Adsorption Behavior and Mechanism for the Uptake of Fluoride Ions by Reed Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Pre-Treatment
2.2. Adsorption Experiment Methods
2.3. Analysis
3. Results and Discussion
3.1. Characteristic of Adsorption Behavior
3.1.1. Characteristic of Kinetic Adsorption
3.1.2. Characteristic of Isothermal Adsorption
3.1.3. Characteristic of Thermodynamic Adsorption
3.2. Analysis of Adsorption Mechanism
3.2.1. Analysis of the Elemental Composition
3.2.3. Analysis of FTIR
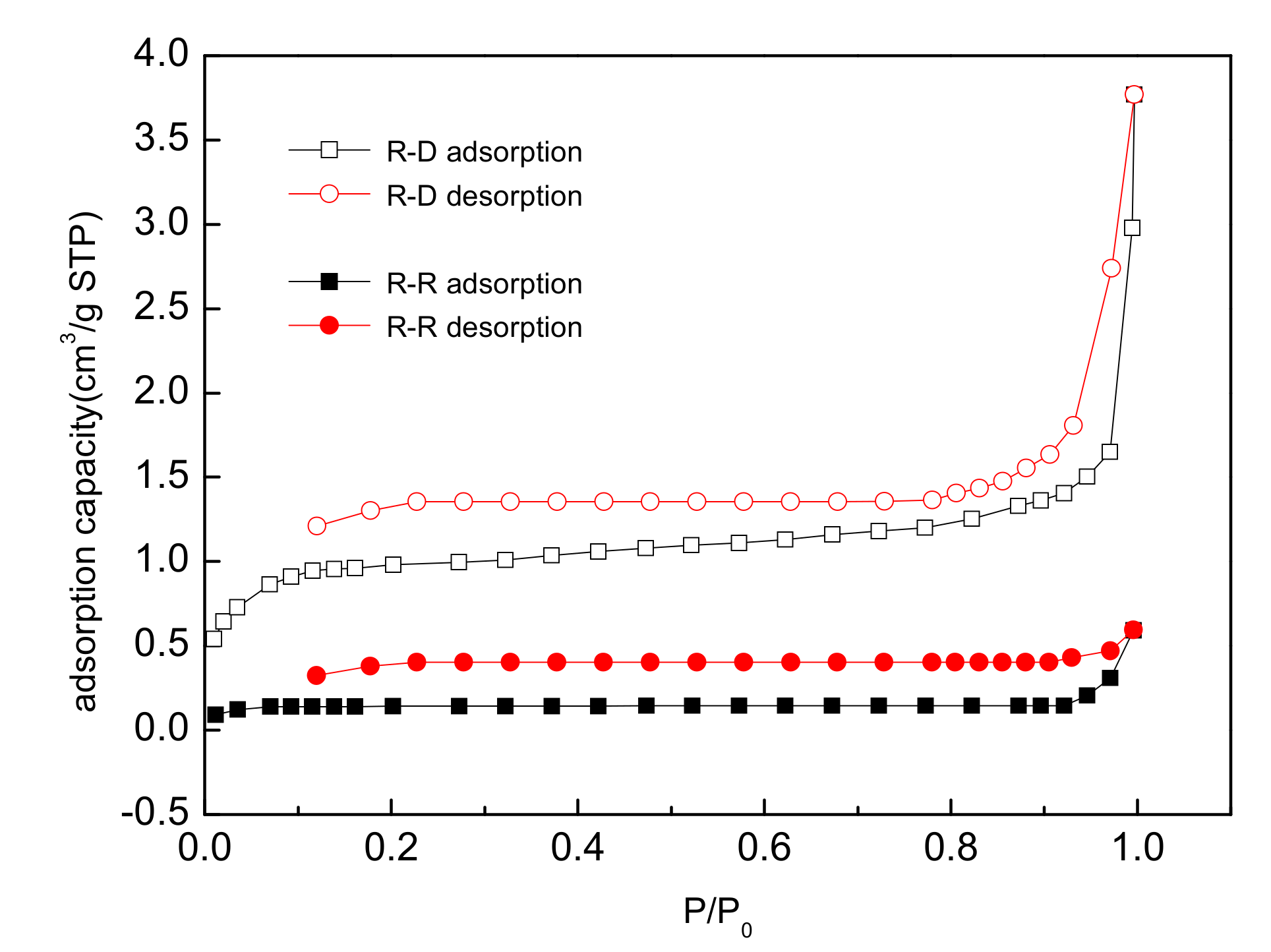
3.2.4. Analysis of Surface Area
4. Conclusions
- (1)
- Fluoride ions could be adsorbed by untreated reed residues, and the adsorption capacities were 191 mg/kg for roots, 175 mg/kg for stems and 150 mg/kg for leaves;
- (2)
- Adsorption capacities after desugarization increased significantly by 8~12 times (roots 2136 mg/kg, stems 1551 mg/kg and leaves 1825 mg/kg);
- (3)
- Desugared reed roots had high adsorption capacity for N2 compared with untreated roots that didn’t adsorb N2, and its surface area increased by 14.27 times. After desugarization, the surface area and aromaticity of reed residues increased, while the polarity and hydrophilicity decreased. This was the reason for the increase in the adsorption capacities.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, D.Y.; Huang, Y.; Xu, R.C.; Hu, J.L.; Zhang, C. Adsorption behavior of low concentration phosphorus from water onto modified reed biochar. Chin. J. Environ. Sci. 2016, 37, 2195–2201. [Google Scholar]
- Bonvicini, G.; Fregni, A.; Palmonari, C. Chapter 7: Fluorine Compounds in Gaseous Emissions from Industrial Sources: The Case of Ceramic Industries. Adv. Fluor. Sci. 2006, 1, 225–249. [Google Scholar]
- Feng, Y.W.; Ogura, N.; Feng, Z.W.; Zhang, F.Z.; Shimizu, H. The Concentrations and Sources of Fluoride in Atmospheric Depositions in Beijing, China. Water Air Soil Pollut. 2003, 145, 95–107. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Smedley, P.L. Fluoride in Natural Waters. In Essentials of Medical Geology; Springer: Berlin, Germany, 2013; pp. 311–336. [Google Scholar]
- Lin, Z.H.; Ou, S.J.; Duan, C.Y.; Zhang, B.-G.; Bai, Z.-P. Naked-eye detection of fluoride ion in water: A remarkably selective easy-to-prepare test paper. Chem. Commun. 2006, 6, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Anazawa, K. Chapter 6: Fluorine and Coexisting Volatiles in the Geosphere: The Role in Japanese Volcanic Rocks. Adv. Fluor. Sci. 2006, 1, 187–224. [Google Scholar]
- Cronin, S.J.; Manoharan, V.; Hedley, M.J.; Loganathan, P. Fluoride: A review of its fate, bioavailability, and risks of fluorosis in grazed-pasture systems in New Zealand. N. Z. J. Agric. Res. 2000, 43, 295–321. [Google Scholar] [CrossRef]
- Ellis, A.J.; Mahon, W.A.J. Chemistry and Geothermal Systems; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Heikens, A.; Sumarti, S.; Van, B.M.; Widianarko, B.; Fokkert, L.; van Leeuwen, K.; Seinen, W. The impact of the hyperacid Ijen Crater Lake: Risks of excess fluoride to human health. Sci. Total Environ. 2005, 346, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Waldbott, G.L. Fluoride in food. Am. J. Clin. Nutr. 1963, 12, 455–462. [Google Scholar] [PubMed]
- Pan, J.-M.; Zhang, H.-S.; Liu, X.-Y. The fluorine mechanism of Euphausia superba. Acta Oceanol. Sin. 2000, 22, 58–64. (In Chinese) [Google Scholar]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Alqadami, A.A.; Alshehri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient. Chem. Eng. J. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]
- Yang, M.Q.; Sun, X.F.; Wang, S.G. Preparation of carboxymethyl chitosan-glucan composite microspheres and its adsorption behavior to fluorine ion. Acta Sci. Circumst. 2017, 37, 4562–4568. (In Chinese) [Google Scholar] [CrossRef]
- Sun, Y.; Fang, Q.; Dong, J.; Cheng, X.; Xu, J. Remove of fluoride from drinking water by natural stilbite zeolite modified with Fe(III). Desalination 2011, 277, 121–127. [Google Scholar] [CrossRef]
- Wei, H.; Li, K.-B.; Shi, J.-Z. Experimental study of modified chitosan for removing fluoride ion from drinking water. J. Northwest AF Univ. (Nat. Sci. Ed.) 2010, 38, 209–213. (In Chinese) [Google Scholar] [CrossRef]
- Liang, P.; Zhang, Y.; Wang, D.F. Preparation of mixed rare earths modified chitosan for fluoride adsorption. J. Rare Earths 2013, 31, 817–822. [Google Scholar] [CrossRef]
- Wang, C.; Ji, X.; Xu, Y.-W.; Jia, Q.-Z.; Lv, X.-C. Adsorption of Fluorine ions by Modified Clay. Technol. Water Treat. 2010, 36, 48–52. (In Chinese) [Google Scholar] [CrossRef]
- Ma, L.-F.; Ruan, J.-Y.; Shi, Y.-Z.; Han, W.-Y. Study on accumulation characteristics of fluorine in tea plants. Acta Agric. Zhejiangensis 2004, 16, 96–98. (In Chinese) [Google Scholar]
- He, F.; Duan, C.-Q.; Hou, Y.-P. Characteristics of Fluoride Accumulation in Some Vegetables in Yunnan and Its Genesis. Ecol. Environ. 2004, 13, 327–329. (In Chinese) [Google Scholar] [CrossRef]
- Wang, R.; Li, J.-J.; Sun, L.-N.; Li, H.-J. Experiment Study of Biosorption and Bioaccumulation of Heavy Metals Mn and Pb by Phragmites Australis at Normal Temperature. Energy Conserv. 2007, 16, 57–61. (In Chinese) [Google Scholar]
- Chen, B.; Li, Y.; Guo, Y.; Zhu, L.; Schnoor, J.L. Role of the Extractable lipids and Polymeric lipids in Sorption of Organic Contaminants onto Plant Cuticles. Environ. Sci. Technol. 2008, 42, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhan, Y.; Zhu, Z.; Xing, Y. Adsorption of tannic acid from aqueous solution onto surfactant-modified zeolite. J. Hazard. Mater. 2011, 193, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, L.; Pu, L.; Wang, L. Adsorption Characteristics of Cr(VI) by Wheat Straw Including Kinetic and Thermodynamics Analysis. Res. Environ. Sci. 2010, 23, 1547–1552. (In Chinese) [Google Scholar]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Yenkie, M.K.N.; Labhsetwar, N.; Rayalu, S. Defluoridation of drinking water using chitosan based mesoporous alumina. Microporous Mesoporous Mater. 2011, 142, 454–463. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly(alginic acid-cl-acrylamide) nanohydrogel. Process Saf. Environ. Prot. 2017, 109, 301–310. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J. Application of ASAP2020 Surface Area and Porosity Analyzer. Anal. Instrum. 2009. (In Chinese) [Google Scholar] [CrossRef]
- HO, Y.S.; Mckay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Int. J. Chem. React. Eng. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Sepehr, M.N.; Sivasankar, V.; Zarrabi, M.; Kumar, M.S. MansuSurface modification of pumice enhancing its fluoride adsorption. Chem. Eng. J. 2013, 228, 192–204. [Google Scholar] [CrossRef]
- Dehghani, M.; Faraji, M.; Mohammadi, A.; Kamani, H. Optimization of fluoride adsorption onto natural and modified pumice using response surface methodology: Isotherm, kinetic and thermodynamic studies. Korean J. Chem. Eng. 2017, 34, 454–462. [Google Scholar] [CrossRef]
- Sahin, R.; Tapadia, K.; Sharma, A. Kinetic and isotherm studies on adsorption of fluoride by limonite with batch technique. J. Environ. Biol. 2016, 37, 919–926. [Google Scholar] [PubMed]
- Sujana, M.G.; Pradhan, H.K.; Anand, S. Studies on sorption of some geomaterials for fluoride removal from aqueous solutions. J. Hazard. Mater. 2009, 161, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Tor, A. Removal of fluoride from an aqueous solution by using montmorillonite. Desalination 2006, 201, 267–276. [Google Scholar] [CrossRef]
- García-Sánchez, J.J.; Solache-Ríos, M.; Martínez-Gutiérrez, J.M.; Arteaga-Larios, N.V.; Ojeda-Escamilla, M.C.; Rodríguez-Torres, I. Modified natural magnetite with Al and La ions for the adsorption of fluoride ions from aqueous solutions. J. Fluor. Chem. 2016, 186, 115–124. [Google Scholar] [CrossRef]
- Bia, G.; De Pauli, C.P.; Borgnino, L. The role of Fe(III)modified montmorillonite on fluoride mobility: Adsorptionexperiments and competition with phosphate. J. Environ. Manag. 2012, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Meng, F.; Zhang, L.; Ma, D.; Wang, M. Defluoridation of water using neodymium-modified chitosan. J. Hazard. Mater. 2009, 165, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tan, Y.; Zhong, M. Defluorination of wastewater by calcium chloride modified natural zeolite. Desalination 2011, 276, 246–252. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Jia, Y. Fluoride adsorption on manganese carbonate: Ion-exchange based on the surface carbonate-like groups and hydroxyl groups. J. Colloid Interface Sci. 2018, 510, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Kanrar, S.; Debnath, S.; De, P.; Parashar, K.; Pillay, K.; Sasikumar, P.; Ghosh, U.C. Preparation, characterization and evaluation of fluoride adsorption efficiency from water of iron-aluminium oxide-graphene oxide composite material. Chem. Eng. J. 2016, 306, 269–279. [Google Scholar] [CrossRef]
- Markeb, A.A.; Alonso, A.; Sánchez, A.; Font, X. Adsorption process of fluoride from drinking water with magnetic core-shell Ce-Ti@Fe3O4 and Ce-Ti oxide nanoparticles. Sci. Total Environ. 2017, 598, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-L.; Zhou, D.-D.; Li, Y.-G.; Zhu, L.-Z. Sorption of 1-Naphthol to Plant Cuticular Waxes with Different States. Environ. Sci. 2008, 29, 1671–1675. (In Chinese) [Google Scholar] [CrossRef]

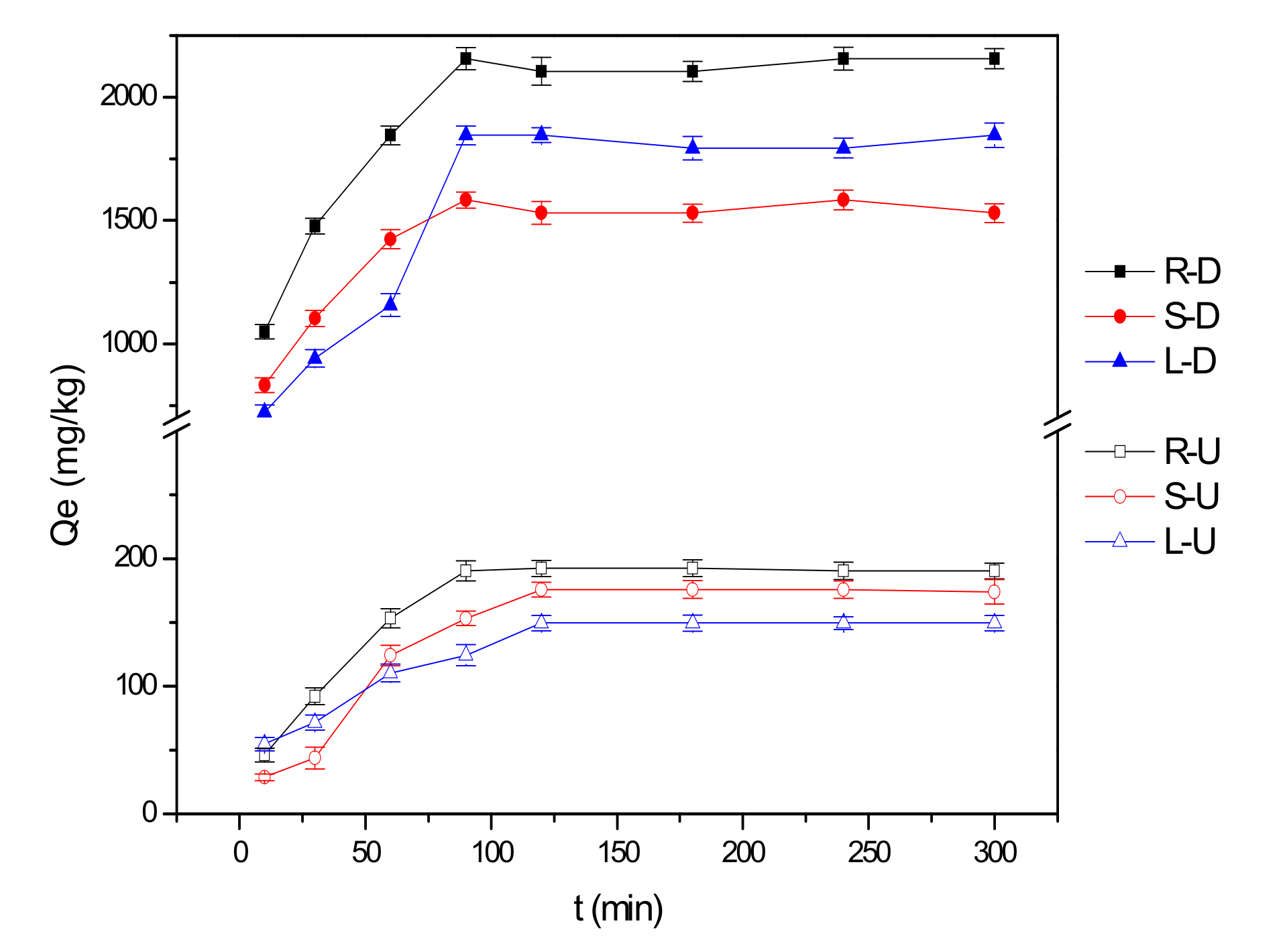

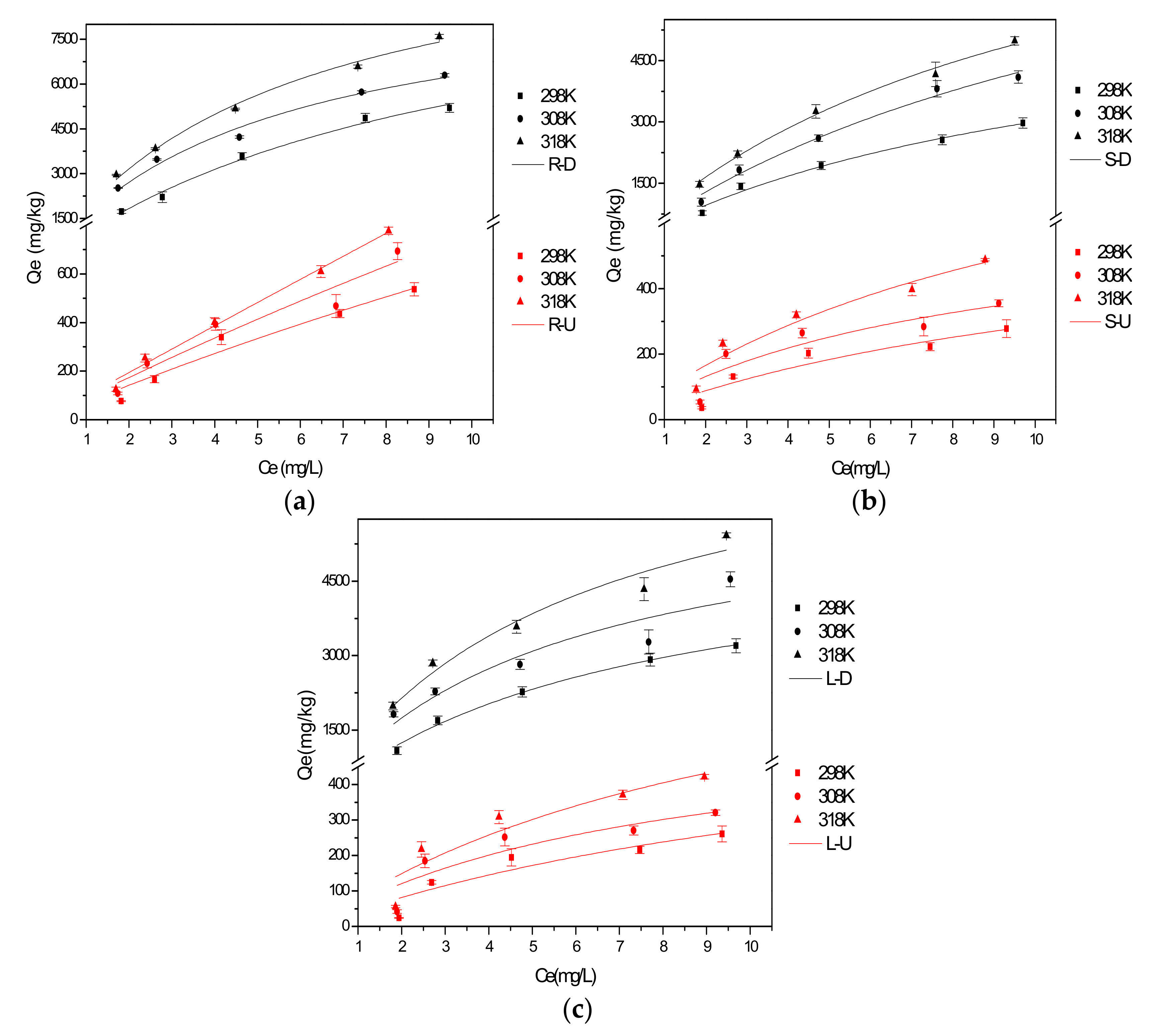


| Samples | Qe, Exp. (mg/kg) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| R2 | K1 (1/min) | Qe, cal (mg/kg) | R2 | K2 (kg/mg·min) | Qe, cal (mg/kg) | ||
| R-U | 191.28 | 0.9764 | 0.0254 | 196.02 | 0.9263 | 1.38 × 10−4 | 226.52 |
| S-U | 175.29 | 0.9401 | 0.0170 | 183.88 | 0.8983 | 7.54 × 10−5 | 227.51 |
| L-U | 149.52 | 0.9208 | 0.0242 | 149.81 | 0.9348 | 1.90 × 10−4 | 170.91 |
| R-D | 2135.66 | 0.8932 | 0.0491 | 2108.14 | 0.9537 | 3.33 × 10−5 | 2291.83 |
| S-D | 1551.42 | 0.8668 | 0.0578 | 1530.64 | 0.9310 | 5.67 × 10−5 | 1646.98 |
| L-D | 1824.57 | 0.8318 | 0.0258 | 1840.13 | 0.8377 | 1.75 × 10−5 | 2074.31 |
| Samples | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|
| Qm (mg/kg) | KL (L/mg) | R2 | RL | KF (mg/kg) (L/mg) 1/n | n | R2 | |
| R-U | 3547.04 | 0.0208 | 0.9401 | 0.4375 | 72.37 | 1.07 | 0.9351 |
| S-U | 655.37 | 0.0778 | 0.8418 | 0.4190 | 53.10 | 1.34 | 0.8181 |
| L-U | 668.87 | 0.0691 | 0.8116 | 0.4215 | 47.50 | 1.27 | 0.7867 |
| R-D | 10,859.82 | 0.1018 | 0.9882 | 0.4123 | 1210.72 | 1.50 | 0.9749 |
| S-D | 6404.78 | 0.0886 | 0.9801 | 0.4159 | 622.29 | 1.44 | 0.9695 |
| L-D | 5497.05 | 0.1460 | 0.9917 | 0.4016 | 869.47 | 1.71 | 0.9737 |
| Adsorbent | Isotherm Model | pH | Capacity (mg/g) | Reference | |
|---|---|---|---|---|---|
| Natural materials | Natural pumice | F | 6.0 | 4.50 | [29] |
| Natural pumice | F | 3.0 | 1.170 | [30] | |
| Natural geomaterial limonite (Iron Ore) | L | 7.0 | 0.269 | [31] | |
| Kaolinite clay | L | 1.450 | [32] | ||
| Montmorillonites | F | 6.0 | 3.365 | [33] | |
| Untreated reed root | L | 7.0 | 3.547 | This study | |
| Untreated reed stem | L | 7.0 | 0.655 | This study | |
| Untreated reed leaf | L | 7.0 | 0.669 | This study | |
| Modified materials | Modified pumice with FeCl3 | F | 3.0 | 21.740 | [32] |
| Modified pumice with HDTMA | F | 3.0 | 25.000 | [32] | |
| Modified magnetite ore with aluminum and lanthanum ions | L | 7.8 | M-Al 1.51 M-Na 1.42 | [34] | |
| Modified montmorillonite with Fe(III) | L | 4.5 | 9.696 | [35] | |
| Modified chitosan with neodymium | L | 7.0 | 22.380 | [36] | |
| Modified zeolite with calcium chloride | F/L | 1.766 | [37] | ||
| Desugared reed root | L | 7.0 | 10.860 | This study | |
| Desugared reed stem | L | 7.0 | 6.405 | This study | |
| Desugared reed leaf | L | 7.0 | 5.497 | This study | |
| Synthetic materials | MnCO3 nanowires | L | 7.0 | 11.580 | [38] |
| Graphene oxide (GO)-incorporated iron-aluminium mixed oxide | L | 7.0 | 22.900 | [39] | |
| Ce-Ti oxides nanoparticles | L | 7.0 | 44.370 | [40] | |
| Ce-Ti@Fe3O4 nanoparticles | L | 7.0 | 91.070 | [40] | |
| Samples | Parameters | T (K) | ||
|---|---|---|---|---|
| 298 | 308 | 318 | ||
| R-U | Qm (mg/kg) | 3547.04 | 5313.88 | 5076.931 |
| KL (L/mg) | 0.0208 | 0.0169 | 0.0019 | |
| R2 | 0.9401 | 0.9082 | 0.9879 | |
| S-U | Qm (mg/kg) | 655.37 | 648.05 | 1089.40 |
| KL (L/mg) | 0.0778 | 0.1271 | 0.0896 | |
| R2 | 0.8418 | 0.7687 | 0.9161 | |
| L-U | Qm (mg/kg) | 668.87 | 604.28 | 947.65 |
| KL (L/mg) | 0.0691 | 0.1245 | 0.0933 | |
| R2 | 0.8116 | 0.7465 | 0.8317 | |
| R-D | Qm (mg/kg) | 10,859.82 | 9573.71 | 11,775.19 |
| KL (L/mg) | 0.1018 | 0.1976 | 0.1835 | |
| R2 | 0.9882 | 0.9796 | 0.9899 | |
| S-D | Qm (mg/kg) | 6404.78 | 10,470.99 | 10,280.32 |
| KL (L/mg) | 0.0886 | 0.0702 | 0.0957 | |
| R2 | 0.9801 | 0.9832 | 0.9921 | |
| L-D | Qm (mg/kg) | 5497.05 | 6373.20 | 8185.86 |
| KL (L/mg) | 0.1460 | 0.1879 | 0.1771 | |
| R2 | 0.9917 | 0.8436 | 0.9506 | |
| Samples | T (K) | K0 | ΔG (K·mol−1) | ΔH (KJ·mol−1) | ΔS (KJ·mol−1·K−1) |
|---|---|---|---|---|---|
| R-U | 298 | 3.9785 | −3.4213 | 4.6010 | 0.0270 |
| 308 | 4.3751 | −3.7794 | |||
| 318 | 4.4680 | −3.9577 | |||
| S-U | 298 | 3.4964 | −3.1013 | 8.7097 | 0.0397 |
| 308 | 3.9921 | −3.5450 | |||
| 318 | 4.3598 | −3.8929 | |||
| L-U | 298 | 3.2021 | −2.8834 | 8.3406 | 0.0378 |
| 308 | 3.7504 | −3.3849 | |||
| 318 | 3.9530 | −3.6339 | |||
| R-D | 298 | 6.9271 | −4.7952 | 3.4746 | 0.0278 |
| 308 | 7.4067 | −5.1275 | |||
| 318 | 7.5623 | −5.3490 | |||
| S-D | 298 | 6.2228 | −4.5295 | 3.6013 | 0.0273 |
| 308 | 6.4882 | −4.7885 | |||
| 318 | 6.8193 | −5.0756 | |||
| L-D | 298 | 6.5464 | −4.6551 | 3.5335 | 0.0275 |
| 308 | 6.9914 | −4.9798 | |||
| 318 | 7.1577 | −5.2036 |
| Samples | Qe, Exp. (mg/kg) | C (%) | H (%) | O (%) | H/C | (N + O)/C | O/C | Kd (L/kg) | Koc |
|---|---|---|---|---|---|---|---|---|---|
| R-U | 191.24 | 42.46 | 6.06 | 44.59 | 1.71 | 0.81 | 0.79 | 63.67 | 149.95 |
| R-D | 2135.66 | 51.49 | 5.46 | 38.28 | 1.27 | 0.57 | 0.56 | 472.43 | 917.52 |
| S-U | 175.29 | 44.93 | 6.12 | 44.88 | 1.63 | 0.76 | 0.75 | 27.15 | 60.43 |
| S-D | 1551.42 | 50.96 | 5.91 | 43.43 | 1.39 | 0.64 | 0.64 | 262.42 | 514.95 |
| L-U | 149.52 | 42.13 | 6.10 | 40.43 | 1.74 | 0.76 | 0.72 | 26.52 | 62.95 |
| L-D | 1824.57 | 50.15 | 6.26 | 39.18 | 1.50 | 0.60 | 0.59 | 258.84 | 516.13 |
| Sample Types | Surface Area (m2/g) | Pore Area (m2/g) | Micropore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| R-U | 0.1844 | 2.2274 | 0.0009 | - |
| R-D | 2.6321 | 3.0050 | 0.0013 | 8.8610 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Yang, S.; Xu, H.; Wang, Z.; Chen, Y.; Wang, Y. Adsorption Behavior and Mechanism for the Uptake of Fluoride Ions by Reed Residues. Int. J. Environ. Res. Public Health 2018, 15, 101. https://doi.org/10.3390/ijerph15010101
Song R, Yang S, Xu H, Wang Z, Chen Y, Wang Y. Adsorption Behavior and Mechanism for the Uptake of Fluoride Ions by Reed Residues. International Journal of Environmental Research and Public Health. 2018; 15(1):101. https://doi.org/10.3390/ijerph15010101
Chicago/Turabian StyleSong, Rong, Shengke Yang, Haiyang Xu, Zongzhou Wang, Yangyang Chen, and Yanhua Wang. 2018. "Adsorption Behavior and Mechanism for the Uptake of Fluoride Ions by Reed Residues" International Journal of Environmental Research and Public Health 15, no. 1: 101. https://doi.org/10.3390/ijerph15010101




