Synthesis and Ion-Exchange Properties of Graphene Th(IV) Phosphate Composite Cation Exchanger: Its Applications in the Selective Separation of Lead Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Preparation of the Reagent Solutions
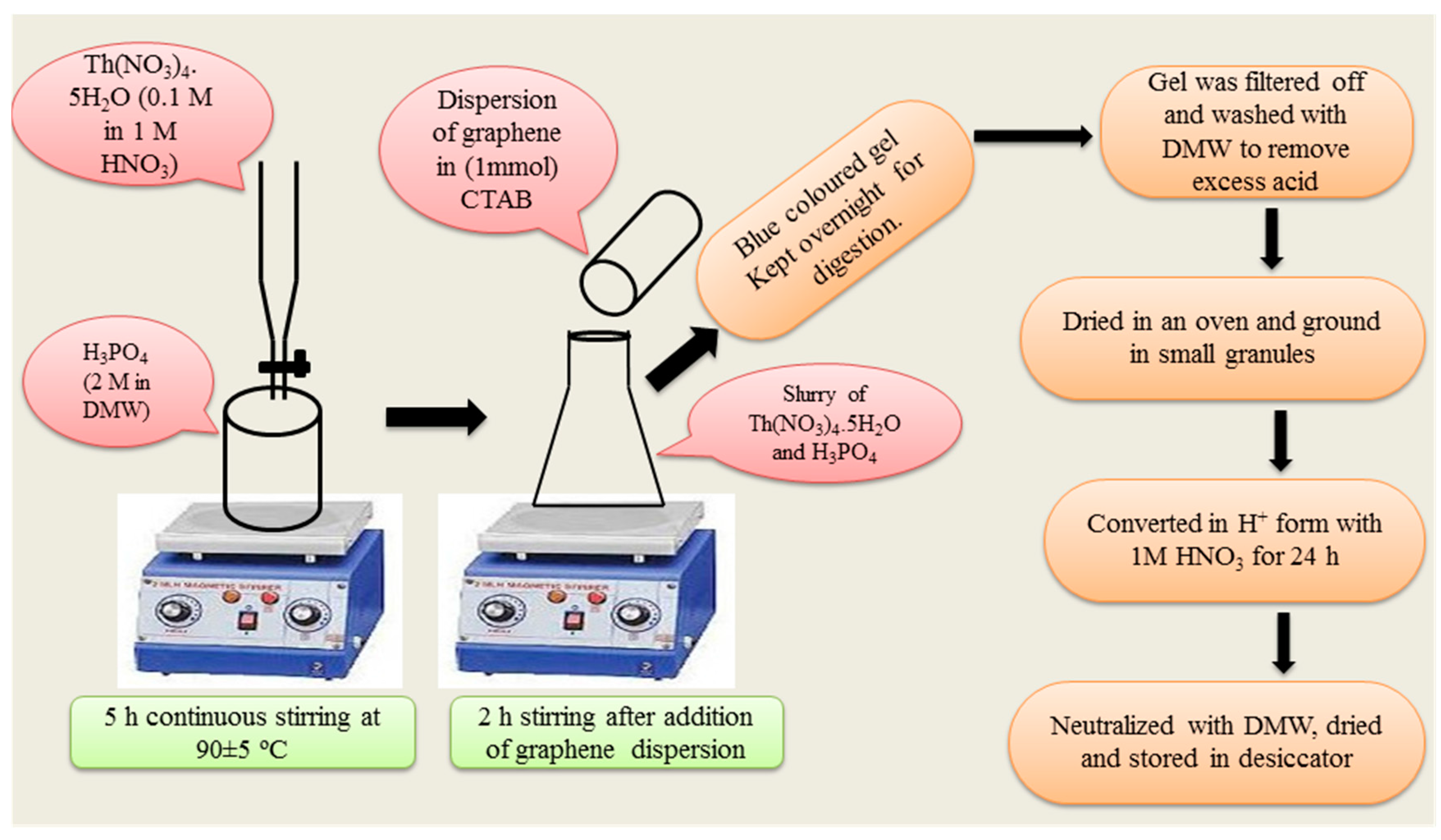
2.3. Synthesis of Composite Cation-Exchanger
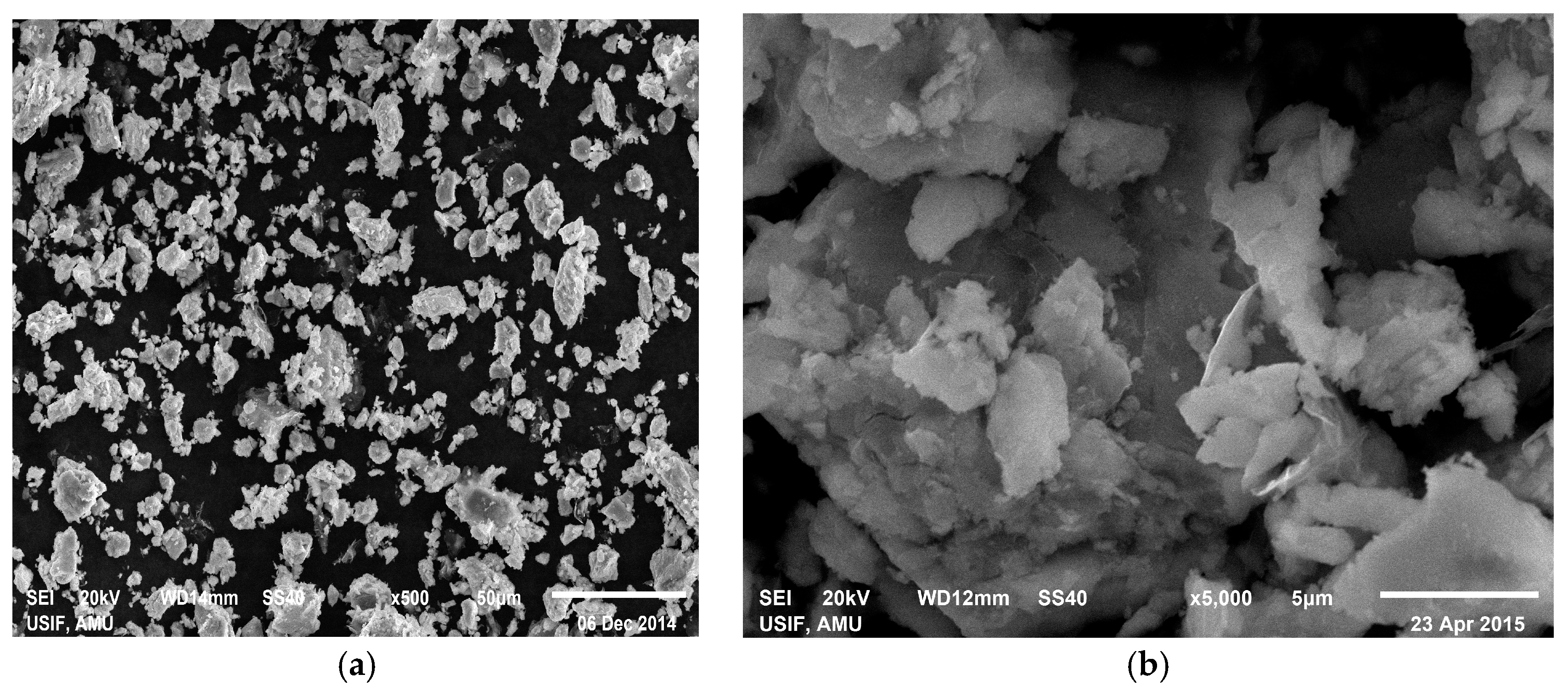
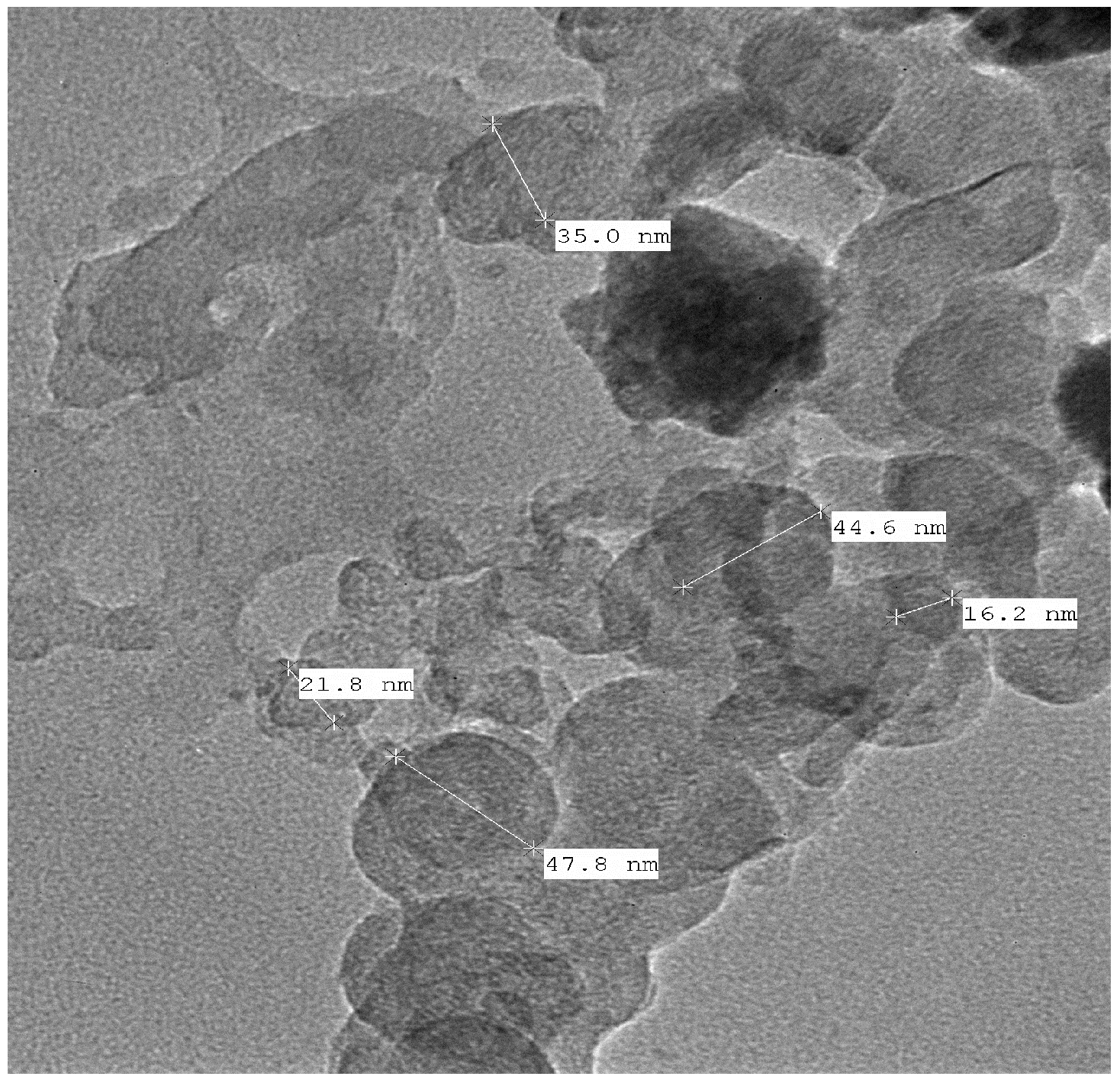
2.4. Physical Characterization
2.5. Determination of Ion-Exchange Capacity
2.5.1. Ion-Exchange Capacity for Alkali and Alkaline Earth Metals
2.5.2. Thermal Effect on Ion-Exchange Capacity
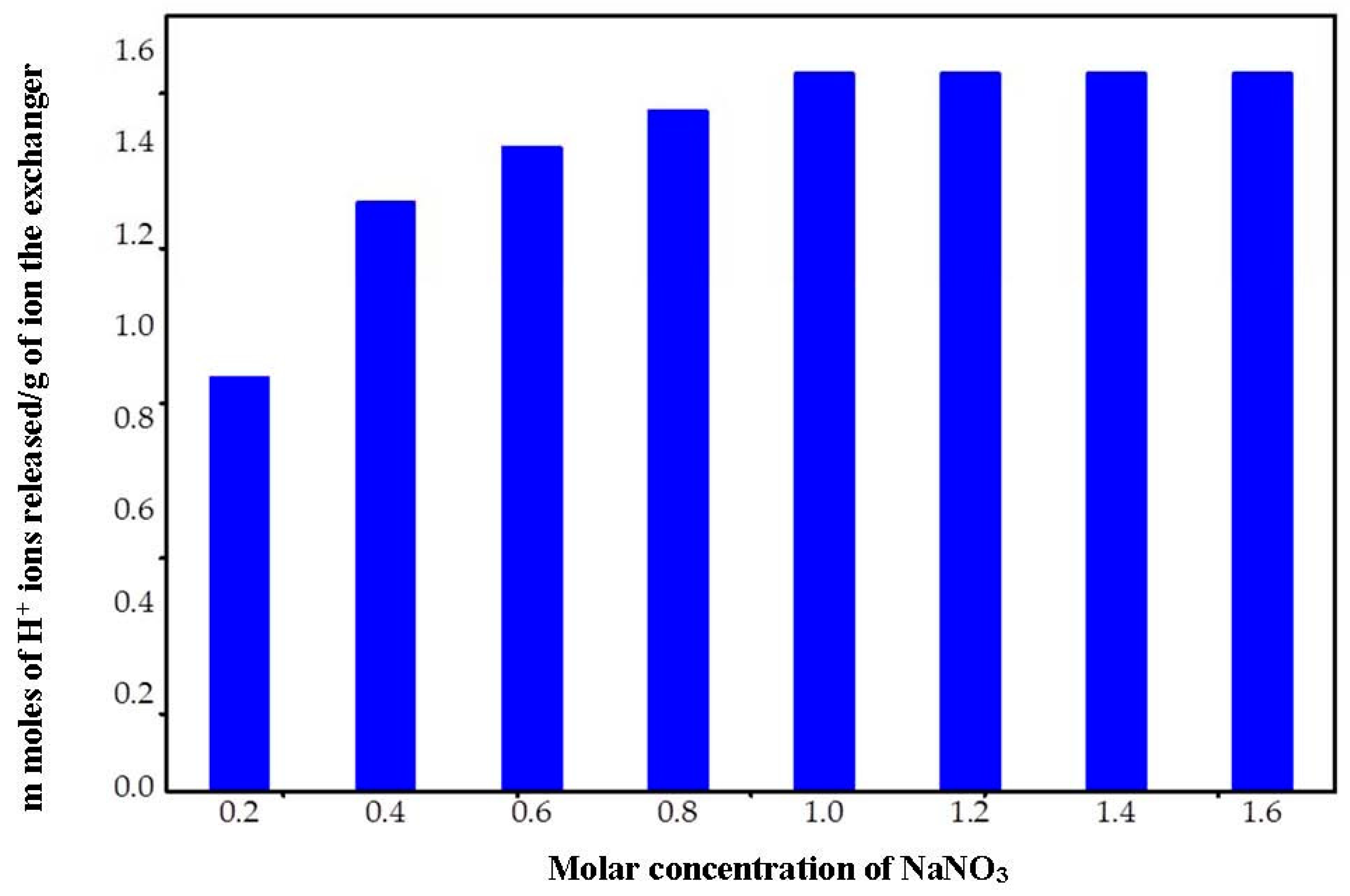
2.5.3. Effect of Eluent Concentration
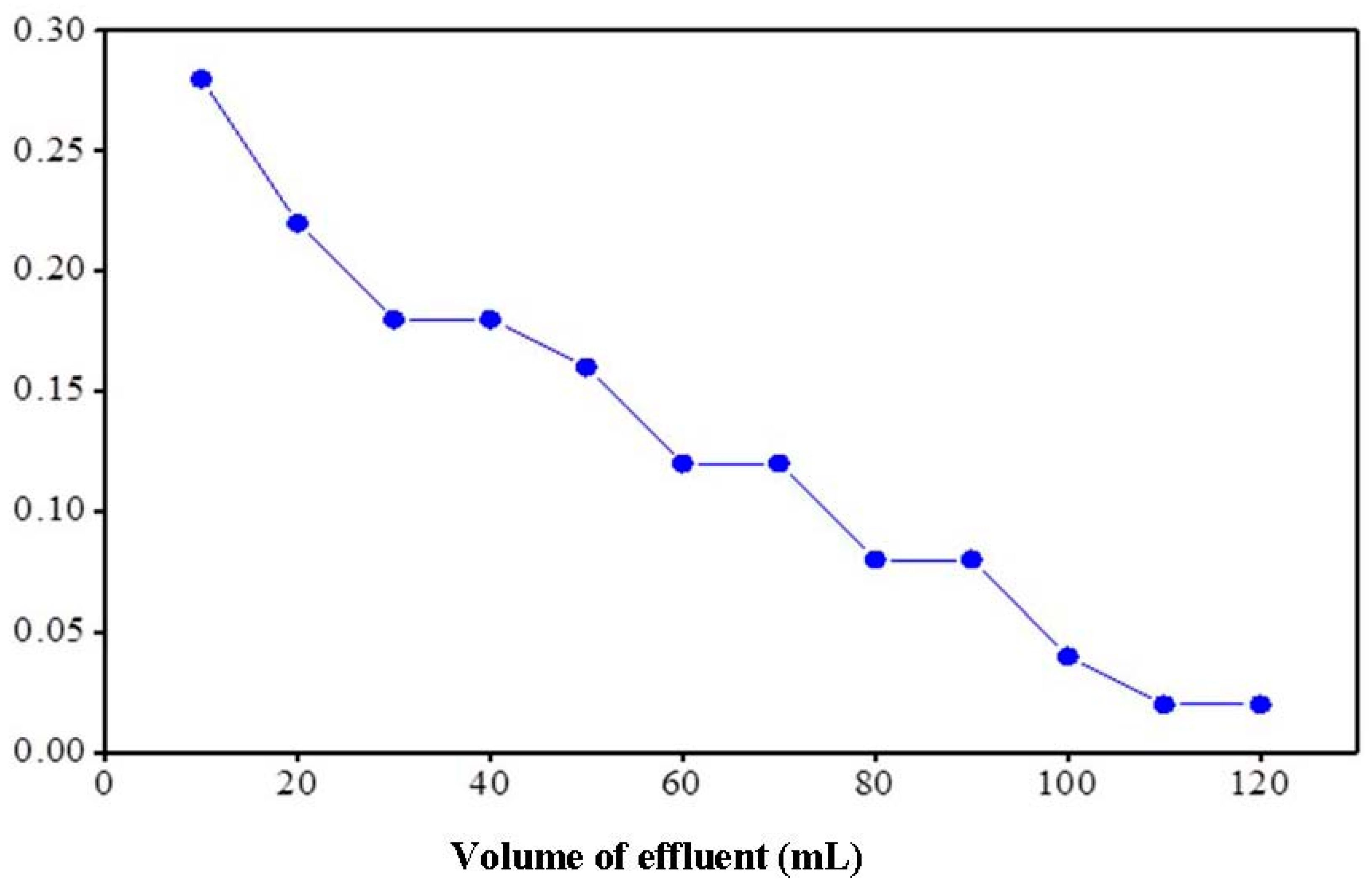
2.5.4. Elution Behavior
2.5.5. Distribution Studies
2.5.6. Separation of Metal Ions
2.5.7. Selective Separation of Metal Ions from a Synthetic Mixture
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hobbelen, P.H.F.; Koolhaas, J.E.; van Gestel, C.A.M. Risk assessment of heavy metal pollution for detritivores in floodplain soils in the Biesbosch, The Netherlands, taking bioavailability into account. Environ. Pollut. 2004, 129, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M. Surfactant assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem. Eng. J. 2014, 235, 100–108. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; Taylor and Francis Group: London, UK, 2005. [Google Scholar]
- Ahamad, T.; Naushad, M.; Inamuddin. Heavy metal ion-exchange kinetic studies over cellulose acetate Zr(IV) molybdophosphate composite cation-exchanger. Desalination Water Treat. 2015, 53, 1675–1682. [Google Scholar] [CrossRef]
- Rahman, N.; Haseen, U.; Rashid, M. Synthesis and characterization of polyacrylamide zirconium (IV) iodate ion-exchanger: Its application for selective removal of lead (II) from wastewater. Arab. J. Chem. 2013, 10, S1765–S1773. [Google Scholar] [CrossRef]
- Feng, Q.; Lin, Q.; Gong, F.; Sugita, S.; Shoya, M. Adsorption of lead and mercury by rice husk ash. J. Colloid Interface Sci. 2004, 278, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, M.M.; Kashani, M.K.; Salavati-niasari, M.; Ensafi, A.A. Lead ion-selective electrode prepared by sol—Gel and PVC membrane techniques. Sens. Actuators B 2005, 107, 438–445. [Google Scholar] [CrossRef]
- Arduini, F.; Calvo, J.Q.; Palleschi, G.; Moscone, D.; Amine, A. Bismuth-modified electrodes for lead detection. Trends Anal. Chem. 2010, 29, 1295–1304. [Google Scholar] [CrossRef]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef] [PubMed]
- Inamuddin; Rangreez, T.A.; Naushad, M.; Al-Ahmad, A. Synthesis and characterisation of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS) Zr(IV) monothiophosphate composite cation exchanger: Analytical application as lead ion selective membrane electrode. Int. J. Environ. Anal. Chem. 2015, 95, 312–323. [Google Scholar] [CrossRef]
- Mousavi, M.F.; Barzegar, M.B.; Sahari, S. A PVC-based capric acid membrane potentiometric sensor for lead (II) ions. Sens. Actuators B 2001, 73, 1–6. [Google Scholar] [CrossRef]
- Yuan, X.-J.; Wang, R.-Y.; Mao, C.-B.; Wu, L.; Chu, C.-Q.; Yao, R.; Gao, Z.-Y.; Wu, B.-L.; Zhang, H.-Y. New Pb(II)-selective membrane electrode based on a new Schiff base complex. Inorg. Chem. Commun. 2012, 15, 29–32. [Google Scholar] [CrossRef]
- Guo, L.; Hong, S.; Lin, X.; Xie, Z.; Chen, G. An organically modified sol–gel membrane for detection of lead ion by using 2-hydroxy-1-naphthaldehydene-8-aminoquinoline as fluorescence probe. Sens. Actuators B 2008, 130, 789–794. [Google Scholar] [CrossRef]
- Rangreez, T.A.; Inamuddin; Naushad, M.; Ali, H. Synthesis and characterisation of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS) Zr(IV) monothiophosphate composite cation exchanger: Analytical application in the selective separation of lead metal ions. Int. J. Environ. Anal. Chem. 2015, 95, 556–568. [Google Scholar] [CrossRef]
- Bureau of Indian Standards. Drinking Water Specification; BIS: New Delhi, India, 2012. [Google Scholar]
- Ghaedi, M.; Hekmati Jah, A.; Khodadoust, S.; Sahraei, R.; Daneshfar, A.; Mihandoost, A.; Purkait, M.K. Cadmium telluride nanoparticles loaded on activated carbon as adsorbent for removal of sunset yellow. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2012, 90, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Inamuddin; Alam, M.M. Preparation, characterization and analytical applications of a new and novel electrically conducting fibrous type polymeric–inorganic composite material: Polypyrrole Th(IV) phosphate used as a cation-exchanger and Pb(II) ion-selective membrane electrode. Mater. Res. Bull. 2005, 40, 289–305. [Google Scholar]
- Khan, A.A.; Khan, A.; Inamuddin. Preparation and characterization of a new organic–inorganic nano-composite poly-o-toluidine Th(IV) phosphate: Its analytical applications as cation-exchanger and in making ion-selective electrode. Talanta 2007, 72, 699–710. [Google Scholar] [PubMed]
- Inamuddin, M.L. (Ed.) Ion Exchange Technology I: Theory and Materials; Springer: London, UK, 2012. [Google Scholar]
- Sizgek, G.D.; Griffith, C.S.; Sizgek, E.; Luca, V. Mesoporous Zirconium Titanium Oxides. Part 3. Synthesis and Adsorption Properties of Unfunctionalized and Phosphonate-Functionalized Hierarchical Polyacrylonitrile-F-127-Templated Beads. Langmuir 2009, 25, 11874–11882. [Google Scholar] [PubMed]
- Khan, S.N.; Fazal, U.-R. Therapeutic Applications of Ion. Exchange Resins; Inamuddin, M.L., Ed.; Ion Exchange Technology II: Applications; Springer: London, UK, 2012. [Google Scholar]
- Wang, Z.H.; Yue, B.Y.; Teng, J.; Jia, F.P.; Jiang, X.Y.; Yu, J.G.; Zhong, M.; Chen, X.Q. Tartaric acid modified graphene oxide as a novel adsorbent for high-efficiently removal of Cu(II) and Pb(II) from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2016, 66, 181–190. [Google Scholar]
- Ge, H.; Zou, W. Preparation and characterization of L-glutamic acid-functionalized graphene oxide for adsorption of Pb(II). J. Dispers. Sci. Technol. 2017, 38, 241–247. [Google Scholar] [CrossRef]
- Yong, L.H.; Yu, Y.B.; Gang, Y.J.; Wei, W.X.; Xin, Z.W.; Nan, Z.; Jie, T.; Ming, Z. Diiodocarbene modified graphene: Preparation, characterization and its application as a novel adsorbent for aqueous removal of Pb(II). Nanosci. Nanotechnol. Lett. 2016, 8, 387–392. [Google Scholar]
- Jie, T.; Xiang, Z.; Yu, Y.B.; Hui, Z.X.; Hang, W.Z.; Gang, Y.J.; Ming, X.X.; Ming, Z.; Wei, W.X.; Xin, Z.W.; et al. Dibromocarbene modified graphene: Preparation, characterization and its application in removal of Pb(II) from aqueous solutions. Nanosci. Nanotechnol. 2016, 8, 226–231. [Google Scholar]
- Xiang, B.; Ling, D.; Lou, H.; Gu, H. 3D hierarchical flowerlike nickel ferrite/manganese dioxide toward lead (II) removal from aqueous water. J. Hazard. Mater. 2017, 325, 178–188. [Google Scholar] [PubMed]
- Reilley, C.N.; Schmid, R.W.; Sadek, F.S. Chelon approach to analysis: I. Survey of theory and application. J. Chem. Educ. 1959, 36, 555–564. [Google Scholar]
- Nabi, S.A.; Shalla, A.H. EDTA-stannic (IV) iodate: Preparation, characterization and its analytical applications for metal content determination in real and synthetic samples. J. Porous Mater. 2009, 16, 587–597. [Google Scholar]
- Nabi, S.A.; Akhter, A.; Khan, M.D.A.; Khan, M.A. Synthesis, characterization and electrical conductivity of Polyaniline-Sn(IV)tungstophosphate hybrid cation exchanger: Analytical application for removal of heavy metal ions from wastewater. Desalination 2014, 340, 73–83. [Google Scholar]
- Rangreez, T.A.; Inamuddin. Synthesis and characterization of graphene Th(IV) phosphate composite cation 12 exchanger: Analytical application as lead ion-selective membrane electrode. Desalination Water Treat. 2016, 57, 23893–23902. [Google Scholar]
- Rawat, J.P.; Singh, J.P. Studies on inorganic ion exchangers. II. Synthesis ion exchange properties and applications of ferric arsenate. Can. J. Chem. 1976, 54, 2534–2539. [Google Scholar]
- Gupta, A.P.; Agarwal, H.; Ikram, S. Studies on a new composites material polyanilinezirconium (IV) tungstophosphate: A thorium (IV) selective cation exchanger. J. Indian Chem. Soc. 2003, 80, 57–59. [Google Scholar]
- Nachod, F.C.; Wood, W. The Reaction Velocity of Ion Exchange. J. Am. Chem. Soc. 1944, 66, 1380–1384. [Google Scholar]
| S. No. | Mixing Volume Ratios (v/v) | Graphene (mg) Dispersion in 1 mmol CTAB | Appearance of Beads after Drying | Na+ Ion Exchange Capacity (meq·dry·g−1) | |
|---|---|---|---|---|---|
| 0.1 M ThPO4·5H2O in 1 M HNO3 | 2 M H3PO4 | ||||
| S-1 | 1 | 1 | 50 | Light Blue | 1.48 |
| S-2 | 1 | 1.5 | 100 | Light Blue | 1.56 |
| S-3 | 1 | 2 | 150 | Light Blue | 1.56 |
| S-4 | 1 | 2.5 | 200 | Light Blue | 1.56 |
| S. No. | Ion-Exchanger | Ion-Exchange Capacity (meq·dry·g−1) | Reference |
|---|---|---|---|
| 1 | EDTA-stannic (IV) iodate | 0.74 | [34] |
| 2 | Polypyrrole Th(IV) phosphate | 1.56 | [23] |
| 3 | Polyaniline Sn(IV) tungstophosphate | 1.10 | [35] |
| 5 | Graphene Thorium(IV) phosphate | 1.56 | Present Study |
| Exchanging Ions | pH of the Metal Solution | Ionic Radii (Å) | Hydrated Ionic Radii (Å) | I.E.C. (meq·dry·g−1) |
|---|---|---|---|---|
| Li+ | 6.7 | 0.68 | 3.40 | 1.30 |
| Na+ | 6.7 | 0.97 | 2.76 | 1.56 |
| K+ | 6.8 | 1.33 | 2.32 | 1.70 |
| Mg2+ | 6.5 | 0.78 | 7.00 | 1.20 |
| Ca2+ | 6.5 | 1.06 | 6.30 | 1.34 |
| Sr2+ | 6.3 | 1.27 | - | 1.50 |
| Ba2+ | 6.3 | 1.43 | 5.90 | 1.68 |
| Heating Temperature (°C) | Appearance (Color) | Weight Loss (%) | Na+ Ion-Exchange Capacity (meq·dry·g−1) | % Retention of I.E.C. |
|---|---|---|---|---|
| 50 | Light Blue | - | 1.56 | 100 |
| 100 | Light Blue | 9.0 | 1.56 | 100 |
| 150 | Storm Grey | 11.0 | 1.40 | 90.03 |
| 200 | 12.0 | 1.25 | 80.12 | |
| 250 | Charcoal grey | 12.5 | 1.08 | 69.59 |
| 300 | Mist Grey | 13.5 | 1.0 | 64.10 |
| 350 | Mist Grey | 15.0 | 0.90 | 60.33 |
| 400 | Mist Grey | 15.0 | 0.88 | 56.56 |
| 450 | Mist Grey | 15.5 | 0.82 | 53.10 |
| Solvents Metal Ions | Kd-Values (mL/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demineralized Water (DDW) | 10−2 M HClO4 | 10−1 M HClO4 | 1 M HClO4 | 10−2 M HNO3 | 10−1 M HNO3 | 1 M HNO3 | 10−2 M HCl | 10−1 M HCl | 1 M HCl | |
| Mg(II) | 95 | 46 | 28 | 17 | 30 | 26 | 22 | 25 | 20 | 14 |
| Ca(II) | 134 | 140 | 107 | 83 | 112 | 94 | 81 | 104 | 85 | 67 |
| Cd(II) | 80 | 85 | 69 | 45 | 92 | 77 | 52 | 81 | 63 | 47 |
| Pb(II) | 560 | 640 | 593 | 548 | 580 | 523 | 470 | 500 | 430 | 380 |
| Co(II) | 135 | 155 | 128 | 117 | 137 | 123 | 115 | 140 | 117 | 104 |
| Hg(II) | 380 | 470 | 426 | 377 | 390 | 362 | 284 | 360 | 337 | 246 |
| Mn(II) | 75 | 87 | 71 | 46 | 73 | 56 | 37 | 78 | 52 | 33 |
| Sr(II) | 127 | 133 | 114 | 92 | 167 | 142 | 109 | 125 | 103 | 72 |
| Cu(II) | 66 | 57 | 39 | 28 | 62 | 45 | 32 | 50 | 31 | 26 |
| Ba(II) | 110 | 116 | 94 | 81 | 100 | 86 | 71 | 110 | 93 | 79 |
| Separation Achieved | Amount Loaded (mg) | Amount Found (mg) | % Error | Eluent Used | Volume of Eluent (mL) |
|---|---|---|---|---|---|
| Cu(II), Pb(II) | 1.2710, 4.1440 | 1.2616, 4.1440 | −0.74, 0.00 | 1 M HCl, 1 M HCl | 50, 60 |
| Ba(II), Pb(II) | 2.7460, 4.1440 | 2.7246, 4.1440 | −0.80, 0.00 | 1 M HNO3, 1 M HCl | 60, 60 |
| Cd(II), Pb(II) | 2.2482, 4.1440 | 2.2257, 4.0881 | −1.00, +1.35 | 1 M HClO4, 1 M HCl | 50, 60 |
| Ca(II), Pb(II) | 4.7230, 4.1440 | 4.6777, 4.1179 | +0.96, −0.63 | 1 M HCl, 1 M HCl | 60, 60 |
| Sr(II), Pb(II) | 1.7524, 4.1440 | 1.7121, 4.1440 | −2.30, 0.00 | 1 M HCl, 1 M HCl | 50, 60 |
| S. No. | Amount Loaded (mg) | Amount Found (mg) | Recovery (%) | Error (%) | Volume of Eluent (0.1 M HCl) Used (mL) |
|---|---|---|---|---|---|
| 1 | 2.0720 | 2.0447 | 98.68 | −1.32 | 50 |
| 2 | 4.1440 | 4.1150 | 99.30 | −0.70 | 60 |
| 3 | 6.2160 | 6.0850 | 97.89 | −2.11 | 65 |
| 4 | 8.2880 | 8.1390 | 98.20 | −1.80 | 75 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangreez, T.A.; Inamuddin; Asiri, A.M.; Alhogbi, B.G.; Naushad, M. Synthesis and Ion-Exchange Properties of Graphene Th(IV) Phosphate Composite Cation Exchanger: Its Applications in the Selective Separation of Lead Metal Ions. Int. J. Environ. Res. Public Health 2017, 14, 828. https://doi.org/10.3390/ijerph14070828
Rangreez TA, Inamuddin, Asiri AM, Alhogbi BG, Naushad M. Synthesis and Ion-Exchange Properties of Graphene Th(IV) Phosphate Composite Cation Exchanger: Its Applications in the Selective Separation of Lead Metal Ions. International Journal of Environmental Research and Public Health. 2017; 14(7):828. https://doi.org/10.3390/ijerph14070828
Chicago/Turabian StyleRangreez, Tauseef Ahmad, Inamuddin, Abdullah M. Asiri, Basma G. Alhogbi, and Mu. Naushad. 2017. "Synthesis and Ion-Exchange Properties of Graphene Th(IV) Phosphate Composite Cation Exchanger: Its Applications in the Selective Separation of Lead Metal Ions" International Journal of Environmental Research and Public Health 14, no. 7: 828. https://doi.org/10.3390/ijerph14070828
APA StyleRangreez, T. A., Inamuddin, Asiri, A. M., Alhogbi, B. G., & Naushad, M. (2017). Synthesis and Ion-Exchange Properties of Graphene Th(IV) Phosphate Composite Cation Exchanger: Its Applications in the Selective Separation of Lead Metal Ions. International Journal of Environmental Research and Public Health, 14(7), 828. https://doi.org/10.3390/ijerph14070828






