CRP Genotypes Predict Increased Risk to Co-Present with Low Vitamin D and Elevated CRP in a Group of Healthy Black South African Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Research Design and Study Population
2.3. Biochemical and Blood Pressure Measurements
2.4. Anthropometric Measurements
2.5. Factors Pertaining to Lifestyle
2.6. Genetic Analyses
2.7. Environmental Data
2.8. Statistical Analyses
3. Results
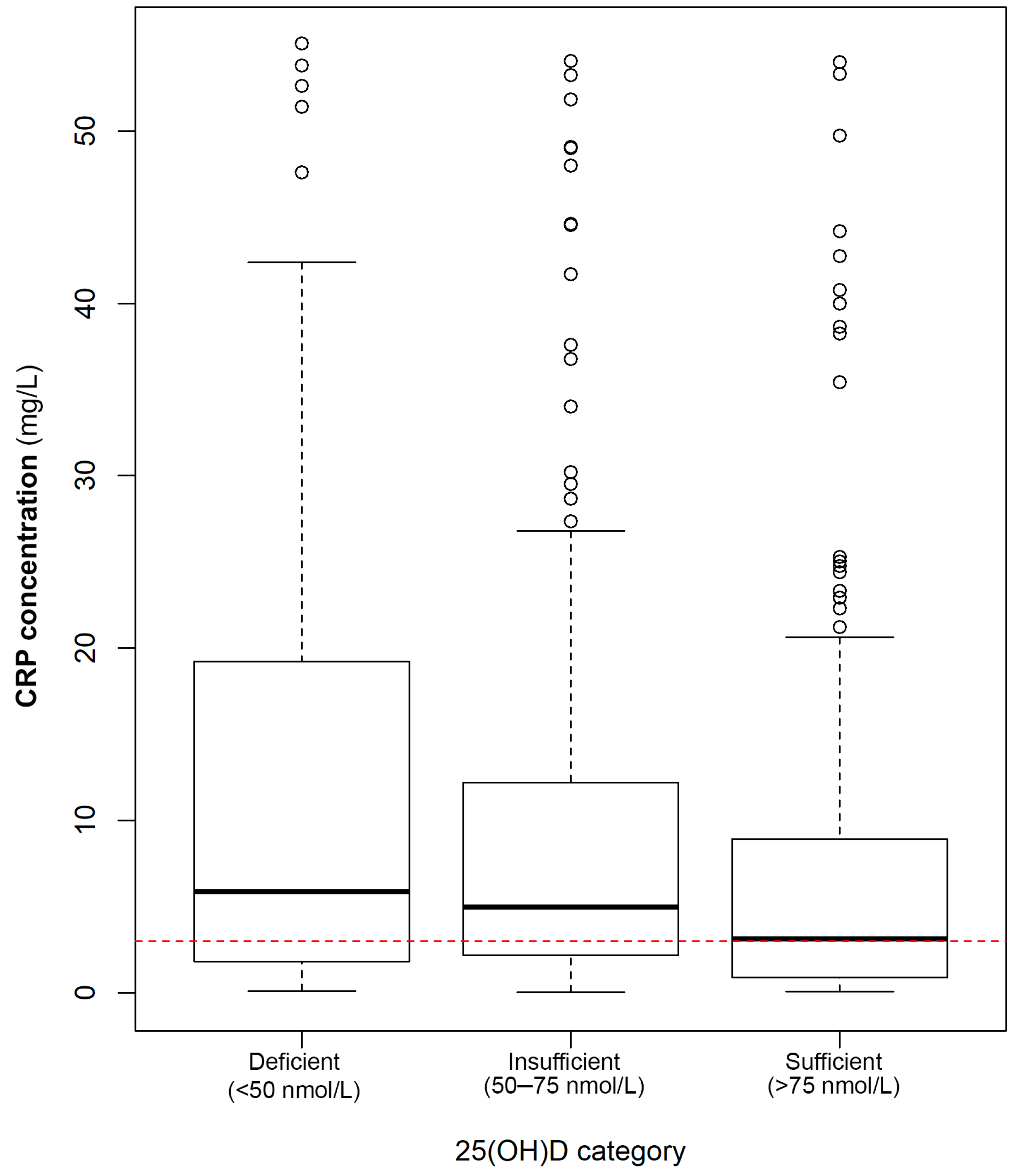
3.1. Association of 25(OH)D Concentrations/Status with Circulating CRP Concentrations
3.2. Quantification of the Associations of 25(OH)D with CRP Concentrations
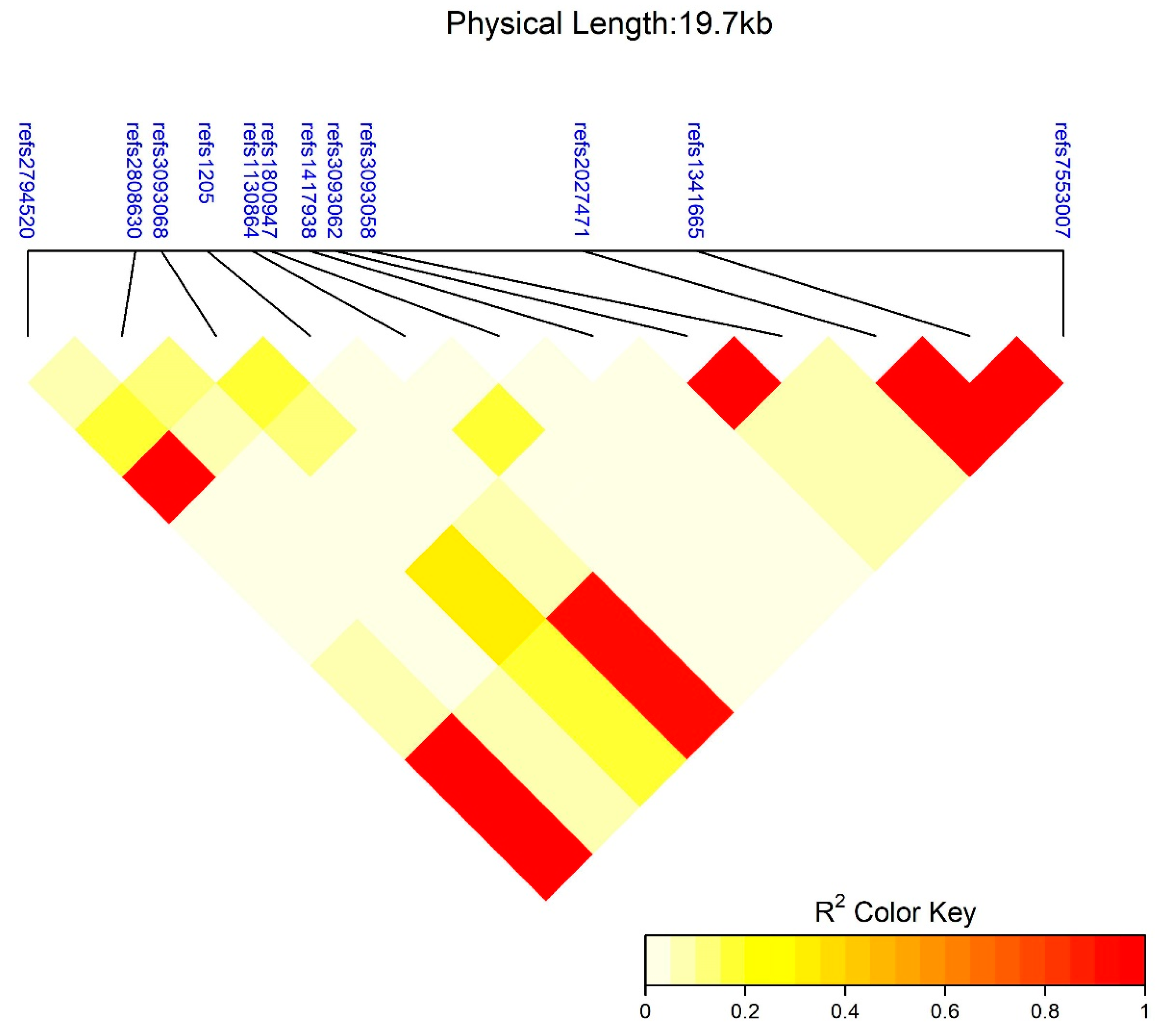
3.3. SNP Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nyirenda, M.J. Non-communicable diseases in sub-Saharan Africa: Understanding the drivers of the epidemic to inform intervention strategies. Int. Health 2016, 8, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Wilkinson, D.; Hansen, C.; Ntyintyane, L.; Tibazarwa, K.; Becker, A.; Stewart, S. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): A cohort study. Lancet 2008, 371, 915–922. [Google Scholar] [CrossRef]
- Statistics South Africa. Mortality and Causes of Death in South Africa, 2015: Findings from Death Notification; Statistics South Africa: Pretoria, Gauteng, 2017; p. 140. [Google Scholar]
- Ruiz-Núñez, B.; Pruimboom, L.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Lifestyle and nutritional imbalances associated with Western diseases: Causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J. Nutr. Biochem. 2013, 24, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K. C-reactive protein, inflammation and coronary heart disease. Egypt. Heart J. 2015, 67, 89–97. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-reactive protein to interleukin-6 to interleukin-1. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Strang, F.; Schunkert, H. C-reactive protein and coronary heart disease: All said, is not it? Mediat. Inflamm. 2014, 2014, 757123. [Google Scholar] [CrossRef] [PubMed]
- King, K. Neighborhood walkable urban form and C-reactive protein. Prev. Med. 2013, 57, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.A.; Glynn, R.J.; Ridker, P.M. Alcohol consumption and plasma concentration of C-reactive protein. Circulation 2003, 107, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Deverts, D.J.; Cohen, S.; Kalra, P.; Matthews, K.A. The prospective association of socioeconomic status with C-reactive protein levels in the CARDIA study. Brain Behav. Immun. 2012, 26, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ordovas, J.M. Impact of genetics and environmental factors on CRP levels and response to therapeutic agents. Clin. Chem. 2009, 55, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wener, M.H.; Daum, P.R.; McQuillan, G.M. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J. Rheumatol. 2000, 27, 2351–2359. [Google Scholar] [PubMed]
- Liefaard, M.C.; Ligthart, S.; Vitezova, A.; Hofman, A.; Uitterlinden, A.G.; Kiefte-de Jong, J.C.; Franco, O.H.; Zillikens, M.C.; Dehghan, A. Vitamin D and C-reactive protein: A Mendelian randomization study. PLoS ONE 2015, 10, e0131740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, N.; Mah, R.; Aburto, M.; Peters, M.J.; Dupper, M.V.; Chen, L.H. Hypovitaminosis D correction and high-sensitivity C-reactive protein levels in hypertensive adults. Perm. J. 2013, 17, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wan, Z.; Han, S.F.; Li, B.Y.; Zhang, Z.L.; Qin, L.Q. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: A meta-analysis of randomized controlled trials. Nutrients 2014, 6, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Pankow, J.S.; Folsom, A.R.; Cushman, M.; Borecki, I.B.; Hopkins, P.N.; Eckfeldt, J.H.; Tracy, R.P. Familial and genetic determinants of systemic markers of inflammation: The NHLBI family heart study. Atherosclerosis 2001, 154, 681–689. [Google Scholar] [CrossRef]
- Nienaber-Rousseau, C.; Swanepoel, B.; Dolman, R.C.; Pieters, M.; Conradie, K.R.; Towers, G.W. Interactions between C-reactive protein genotypes with markers of nutritional status in relation to inflammation. Nutrients 2014, 6, 5034–5050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bipath, P.; Viljoen, M.; Levay, P.F. Levels of procalcitonin, C-reactive protein and neopterin in patients with advanced HIV-1 infection. S. Afr. J. HIV Med. 2012, 13, 78–82. [Google Scholar] [CrossRef]
- Nazmi, A.; Victora, C.G. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: A systematic review of population-based studies. BMC Public Health 2007, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Newcombe, P.; Smeeth, L.; Addo, J.; Casas, J.P.; Whittaker, J.; Miller, M.A.; Tinworth, L.; Jeffery, S.; Strazzullo, P.; et al. Ancestry as a determinant of mean population C-reactive protein values: Implications for cardiovascular risk prediction. Circ. Cardiovasc. Genet. 2010, 3, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Mokhaneli, M.C.; Fourie, C.M.; Botha, S.; Mels, C.M. The association of oxidative stress with arterial compliance and vascular resistance in a bi-ethnic population: The SABPA study. Free Radic. Res. 2016, 50, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, N.G.; Chaplin, G. Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. USA 2010, 107, 8962–8968. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S. Vitamin D and African Americans. J. Nutr. 2006, 136, 1126–1129. [Google Scholar] [PubMed]
- Wright, C.Y.; Norval, M.; Summers, B.; Davids, L.M.; Coetzee, G.; Oriowo, M. Solar ultraviolet radiation exposure and human health in South Africa: Finding a balance. S. Afr. Med. J. 2012, 102, 665–666. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.Y.; Thoreson, C.K.; Ramsey, N.L.M.; Ricks, M.; Sumner, A.E. The Uncertain Significance of Low Vitamin D levels in African Descent Populations: A Review of the Bone and Cardiometabolic Literature. Prog. Cardiovasc. Dis. 2013, 56, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Coussens, A.K.; Wilkinson, R.J.; Bornman, L.; Lucas, R.M.; Wright, C.Y. Vitamin D Status and Its Consequences for Health in South Africa. Int. J. Environ. Res. Public Health 2016, 13, 1019. [Google Scholar] [CrossRef] [PubMed]
- Tsiaras, W.G.; Weinstock, M.A. Factors Influencing Vitamin D Status. Acta Derm.-Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Je, L.; Js, A. Vitamin D in HIV-infected patients. Curr. HIV/AIDS Rep. 2011, 8, 133–141. [Google Scholar]
- Kassi, E.N.; Stavropoulos, S.; Kokkoris, P.; Galanos, A.; Moutsatsou, P.; Dimas, C.; Papatheodorou, A.; Zafeiris, C.; Lyritis, G. Smoking is a significant determinant of low serum vitamin D in young and middle-aged healthy males. Hormones 2015, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The nonskeletal effects of vitamin D: An Endocrine Society scientific statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Brondum-Jacobsen, P.; Benn, M.; Jensen, G.B.; Nordestgaard, B.G. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: Population-based study and meta-analyses of 18 and 17 studies. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Brondum-Jacobsen, P.; Nordestgaard, B.G.; Schnohr, P.; Benn, M. 25-hydroxyvitamin D and symptomatic ischemic stroke: An original study and meta-analysis. Ann. Neurol. 2013, 73, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickie, L.J.; Church, L.D.; Coulthard, L.R.; Mathews, R.J.; Emery, P.; McDermott, M.F. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology 2010, 49, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, M.; Samols, D.; Kushner, I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J. Biol. Chem. 1996, 271, 9503–9509. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.G.; Albrecht, U.; Haussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins—Regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell Biol. 2012, 91, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.V.; Boyd, K.M.; Webb, D.J. The revision of the Declaration of Helsinki: Past, present and future. Br. J. Clin. Pharmacol. 2004, 57, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.; Chow, C.K.; Vaz, M.; Rangarajan, S.; Yusuf, S. The Prospective Urban Rural Epidemiology (PURE) study: Examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am. Heart J. 2009, 158, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vorster, H.H.; Kruger, A.; Wentzel-Viljoen, E.; Kruger, H.S.; Margetts, B.M. Added sugar intake in South Africa: Findings from the Adult Prospective Urban and Rural Epidemiology cohort study. Am. J. Clin. Nutr. 2014, 99, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- MacIntyre, U.E.; Venter, C.S.; Vorster, H.H.; Steyn, H.S. A combination of statistical methods for the analysis of the relative validation data of the quantitative food frequency questionnaire used in the THUSA study. Transition, Health and Urbanisation in South Africa. Public Health Nutr. 2001, 4, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Acker, J.G.; Leptoukh, G. Online analysis enhances use of NASA Earth science data. Eos Trans. Am. Geophys. Union 2007, 88, 14–17. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Subirana, I.; Sanz, H.; Vila, J. Building bivariate tables: The compareGroups package for R. J. Stat. Softw. 2014, 57, 1–16. [Google Scholar] [CrossRef]
- Gonzalez, J.R.; Armengol, L.; Sole, X.; Guino, E.; Mercader, J.M.; Estivill, X.; Moreno, V. SNPassoc: An R package to perform whole genome association studies. Bioinformatics 2007, 23, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Tamez, H.; Kalim, S.; Thadhani, R.I. Does vitamin D modulate blood pressure? Curr. Opin. Nephrol. Hypertens. 2013, 22, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, J.D.; Camargo, C.A., Jr.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Khaw, K.T.; Thom, S.A.M.; Hametner, B.; Wassertheurer, S.; et al. Effect of Monthly, High-Dose, Long-Term Vitamin D Supplementation on Central Blood Pressure Parameters: A Randomized Controlled Trial Substudy. J. Am. Heart Assoc. 2017, 6, e006802. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C. Vitamin D regulation of the renin-angiotensin system. J. Cell. Biochem. 2003, 88, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Grammer, T.; Drechsler, C.; Boehm, B.O.; Marz, W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Gafane, L.F.; Schutte, R.; Kruger, I.M.; Schutte, A.E. Large artery stiffness and carotid intima-media thickness in relation to markers of calcium and bone mineral metabolism in African women older than 46 years. J. Hum. Hypertens. 2015, 29, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kruger, I.M.; Kruger, M.C.; Doak, C.M.; Schutte, A.E.; Huisman, H.W.; Van Rooyen, J.M.; Schutte, R.; Malan, L.; Malan, N.T.; Fourie, C.M.T.; et al. The association of 25(OH)D with blood pressure, pulse pressure and carotid-radial pulse wave velocity in African women. PLoS ONE 2013, 8, e54554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizieh, F.; Alyahya, K.O.; Raghupathy, R. Association between levels of vitamin D and inflammatory markers in healthy women. J. Inflamm. Res. 2016, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Nienaber-Rousseau, C.; Sotunde, O.; Ukegbu, P.; Myburgh, P.; Wright, H.; Havemann-Nel, L.; Moss, S.; Kruger, I.; Kruger, H. Socio-demographic and lifestyle factors predict 5-year changes in adiposity among a group of black South African adults. Int. J. Environ. Res. Public Health 2017, 14, 1089. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Annweiler, C.; Duval, G.; Karras, S.; Tirabassi, G.; Salvio, G.; Balercia, G.; Kimball, S.; Kotsa, K.; Mascitelli, L.; et al. Vitamin D and cardiovascular disease: From atherosclerosis to myocardial infarction and stroke. Int. J. Cardiol. 2017, 230, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Quyyumi, A.A. Vitamin D and cardiovascular disease. J. Am. Coll. Cardiol. 2017, 70, 89–100. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C. Vitamin D and aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S.; Dawson-Hughes, B.; Perrone, G.A. Plasma 25-hydroxyvitamin D responses of younger and older men to three weeks of supplementation with 1800 IU/day of vitamin D. J. Am. Coll. Nutr. 1999, 18, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Iqbal, M.P.; Azam, I.; Habib, A.; Bhutta, S.; Soofi, S.B.; Bhutta, Z.A. Urban and rural comparison of vitamin D status in Pakistani pregnant women and neonates. J. Obstet. Gynaecol. 2016, 36, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wakayo, T.; Belachew, T.; Vatanparast, H.; Whiting, S.J. Vitamin D deficiency and its predictors in a country with thirteen months of sunshine: The case of school children in central Ethiopia. PLoS ONE 2015, 10, e0120963. [Google Scholar] [CrossRef] [PubMed]
- Manicourt, D.H.; Devogelaer, J.P. Urban tropospheric ozone increases the prevalence of vitamin D deficiency among Belgian postmenopausal women with outdoor activities during summer. J. Clin. Endocrinol. Metab. 2008, 93, 3893–3899. [Google Scholar] [CrossRef] [PubMed]
- Lategan, R.; van den Berg, V.L.; Ilich, J.Z.; Walsh, C.M. Vitamin D status, hypertension and body mass index in an urban black community in Mangaung, South Africa. Afr. J. Prim. Health Care Fam. Med. 2016, 8, 1210. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080s–1086s. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G.; Matsuoka, L.Y.; Hollis, B.W.; Hu, Y.Z.; Wortsman, J. Human plasma transport of vitamin D after its endogenous synthesis. J. Clin. Investig. 1993, 91, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Xiao, L.; Imayama, I.; Duggan, C.; Wang, C.-Y.; Korde, L.; McTiernan, A. Vitamin D(3) supplementation during weight loss: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.C.; Blaha, M.J.; Blumenthal, R.S. Relation of C-reactive protein to abdominal adiposity. Am. J. Cardiol. 2010, 106, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rexrode, K.M.; Pradhan, A.; Manson, J.E.; Buring, J.E.; Ridker, P.M. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann. Epidemiol. 2003, 13, 674–682. [Google Scholar] [CrossRef]
- Su, H.Y.; Lee, H.C.; Cheng, W.Y.; Huang, S.Y. A calorie-restriction diet supplemented with fish oil and high-protein powder is associated with reduced severity of metabolic syndrome in obese women. Eur. J. Clin. Nutr. 2015, 69, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, R.E.; Tangpricha, V. Evaluation of vehicle substances on vitamin D bioavailability: A systematic review. Mol. Nutr. Food Res. 2010, 54, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Detrick, B.; Cardoso, S.W.; Berendes, S.; Lama, J.R.; Sugandhavesa, P.; Mwelase, N.H.; Campbell, T.B.; Gupta, A.; ACTG A5175 PEARLS; et al. Change in vitamin D levels occurs early after antiretroviral therapy initiation and depends on treatment regimen in resource-limited settings. PLoS ONE 2014, 9, e95164. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, R.; Yang, W.T.; Berendes, S.; Mwelase, N.; Kanyama, C.; Pillay, S.; Samaneka, W.; Santos, B.; Poongulali, S.; Tripathy, S.; et al. Persistently elevated C-reactive protein level in the first year of antiretroviral therapy, despite virologic suppression, is associated with HIV disease progression in resource-constrained settings. J. Infect. Dis. 2016, 213, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Sadeghnia, H.R.; Tabatabaeizadeh, S.A.; Bahrami-Taghanaki, H.; Behboodi, N.; Esmaeili, H.; Ferns, G.A.; Mobarhan, M.G.; Avan, A. Genetic and epigenetic factors influencing vitamin D status. J. Cell. Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
| Variable | Controls (n = 289, 57.2%) | Cases (n = 216, 42.8%) | p-Value |
|---|---|---|---|
| Urban/Rural | 144 (49.8%)/145 (50.2%) | 126 (58.3%)/90 (41.7%) | NS |
| Age (years) | 53.0 (49.0; 59.0) | 56.0 (51.0; 63.0) | <0.001 |
| Smoking status: Former/Current/Abstainer | 6 (2.10%)/138 (48.3%)/142 (49.7%) | 7 (3.24%)/96 (44.4%)/113 (52.3%) | NS |
| HIV positive/negative | 26 (9.03%)/262 (91.0%) | 15 (6.98%)/200 (93.0%) | NS |
| Vitamin D intake (µg/day) | 2.00 (1.02; 3.30) | 2.05 (1.02; 3.66) | NS |
| Menorhea/Amenorhea | 64 (23.0%)/214 (77.0%) | 37 (17.2%)/178 (82.8%) | NS |
| Variable | Controls (n = 289, 57.2%) | Cases (n = 216, 42.8%) | p-Value | Adjusted p-Value | |
|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 133 (118; 148) | 138 (124; 159) | <0.001 | <0.01 | |
| Diastolic blood pressure (mmHg) | 87.0 (78.0; 96.0) | 91.0 (83.8; 101) | <0.001 | <0.01 | |
| Heart rate (BPM) | 73.0 (65.0; 83.0) | 72.0 (63.0; 85.0) | NS | NS | |
| Waist circumference (cm) | 79.3 (70.8; 87.7) | 86.4 (74.8; 95.5) | <0.001 | <0.001 | |
| Hip circumference (cm) | 98.0 (89.5; 106) | 106 (94.1; 119) | <0.001 | 0.01 | |
| WHR | 0.81 (0.76–0.87) | 0.81 (0.76–0.86) | NS | NS | |
| Weight (kg) | 60.2 (52.1; 72.8) | 72.0 (56.5; 85.5) | <0.001 | <0.01 | |
| BMI (kg/m2) | 24.7 (21.3; 29.0) | 29.9 (23.3; 35.2) | <0.001 | <0.001 | |
| BMI category | Underweight | 29 (10.0%) | 22 (10.2%) | <0.001 | <0.001 |
| Healthy | 122 (42.2%) | 45 (20.8%) | |||
| Overweight | 80 (27.7%) | 42 (19.4%) | |||
| Obese | 58 (20.1%) | 107 (49.5%) | |||
| Total cholesterol (mmol/L) | 5.21 (4.38; 6.33) | 5.30 (4.52; 6.20) | NS | NS | |
| High-density lipoprotein cholesterol (mmol/L) | 1.48 (1.14; 1.92) | 1.36 (1.07; 1.76) | 0.03 | NS | |
| Low-density lipoprotein cholesterol (mmol/L) | 3.34 (2.64; 4.23) | 3.58 (2.69; 4.41) | NS | NS | |
| Triglycerides (mmol/L) | 1.22 (0.90; 1.79) | 1.32 (0.92; 1.78) | NS | NS | |
| Dietary intake (kJ) | 6620 (5056; 9265) | 7432 (5294; 9283) | NS | NS | |
| SNP ID | Allele | Control Phenotypes | % | Case Phenotypes | % | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| rs2794520 | C/C | 166 | 57.4 | 141 | 66.8 | 0.03 | |
| C/T–T/T | 123 | 42.6 | 70 | 33.2 | 0.65 (0.44–0.95) | ||
| rs2808630 | T/T | 198 | 68.5 | 154 | 71.6 | NS | |
| C/T–C/C | 91 | 31.5 | 61 | 28.4 | 0.79 (0.53–1.18) | ||
| rs3093068 | C/C | 123 | 42.9 | 72 | 33.5 | 0.03 | |
| C/G–G/G | 164 | 57.1 | 143 | 66.5 | 1.54 (1.05–2.26) | ||
| rs1205 | C/C | 171 | 59.2 | 142 | 66 | NS | |
| C/T–T/T | 118 | 40.8 | 73 | 34 | 0.72 (0.49–1.05) | ||
| rs1130864 | C/C | 216 | 74.7 | 170 | 79.1 | NS | |
| C/T–T/T | 73 | 25.3 | 45 | 20.9 | 0.86 (0.56–1.34) | ||
| rs1800947 | C/C | 289 | 100 | 213 | 99.1 | NS | |
| C/G | 0 | 0 | 2 | 0.9 | 0 | ||
| rs1417943 | A/A | 277 | 95.8 | 203 | 95.3 | NS | |
| A/T | 12 | 4.2 | 10 | 4.7 | 1.7 (0.70–4.13) | ||
| rs3093062 | G/G | 214 | 74.3 | 137 | 63.7 | 0.02 | |
| A/G–A/A | 74 | 25.7 | 78 | 36.3 | 1.64 (1.10–2.45) | ||
| rs3093058 | A/A | 215 | 74.4 | 135 | 63.4 | 0.01 | |
| A/T–T/T | 74 | 25.6 | 78 | 36.6 | 1.67 (1.12–2.50) | ||
| rs2027471 | T/T | 167 | 57.8 | 142 | 66 | 0.05 | |
| A/T–A/A | 122 | 42.2 | 73 | 34 | 0.68 (0.46–1.00) | ||
| rs1341665 | G/G | 167 | 57.8 | 142 | 66 | 0.05 | |
| A/G–A/A | 122 | 42.2 | 73 | 34 | 0.68 (0.46–1.00) | ||
| rs7553007 | G/G | 164 | 56.7 | 141 | 65.6 | 0.04 | |
| A/G–A/A | 125 | 43.3 | 74 | 34.4 | 0.67 (0.46–0.98) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myburgh, P.H.; Towers, G.W.; Kruger, I.M.; Nienaber-Rousseau, C. CRP Genotypes Predict Increased Risk to Co-Present with Low Vitamin D and Elevated CRP in a Group of Healthy Black South African Women. Int. J. Environ. Res. Public Health 2018, 15, 111. https://doi.org/10.3390/ijerph15010111
Myburgh PH, Towers GW, Kruger IM, Nienaber-Rousseau C. CRP Genotypes Predict Increased Risk to Co-Present with Low Vitamin D and Elevated CRP in a Group of Healthy Black South African Women. International Journal of Environmental Research and Public Health. 2018; 15(1):111. https://doi.org/10.3390/ijerph15010111
Chicago/Turabian StyleMyburgh, Pieter H., G. Wayne Towers, Iolanthé M. Kruger, and Cornelie Nienaber-Rousseau. 2018. "CRP Genotypes Predict Increased Risk to Co-Present with Low Vitamin D and Elevated CRP in a Group of Healthy Black South African Women" International Journal of Environmental Research and Public Health 15, no. 1: 111. https://doi.org/10.3390/ijerph15010111
APA StyleMyburgh, P. H., Towers, G. W., Kruger, I. M., & Nienaber-Rousseau, C. (2018). CRP Genotypes Predict Increased Risk to Co-Present with Low Vitamin D and Elevated CRP in a Group of Healthy Black South African Women. International Journal of Environmental Research and Public Health, 15(1), 111. https://doi.org/10.3390/ijerph15010111






