1. Introduction
Lithium is an uncommon parameter for drinking water analysis. Its spatial and temporal distributions are not commonly studied and are often overlooked because it appears at very low levels. Its concentrations in natural waters vary depending on geology, topography, hydrogeology and other variables. Despite this, there are some generally referred to ranges of lithium concentrations in waters. In sea water, where it is the fourteenth most abundant element, it occurs at 0.14 to 0.20 mg/L [
1,
2,
3] and in fresh waters it occurs at 0.001 to 0.020 mg/L [
3,
4,
5,
6,
7,
8,
9,
10,
11]. Shand et al. report medium levels of lithium in groundwater, associated with different bedrock geologies in England: Chalk at 0.0008 mg/L and Permo-Triassic Sandstone at 0.001 mg/L [
12]. There is no legislative requirement for lithium to be monitored in Irish drinking water, therefore it has no safety standard. This does not mean that concentrations cannot reach toxic levels. The high altitude continental brine aquifers of Argentina, Chile and Bolivia contain most of the world’s accessible lithium; Bolivia alone is said to have jurisdiction over a third of the world’s recoverable lithium [
13]. The lithium levels in the natural waters in these areas are very high: ≥5 mg/L. Northern Chile has recorded the highest levels of lithium in surface waters in the world, at levels of 2 to 20 mg/L, 2–3 orders of magnitude higher than most rivers. Because of this, there has been some work in the area concerning the high exposure of lithium in the population [
11,
14,
15,
16].
Lithium has recently come into public awareness because of its use in lithium-ion batteries (LIBs). LIBs are already powering most of the electronic devices we use every day, such as our phones and laptops, a market that did not exist 20 years ago. The fact that lithium is set to power the electric vehicles of the future makes it a key resource for both the industrialized world and emerging economies. Other uses of lithium include as lithium carbonate, a medication to treat mental disorders; as lithium stearate, an all-purpose high temperature lubricant; as a fluxing agent in the ceramics industry; as a light-weight alloy; and as lithium chloride, a hygroscopic agent in air conditioning systems. The emerging electric vehicle market is pushing this ordinary alkali metal to become the new “white petroleum” of the 21st century. It is the objective of this work to establish a background level of lithium in the waters of Carlow, prior to any future commercial abstraction.
Lithium has been used as a mood stabilizing drug in people with mood disorders such as bipolar and major depression for over 150 years. It has also been used successfully to treat people with suicidal tendencies. There are several studies which suggest a negative association between lithium levels in drinking water and suicide rates in populations [
17,
18,
19,
20]. Large doses of lithium (10 mg/L in serum) are used in patients to treat these disorders. Lithium has a narrow therapeutic index: at 10 mg/L of blood, a person is considered mildly lithium poisoned; at 15 mg/L, they experience confusion and speech impairment; at 20 mg/L, there is a risk of death [
11]. The lithium ion displays chronic toxicity on the human central nervous system [
11,
21,
22]. A lethal dose of lithium chloride (LiCl) in rats has been measured at 526–840 mg/kg. The amount of lithium in the human body is about 7 mg; a dose of approximately 5 g LiCl can result in fatal poisoning [
11]. On the other hand, lithium from drinking water is not expected to bioaccumulate and its environmental toxicity is low. The lithium requirement in humans is low; available experimental evidence appears to suggest that a provisional recommended daily allowance (RDA) for a 70 kg adult is 1 mg/day. This same evidence also states that the average daily intake of lithium of a 70 kg adult is around 0.65 to 3.1 mg/day from foods such as grains and vegetables [
23,
24].
Geochemically, lithium is a rare metal with average crustal abundances rarely exceeding 10–20 mg/kg. Lithium levels in granite (which makes up about two-thirds of Carlow’s bedrock geology) are higher at around 22 to 65 mg/kg. Lithium tends to be tightly bound in the crystal structure of the rock, therefore it alone does not pose an ecological problem [
11,
23,
25,
26,
27,
28]. Lithium does not appear in its pure form in nature due to its highly reactive properties. The metal occurs predominantly in the silicate matrices of pegmatites; some clay minerals such as Hectorite Na
0.3(Mg,Li)
3Si
4O
10(OH)
2 [
29]; geothermal brines; oilfield brines; and in solution in naturally occurring continental alkaline brine aquifers. The two major exploitable sources are pegmatite minerals such as spodumene and continental brine deposits. Brine deposits are easy to explore, fast to put into production and require less initial capital. Although lithium is easily extracted from brine sources, mineral deposits can contain much greater amounts of lithium. Lithium concentrations in subsurface brines range from 20 to 1500 mg/L [
11,
30], while mineral deposits may contain 530 to 55,100 mg/kg [
31]. Hard rock mineral mining is a more involved process and usually takes the form of open pit mining depending on the depth at which the pegmatites occur. The processing of lithium-containing minerals involves crushing, wet grinding in a ball mill, sizing, gravity concentration followed by flotation using a fatty acid as a collector [
11].
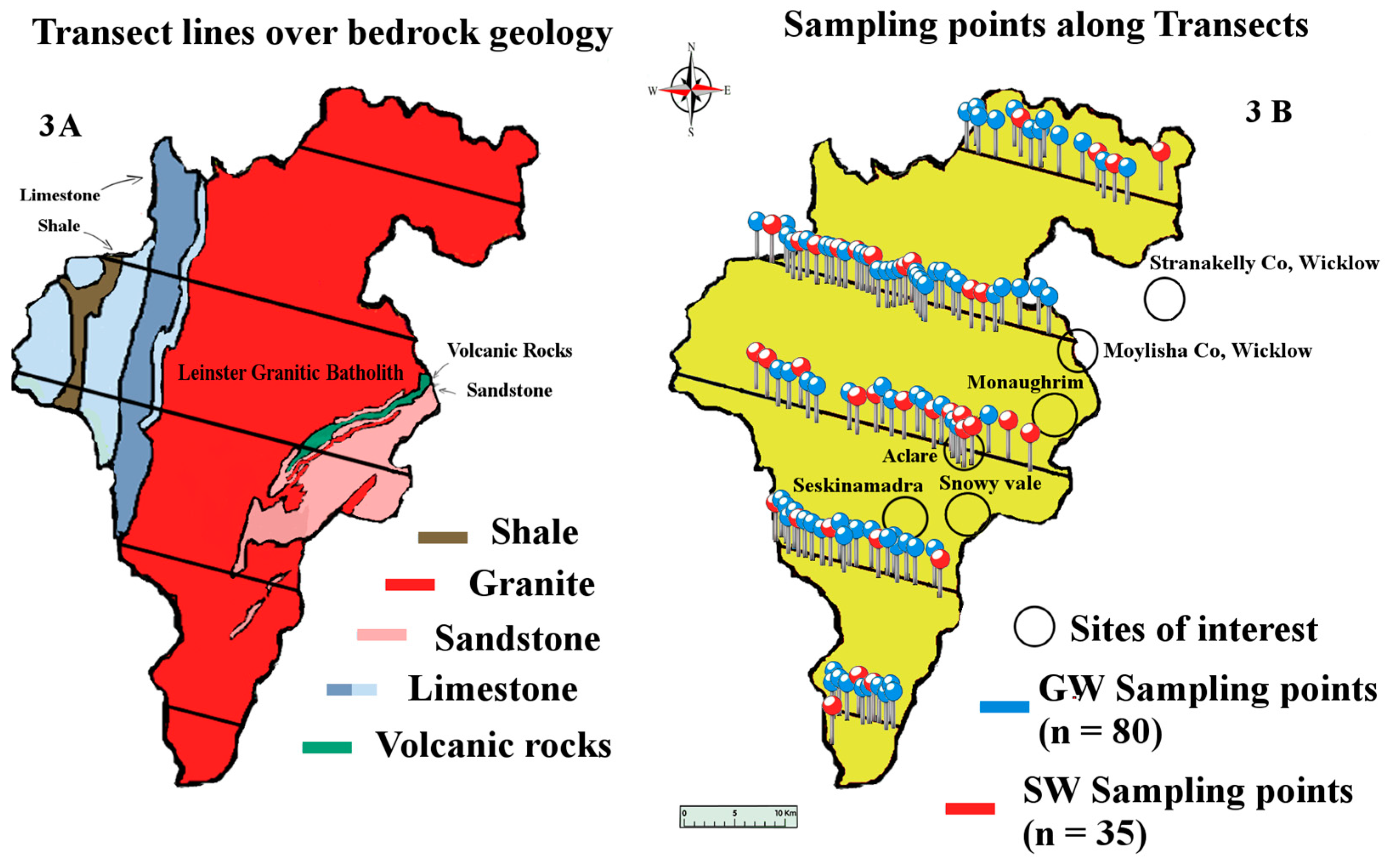
The geology of the South East of Ireland is dominated by the Caledonian Leinster Batholith: a massive felsic intrusion of granite which occurred during the Caledonian orogeny about 419.2 ± 3.2 Mya. It is within this batholith that the lithium-rich pegmatites are found. First discovered in the late 1970s, the lithium deposit at that time was deemed to be of low economic value. Today however, the growth of the LIB market means that mining this once marginal deposit of lithium is a distinct possibility. These spodumene-bearing pegmatites represent one of the largest potential resources of lithium in Western Europe [
31]. There are six known sites along the batholith associated with lithium bearing pegmatites: Aclare, Snowy Vale, Seskinnamadra, Stranakelly, Monaughrim and Moylisha. The largest discovered pegmatite deposit is at Aclare House, Myshall, County Carlow. There are no natural outcrops of the pegmatite deposit at Aclare; its presence was confirmed by drilling and the existence of pegmatite boulders in the area. Pegmatite deposits are extracted in one of two ways: either by open pit mining or underground mining, both of which may place a large load on the environment, i.e., mine tailings, the disposal and treatment of which is an important environmental issue for most mining projects. These tailings contain elements and compounds which are not naturally exposed to the ecological system and therefore have the potential to adversely affect the surrounding environment. The levels of lithium found in the waters of such tailings ponds have been measured at levels approaching 13 mg/L [
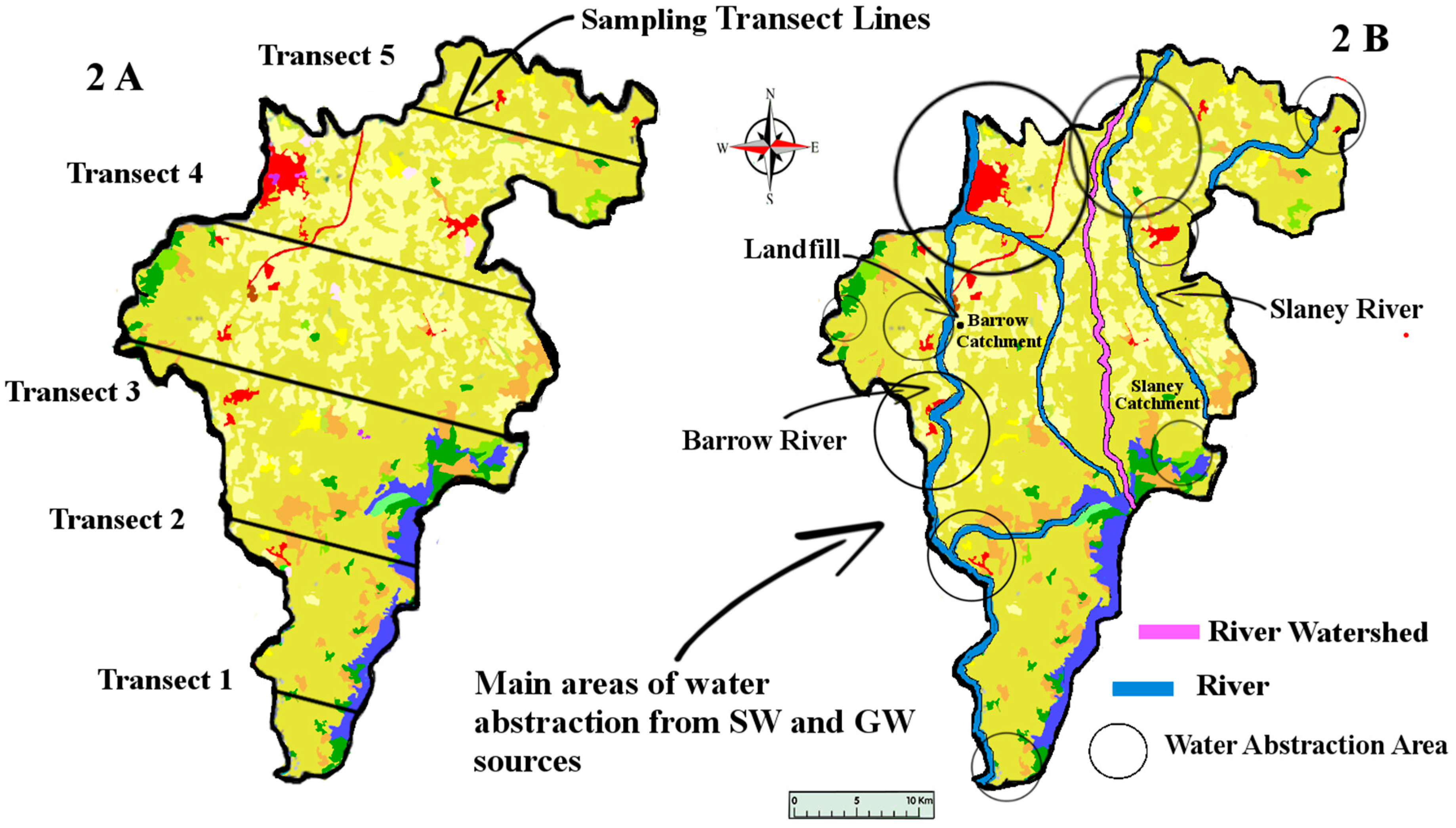
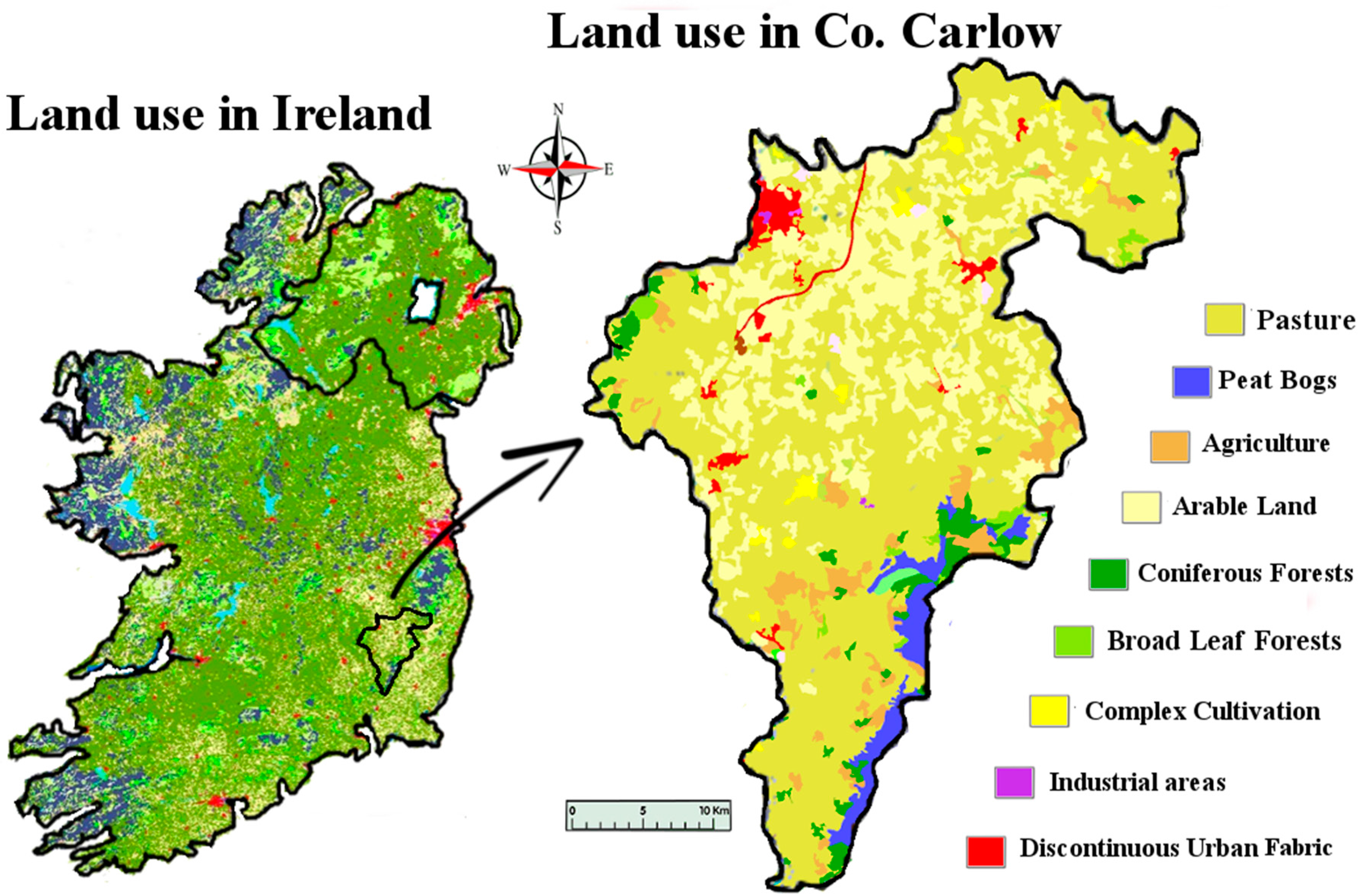
11]. The post-disturbance pathway that these exposed metals take is likely to involve the natural waters of the locality. As well as being found in natural waters, metals originating from mining activities have been found in soil and plants. Farming is the dominant land use and industry in the study area (
Figure 1). Carlow’s economy relies heavily on its agronomic sector, so any potential intrusion of lithium to the land is an important issue for the population. Lithium is readily absorbed by plants, and high levels of lithium have been shown to have harmful effects on plants. In some plants, lithium has been shown to stimulate growth. It has also been shown to cause growth depression in some citrus plants, causing drastic reductions in crop yields. Information on lithium hazard to other economic plants is limited [
13,
14,
15,
16,
17,
20,
28].
It is important to understand the possible environmental impacts of extracting lithium in Ireland. The pegmatite deposits in Ireland exist as elongated tabular dykes. Mining of these pegmatites will probably take the form of open pit mining—one of the most common forms of strategic mineral mining. A good example of hard rock open pit pegmatite mining is the Talison Greenbushes and Wodgina mines in Western Australia which are the world’s leading producer of lithium from hard rock deposits. Open pit mining requires that pegmatite ores which have lain unexposed since deposition are brought to the surface for treatment. The ores are crushed and milled and then separated. The crushing creates dust which can contain radioactive elements, asbestos-like minerals and metallic particles which, absorbed into lung tissue, can cause problems such as pneumoconiosis and silicosis. Prolonged exposure to lithium dust can cause fluid to build up in the lungs, leading to pulmonary oedema. The separation process produces tailings (pulverized rock and liquids) from which toxic elements can leach into the bedrock and nearby water sources if not stored safely. The use of enormous amounts of water in the lithium extraction process is also an issue both for hard rock mining and brine deposits. Lithium brine mining operations in South America have seen several “water conflicts” arise due to the large amounts of water required by the lithium extraction industry [
19]. The extraction of lithium in Ireland could potentially have harmful effects on the local environment through leaching, spills or air emissions. Any mining operation inevitably comes with a lengthy list of environmental concerns and potential issues. Public opinion and activism such as resistance to mining play an important role in deciding whether a deposit is recoverable. It is possible that public opposition may hinder any lithium mining operation in Ireland [
34]. There is a need to measure baseline levels of lithium in the natural waters of the Carlow area prior to any mining operation. The purpose of this research was to establish those baseline levels of dissolved lithium and other metals in the natural waters of Carlow, namely the ground and surface water of the area; the rationale being that if mining were to take place in the region, the data produced here could be used as a benchmark to determine whether disturbed lithium, and by extension other metals we have analyzed, have been leached into the South East’s natural waters.
2. Materials and Methods
2.1. Sampling
Natural waters refer to both surface (SW) and groundwater (GW). This study aimed to sample county Carlow’s GW and SW. Carlow’s land area covers approximately 897 km
2. GW and SW samples were taken along five transect sampling lines (
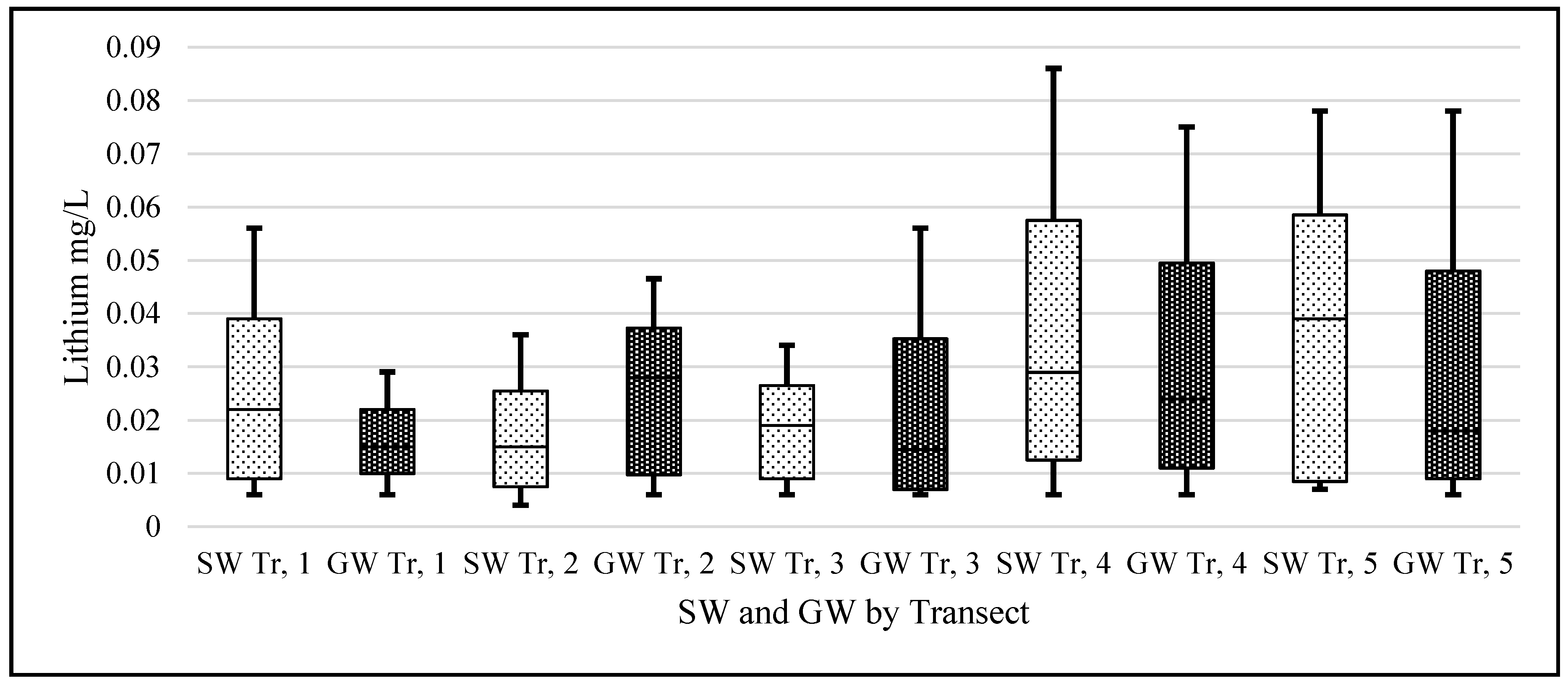
Figure 2) crossing Carlow from East to West. These specific transect lines did not represent any significant geological features but were rather a numerical split of the area taking into account existing road access. This sampling method was chosen to sample the whole county in a cost-efficient way. This method also allowed us to address the question: has the lithium from the pegmatites located in the East/Central part of the county permeated towards the river Barrow in the West of the county.
The study was conducted over a period of seven months during 2015, from March to September. Water samples were collected every two months (four sampling events) from a total of 115 sampling sites, 80 of which were GW and 35 SW, giving a total of 460 water samples (
Figure 3). GW samples were taken directly from household taps that were connected to private boreholes. SW samples were taken from streams and rivers along and adjacent to transect sampling lines. Water samples were collected in acid washed High Density Polyethylene (HDPE) sampling bottles. All bottles were acid washed with dilute HNO
3, then filled with the dilute acid and allowed to sit for approximately one day. Bottles were then rinsed several times with deionized water and placed in sealable bags to prevent any contamination. Parts per billion measurements require rigorous cleaning and sampling methods; because of this, only ultra-pure analytical grade acids were used in this study. Samples collected in the field were filled to the brim leaving very little air space at the top of the bottle. Physico-chemical measurements (pH, Temperature and Conductivity) were taken in the field prior to filtering or acidification. Samples were filtered within 24 h of collection and then acidified. The samples were acidified to arrest any biological activity; dissolve any precipitates present; and discourage the adsorption of lithium onto the walls of the bottles. Trace metals are particularly prone to adsorption. Samples were stored at 4 °C ± 1 °C for the duration of the study.
2.2. Surface Water Sampling
SW samples (n = 140) were taken from rivers and streams where they intersected the sampling transect lines. Sample bottles were rinsed three times with the water being sampled. To avoid non-representative samples caused by surface films and the entrainment of river bottom deposits, a grab sampling method was used. Samples were taken from the center of rivers below the surface of the water while the sampler stood downstream of the sampling point. In some cases, (i.e., shallow streams), several small samples were amalgamated into a sufficiently large sample from which a final sample was taken.
2.3. Ground Water Sampling
GW samples (n = 320) were collected directly from home owners’ plumbing systems (i.e., domestic taps). Only sites which had their own GW well system installed were selected for the study. Care was also taken to avoid any water softening system or other treatment between the GW source and the tap. Prior to sampling from taps, the water was left to flow for two minutes before a sample was taken. When collecting GW samples, the container was rinsed three times with the water to be sampled before taking the final sample. Samples were also taken as close as possible to the source of the supply to minimize any potential influence from the plumbing system.
2.4. Sample Preparation
All water samples and blanks underwent the same preparatory procedures. Firstly, samples were concentrated by a factor of 10 by evaporation (200 mL samples were concentrated down to 20 mL before being analyzed). This ensured that the naturally low lithium levels in our samples would be measured at an order of magnitude greater than they appear naturally and therefore be more easily detected by our instrumentation. Samples were then filtered, using a slow filtration rate filter paper with particle retention at 2–3 µm to remove coarse and gelatinous precipitates from the solution. Further to this, samples were filtered using 0.45 µm pore size cellulose-based membrane syringe filters. These filters trap particles both on the surface of the filter and within the filter; consequently, the retention of small particles increased as the filter became more loaded. Samples were filtered avoiding excessive pressure. Algal cells are known to concentrate trace metals, so rupture of an algal cell could cause inaccurately high results; rupture may also introduce natural chelating agents into the water.
2.5. Reagents
Lithium Standard for AAS (certified reference material), TraceCERT®, 1000 mg/L Li in HNO3 was used to prepare all working standards. Potassium solution as an ionization suppressant, at 2000 mg/L was made up in double deionized water from Potassium hydroxide pellets for analysis (ACS,ISO,Reag. Ph Eur), EMSURE®, and acidified using HNO3. All working solutions and working standards were acidified using nitric acid (HNO3), ≥69.0%, for analysis EMSURE® (ACS,Reag. Ph Eur) to the same percentage as collected samples (i.e., 1%). Glassware was thoroughly soaked in dilute HNO3 and rinsed several times with deionized water before use. No other metals in the analysis required an ionization suppressant. All reagents used were commercially available from Sigma Aldrich Ireland Ltd. Vale Road, Arklow, Wicklow, Ireland.
2.6. Analysis
All water samples were analyzed for lithium, as well as Cd, Cu, Fe, Mn, Ni, Pb and Zn using an Agilent (Tech 200 AA series) flame atomic absorption spectrometer (AAS) in an air-acetylene flame [
35]. K and Na were analyzed using a Flame Emission Spectrophotometer (FES) Sherwood Model 410 Flame photometer. These instruments provided sufficient sensitivity, short analysis time and level of accuracy required for the study. Limit of Detection (LOD) for FES analysis was 0.13 mg/L while limit of quantification (LOQ) was 0.44 mg/L. LOD for all AAS analyses was 0.005 mg/L while LOQ was placed at 0.018 mg/L. The working range for lithium was 0 to 2 mg/L; working ranges were between 0 to 10 mg/L for all other metals. Samples were concentrated by a factor of 10 prior to any instrumental analysis, therefore a concentration factor of 10 was applied to all data before reporting the result. Processed samples were drawn at random before being read on the AAS to minimize procedural bias. After every 50 samples, the instrument was recalibrated using blank samples and working standards. Typical readings obtained from blank samples were 0.0001 to 0.0003 mg/L of lithium. Speciation of the lithium and the other metals analyzed in the water was not a factor in this study; only total dissolved metal content was measured.
2.7. Interferences
Alkali metals are very susceptible to ionization. In an air-acetylene flame, lithium ionization is appreciable. To control this ionization, all solutions were made to contain 2000 mg/L of the easily ionized potassium cation (ionization potential of 4.3 eV vs. 5.39 eV for lithium). At 2000 mg/L, minor changes in the potassium concentration had little or no effect on lithium’s absorbance readings.
2.8. Data Analysis
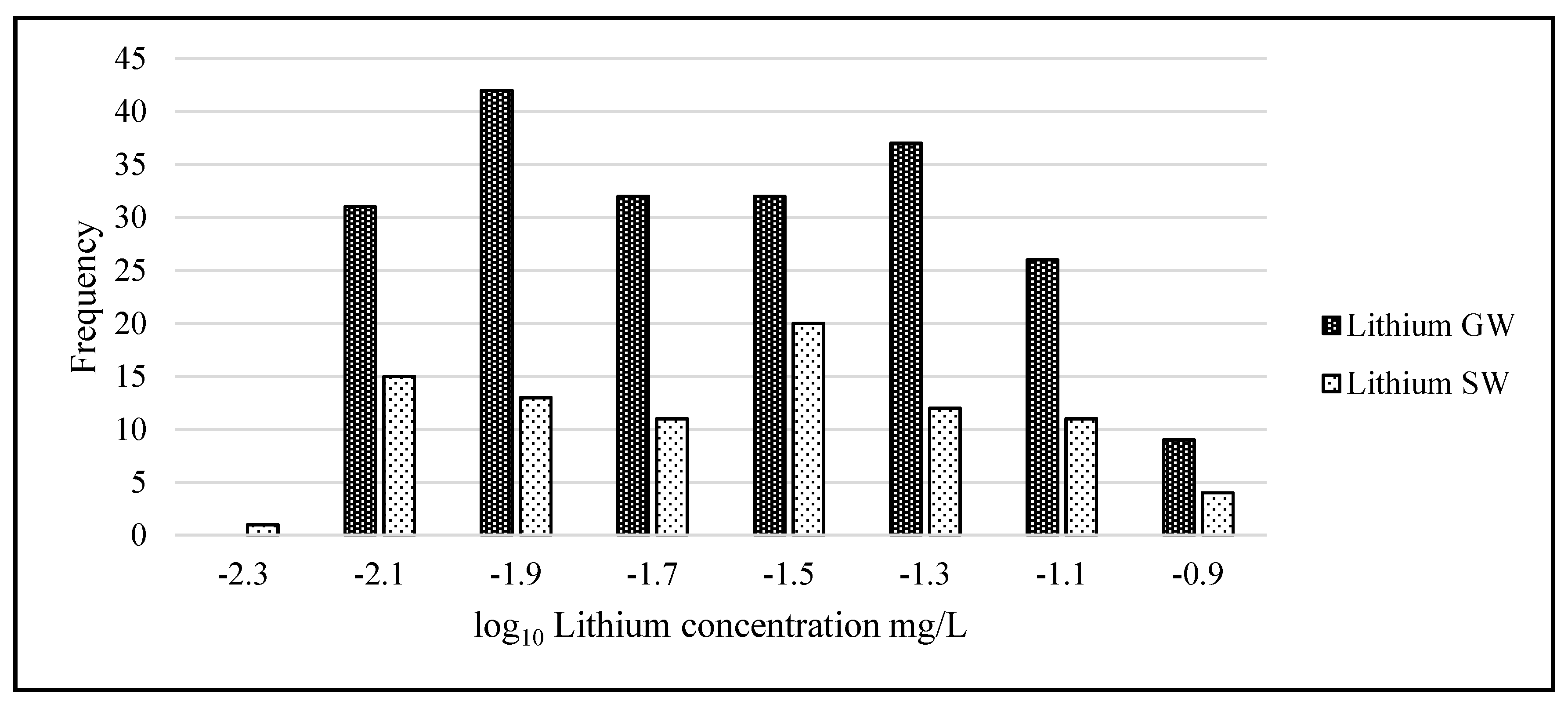
Statistical analysis was carried out using IBM’s statistical package SPSS (Statistical Package for the Social Sciences, version 23.0, IBM Corp, Armonk, NY, USA) and Microsoft Excel 2016. The lithium data were not normally distributed and when graphed showed considerable left skewness (GW skew = 3.89, kurtosis = 18.36; SW skew = 3.25, kurtosis = 12.11). To adjust the data to normality and allow the use of parametric tests, all data underwent a logarithmic transformation (
Figure 4). When comparing lithium means among transects and geographical areas, a one-way ANOVA was used. Where significant differences were found, a post-hoc
t-test was used to identify significant differences between sample means. A
p-value of 0.05 was considered significant. Bonferroni corrections were used as appropriate.
4. Discussion
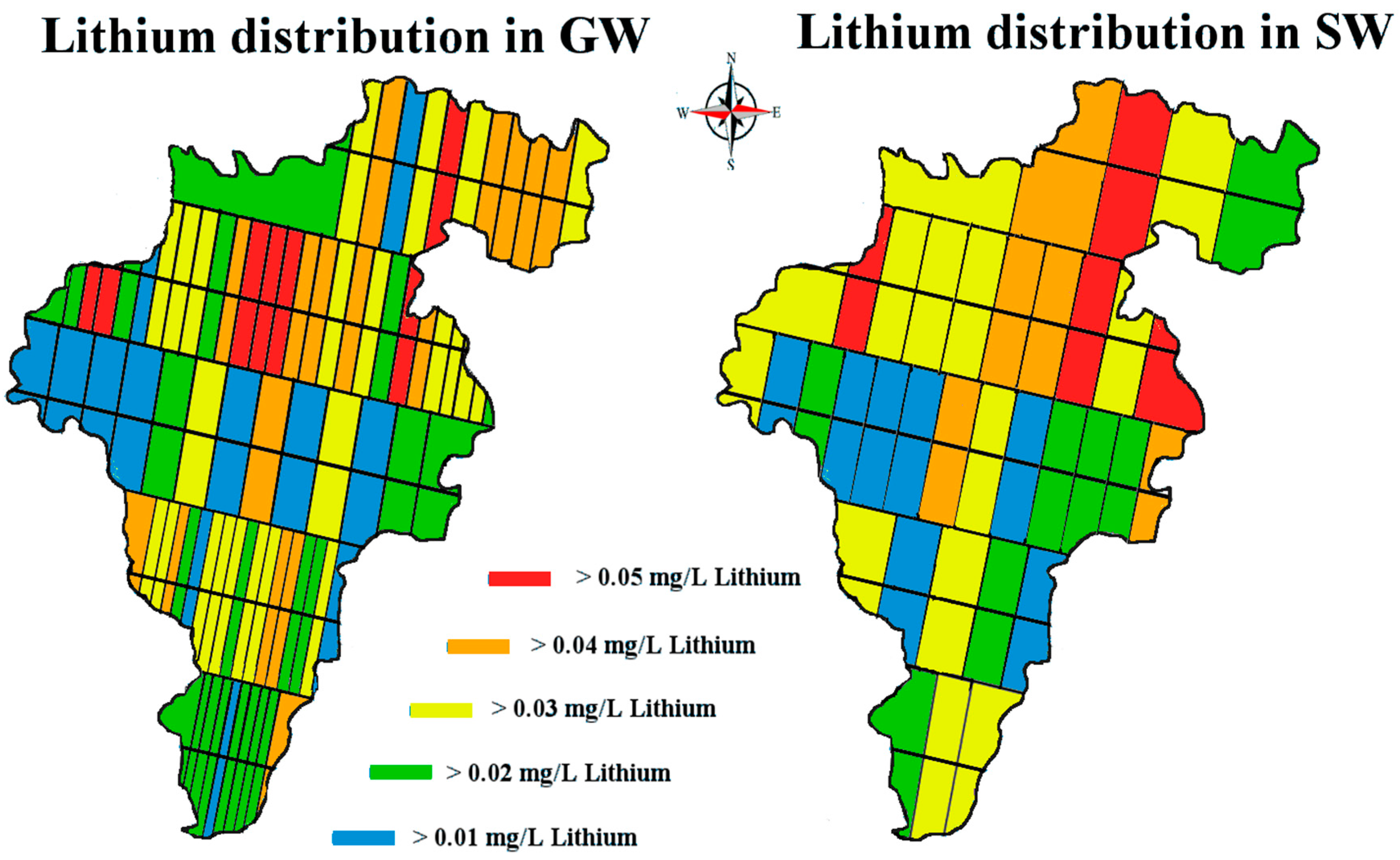
The mean values obtained for lithium levels in county Carlow’s SW at
= 0.02, SD = 0.02 mg/L and GW at
= 0.02, SD = 0.02 mg/L are compatible with published figures for lithium concentrations in drinking waters; for example, drinking water in Texas 0.003 to 0.16 mg/L; in Oita, Japan 0.0007 to 0.059 mg/L; in England 0.001 to 0.021 mg/L; and in Portugal 0.001 to 0.19 mg/L [
17,
18,
19,
20]. It is worth noting that these figures are associated with studies investigating an association between lithium levels in drinking water and suicide rates in the local population. Some of these studies also claim a positive correlation among lithium levels, longevity and general mental health. The theory is that lithium in trace amounts enhances the connectivity among neurons and exposure over a lifetime enhances happiness [
41]. However, data for Ireland, let alone Carlow, in relation to suicide are not currently available at sufficient geographical granularity to allow an investigation of potential associations between suicide rates and lithium levels in drinking water.
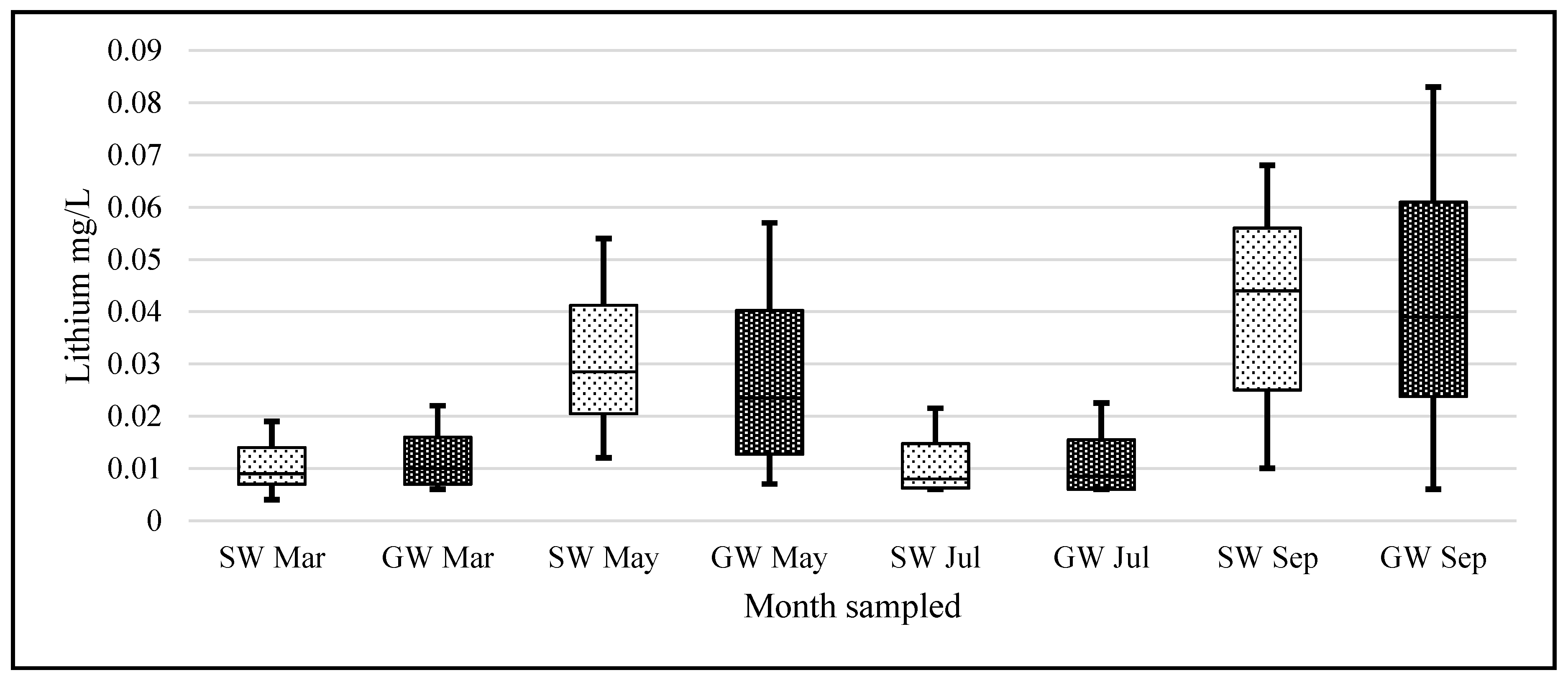
Lithium’s small ionic radius (0.68 Å) means that it has a high ionic potential and therefore has a strong affinity for water molecules. If the lithium from the known pegmatite sources is being slowly eroded and making its way down into the Barrow valley, we should have measured an elevated amount of lithium in our samples. Our data suggest that the lithium levels, although quite variable in the natural waters of Carlow, are within the range of normal ‘background’ levels reported in the literature and not elevated. The passage of water through the pegmatites is low. They are situated at an altitude above the level of the local water table and are unsaturated. The pegmatites also occur within a poor aquifer. The low concentrations found appear to be consistent with limited dissolution of the pegmatites.
When a one-sample t-test assuming unequal variances was carried out on our data—the null hypothesis being that the population mean was 0.010 mg/L; the alternative hypothesis being that our samples are greater than this level—the following was observed, (t = 1.96, df = 459, p = 3.1 × 10−13) rejecting the Ho. This should serve to focus some critical attention away from mean/median values to the range of levels for ‘lithium in natural waters’ when the literature and our data suggest variation through 3 orders of magnitude (0.0007 to 0.19 mg/L).
Finding bedrock outcrops of Ireland’s granitic batholith is quite rare. This is especially the case with the South East’s pegmatites. Neither recent nor historic prospecting have uncovered any cases of exposed pegmatite-bearing bedrock in the area. Pegmatites are very similar in composition to granite in that they both are susceptible to weathering. However, spodumene LiAl(SiO
3)
2 is an aluminosilicate mineral, thus the leaching from its lattice structure is very slow. This fact, the lack of exposed bedrock outcrops in the area and the fact that the pegmatite deposits are unsaturated may account for the low levels of lithium in our samples. Some of the compounds that lithium forms in nature such as its fluoride, carbonate and phosphate compounds also have very low solubility in water [
42,
43,
44,
45].