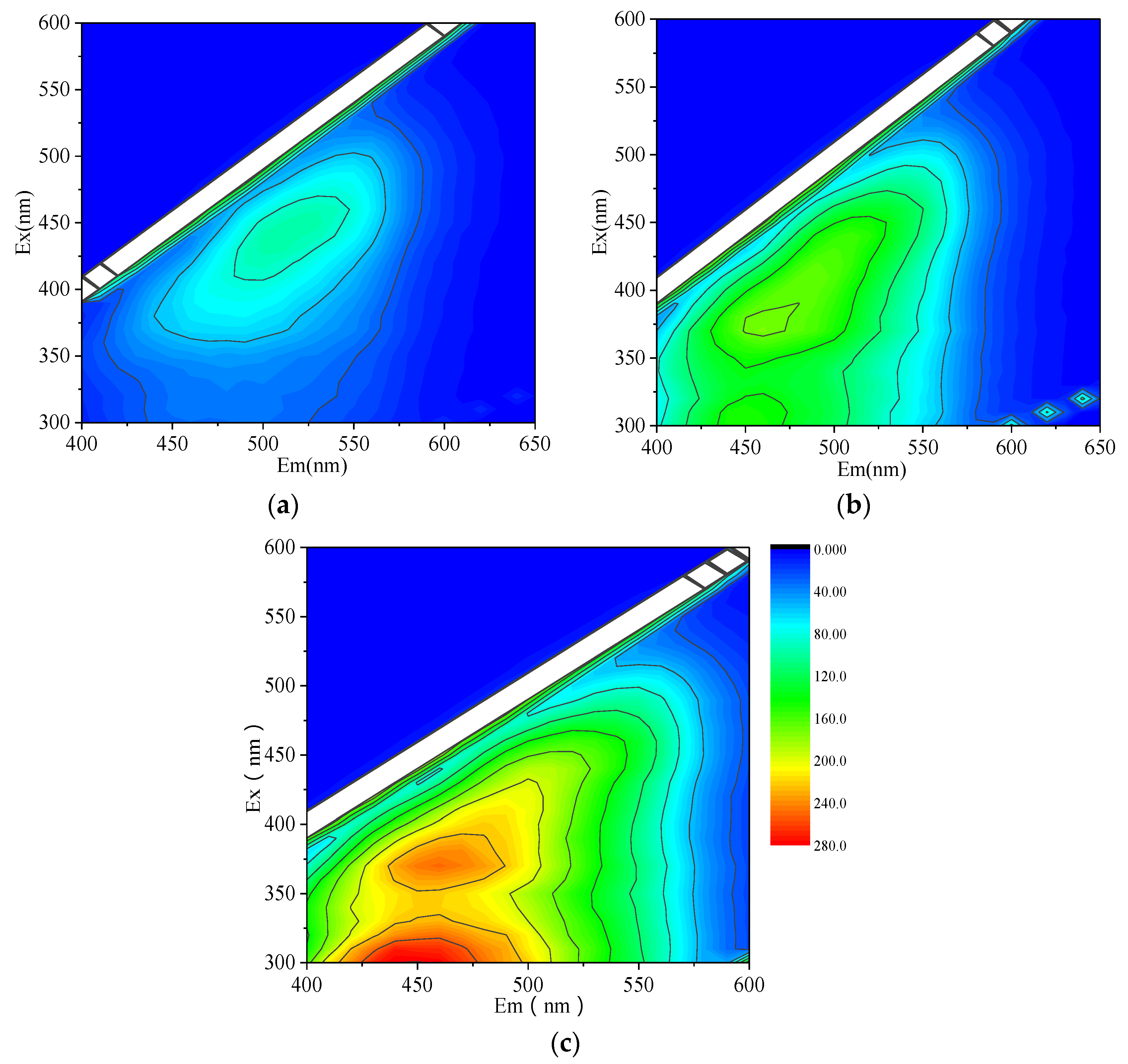
| Peak | Ex | Em | Substances |
|---|---|---|---|
| peak A | 350~440 nm | 430~510 nm | Humic acid-like |
| peak B | 310~360 nm | 370~450 nm | Fulvic acid-like (Visible) |
| peak C | 260~290 nm | 300~350 nm | Protein like |
| peak D | 240~270 nm | 370~440 nm | Fulvic acid-like (UV) |
| Quencher | Ksv (103·L·mol−1) | Kq (1011·L·mol−1·s−1) | Kb (103·L·mol−1) | n | R2 |
|---|---|---|---|---|---|
| OTC | 9.811 | 9.811 | 7.183 | 1.207 | 0.9708 |
| SD | 5.271 | 5.271 | 3.123 | 0.997 | 0.9470 |
| Antibiotics | T (K) | Ksv (103·L·mol−1) | Kq (1011·L·mol−1·s−1) | Kb (103·L·mol−1) | n | R2 |
|---|---|---|---|---|---|---|
| OTC | 288 | 13.174 | 13.174 | 12.784 | 0.974 | 0.9732 |
| 298 | 9.811 | 9.811 | 7.183 | 1.207 | 0.9708 | |
| 313 | 5.055 | 5.055 | 3.223 | 1.023 | 0.9510 | |
| SD | 288 | 7.513 | 7.513 | 5.533 | 1.104 | 0.8763 |
| 298 | 5.271 | 5.271 | 3.123 | 0.997 | 0.9469 | |
| 313 | 3.163 | 3.163 | 2.051 | 1.045 | 0.9617 |
| Antibiotics | T (K) | ΔG (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (J·mol−1·K−1) |
|---|---|---|---|---|
| OTC | 288 | −22.642 | −41.306 | −64.807 |
| 298 | −22.000 | −64.787 | ||
| 313 | −21.021 | −64.807 | ||
| SD | 288 | −20.636 | −29.750 | −31.645 |
| 298 | −19.936 | −32.933 | ||
| 313 | −19.845 | −31.645 |
| Quencher | pH | Ksv (103·L·mol−1) | Kq (1011·L·mol−1·s−1) | Kb (103·L·mol−1) | n | R2 |
|---|---|---|---|---|---|---|
| OTC | 4 | 7.282 | 7.282 | 6.430 | 0.944 | 0.5115 |
| 8 | 10.916 | 10.916 | 9.872 | 0.914 | 0.9217 | |
| 10 | 10.363 | 10.363 | 8.890 | 1.288 | 0.7982 | |
| SD | 4 | 3.894 | 3.894 | 3.724 | 1.340 | 0.8836 |
| 8 | 4.043 | 4.043 | 4.015 | 0.690 | 0.9229 | |
| 10 | 3.946 | 3.946 | 3.824 | 0.810 | 0.8263 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yang, S.; Fang, J.; Wang, Z.; Chen, Y.; Zhang, D.; Yang, C. Characterizing the Interaction between Antibiotics and Humic Acid by Fluorescence Quenching Method. Int. J. Environ. Res. Public Health 2018, 15, 1458. https://doi.org/10.3390/ijerph15071458
Wang R, Yang S, Fang J, Wang Z, Chen Y, Zhang D, Yang C. Characterizing the Interaction between Antibiotics and Humic Acid by Fluorescence Quenching Method. International Journal of Environmental Research and Public Health. 2018; 15(7):1458. https://doi.org/10.3390/ijerph15071458
Chicago/Turabian StyleWang, Runze, Shengke Yang, Jie Fang, Zongzhou Wang, Yangyang Chen, Dan Zhang, and Chunyan Yang. 2018. "Characterizing the Interaction between Antibiotics and Humic Acid by Fluorescence Quenching Method" International Journal of Environmental Research and Public Health 15, no. 7: 1458. https://doi.org/10.3390/ijerph15071458





