Overexpression of SKP2 Inhibits the Radiation-Induced Bystander Effects of Esophageal Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid Construction and Small Interfering RNA Synthesis
2.2. Cell Culture and Transfection
2.3. Western Blot Analyses
2.4. Irradiation and Co-Culture
2.5. MN Assay
2.6. γ-H2AX Focus Formation Assay
2.7. Flow Cytometry
2.8. Statistical Analyses
3. Results
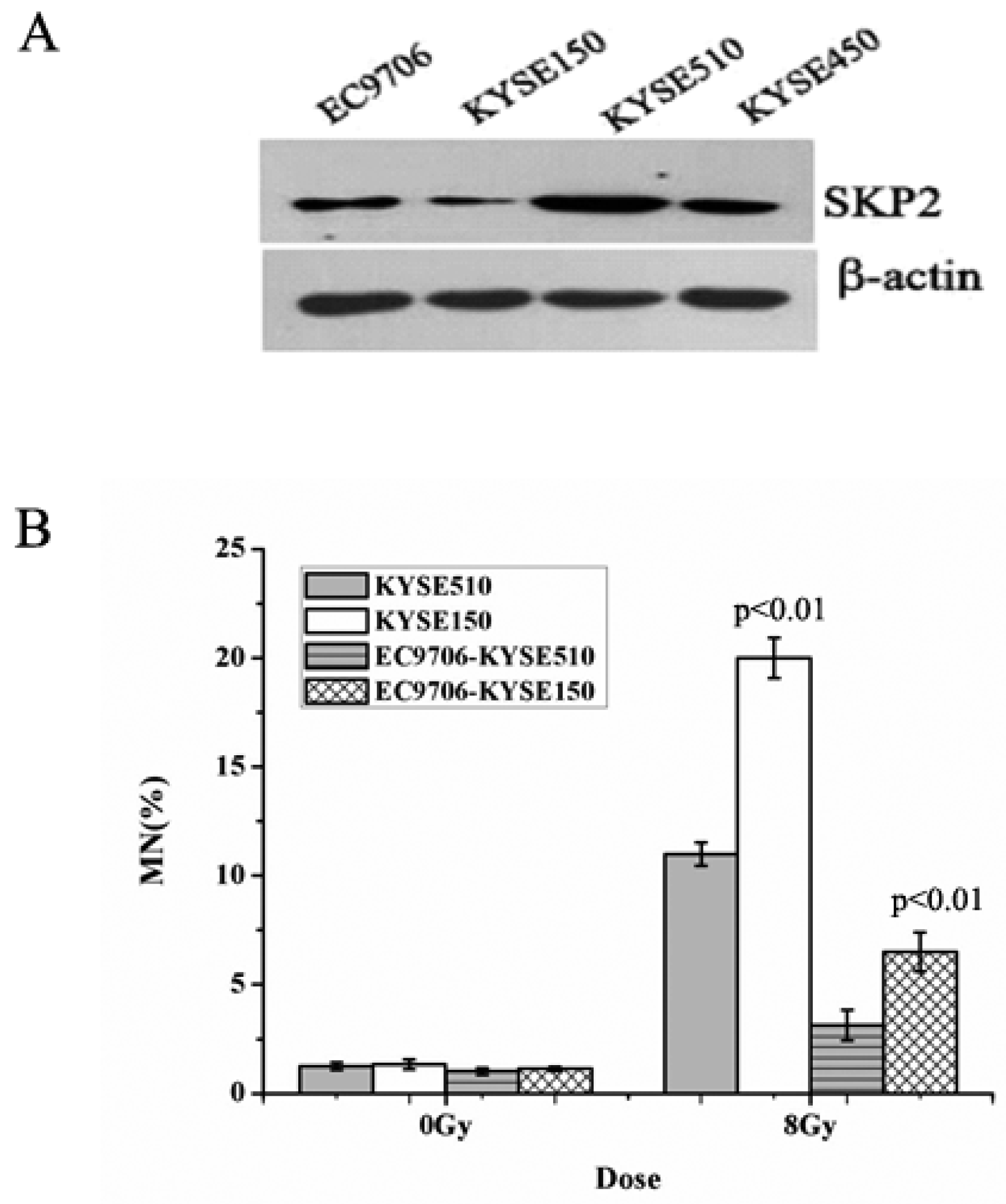
3.1. Endogenous SKP2 Protein Levels in Four EC Cell Lines
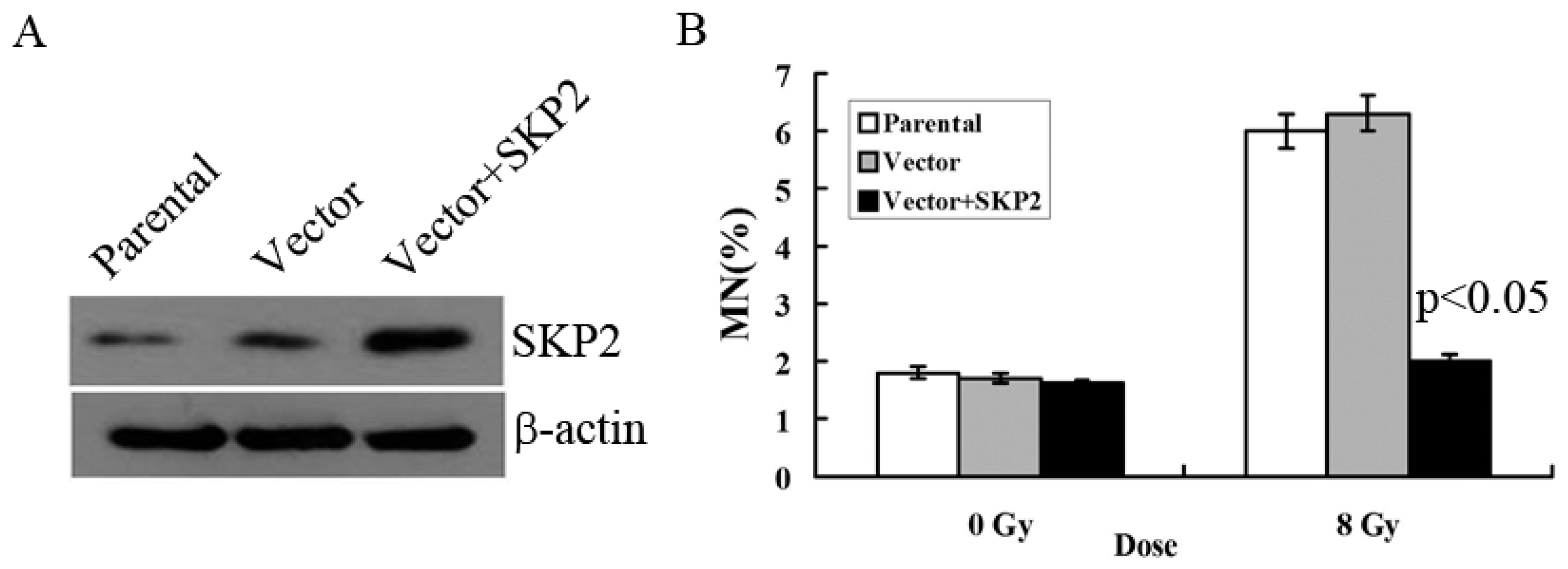
3.2. Relationship between RIBE and Overexpression of SKP2
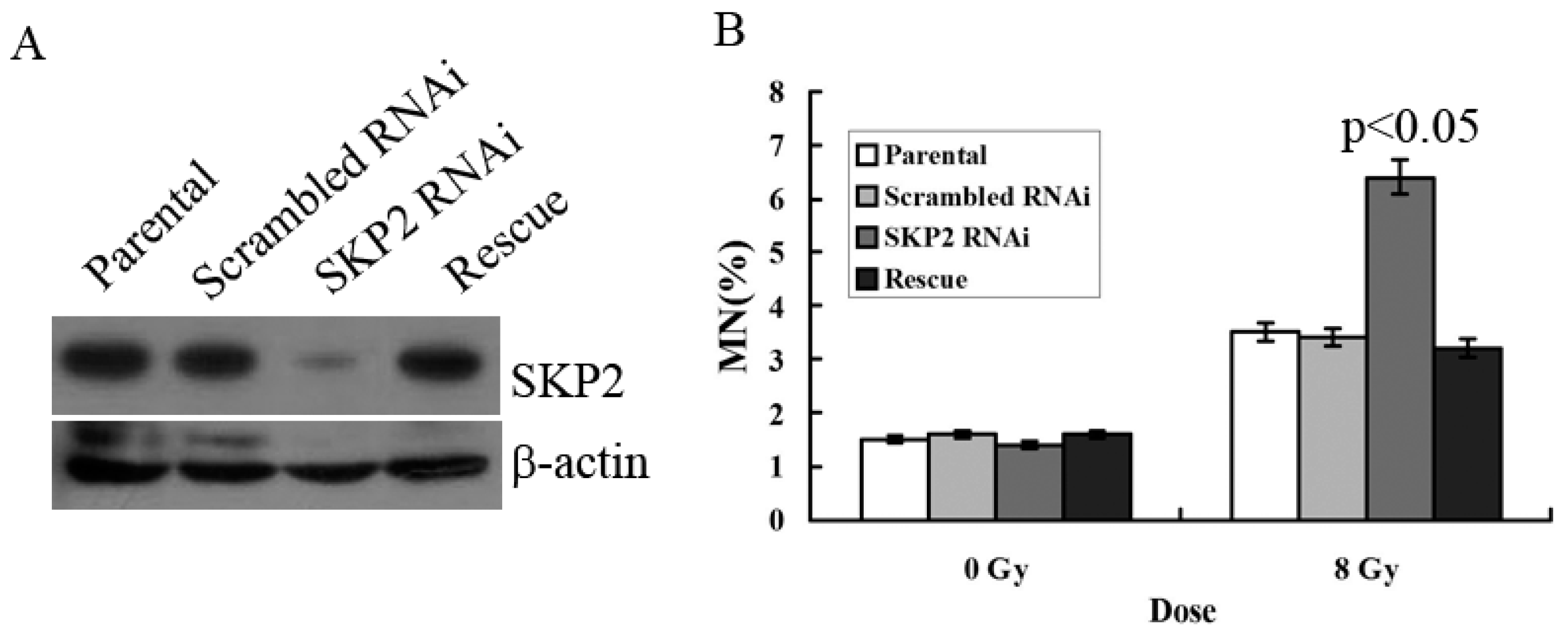
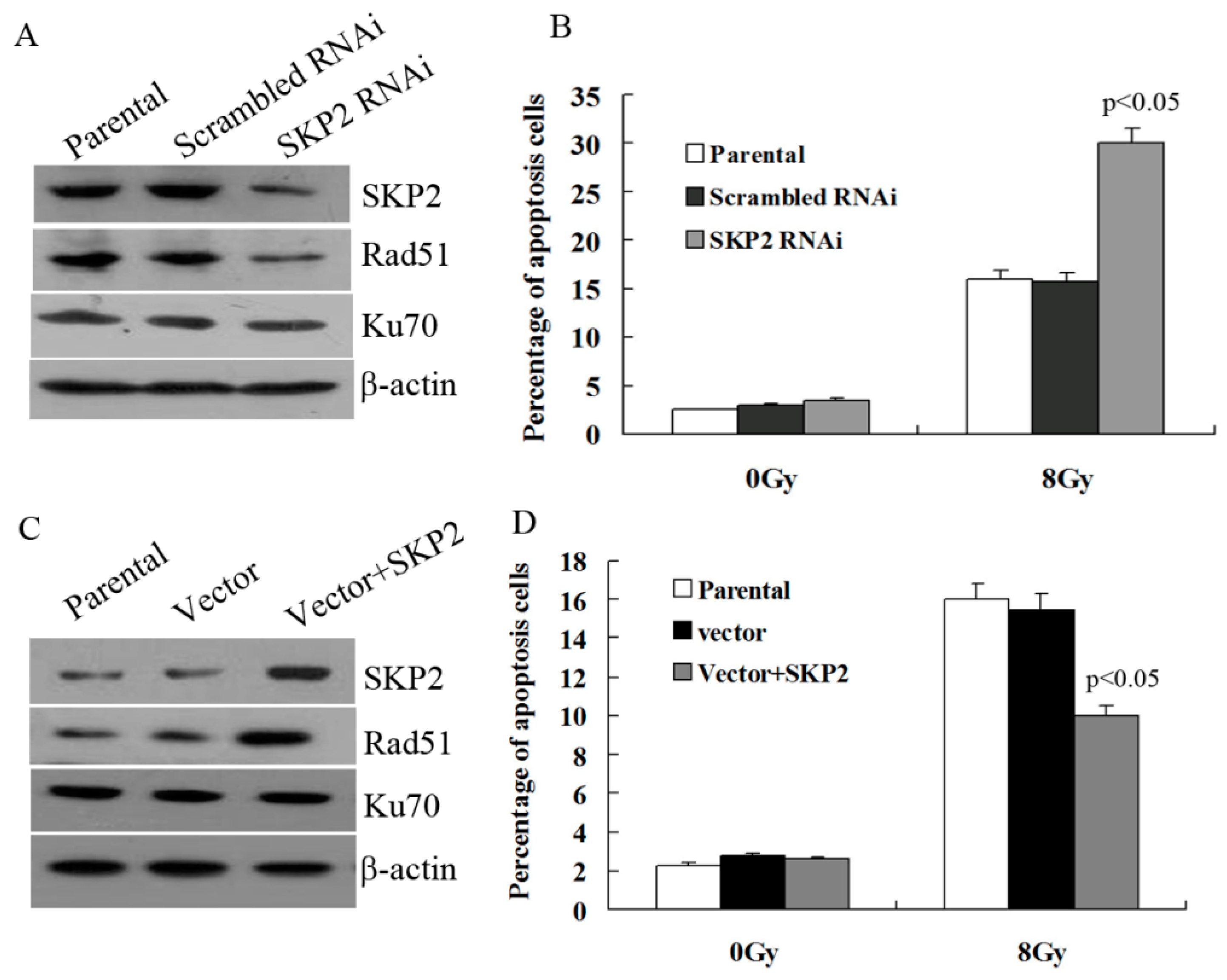
3.3. Relationship between RIBE and Decreased SKP2 Expression
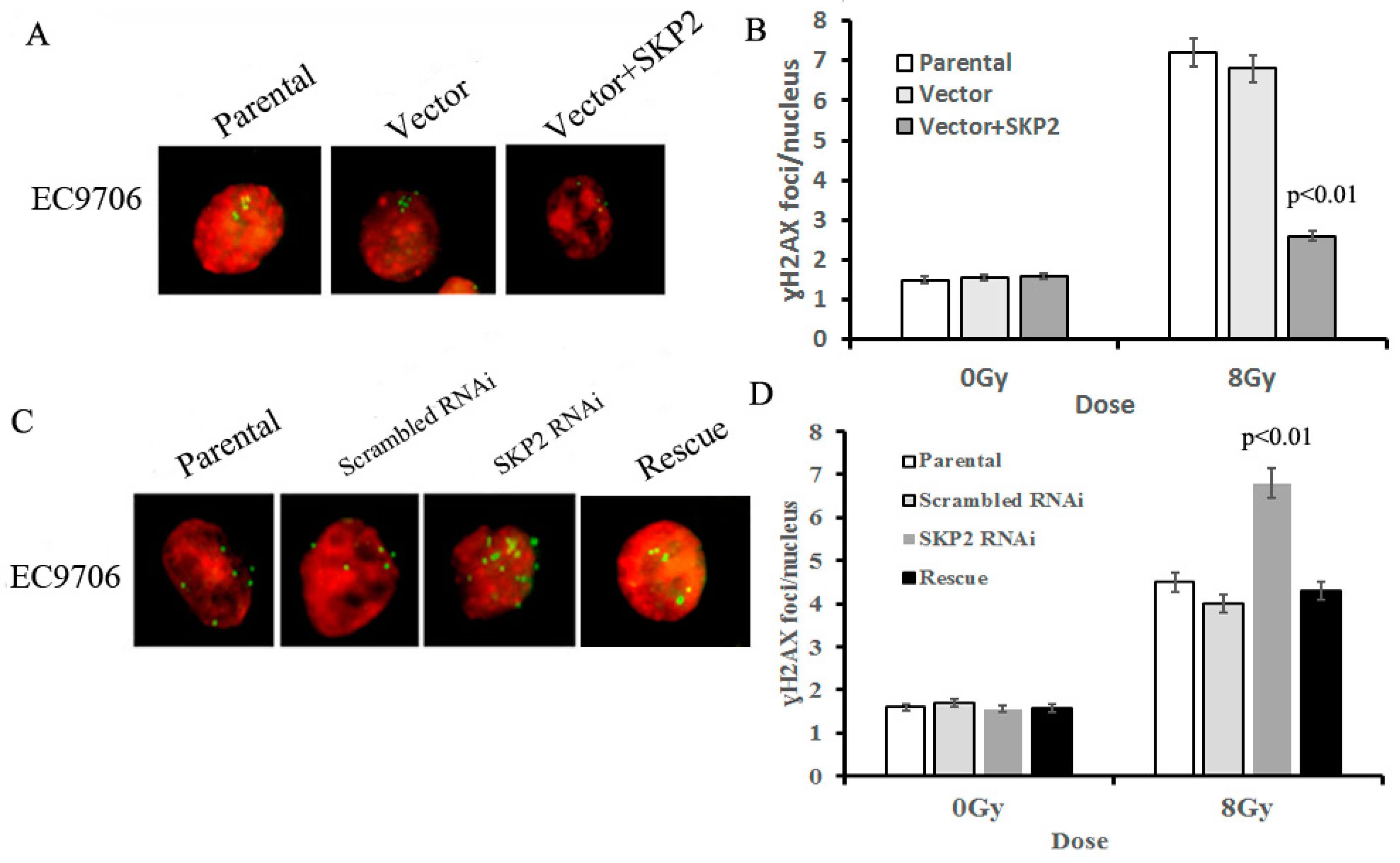
3.4. Effects of SKP2 Expression on the Repair of DSBs Induced by RIBE
3.5. Effects of SKP2 Expression on DSBs Repair Relative Proteins
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar] [PubMed]
- He, M.; Ye, S.; Ren, R.; Dong, C.; Xie, Y.; Yuan, D.; Shao, C. Cytochrome-c mediated a bystander response dependent on inducible nitric oxide synthase in irradiated hepatoma cells. Br. J. Cancer 2012, 106, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Temme, J.; Bauer, G. Low-dose gamma irradiation enhances superoxide anion production by nonirradiated cells through TGF-beta1-dependent bystander signaling. Radiat. Res. 2013, 179, 422–432. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhao, M.; Shen, B.; Prise, K.M.; Shao, C. Radiation-induced intercellular signaling mediated by cytochrome-c via a p53-dependent pathway in hepatoma cells. Oncogene 2011, 30, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Klokov, D.; Leskov, K.; Araki, S.; Zou, Y.; Goetz, E.M.; Luo, X.; Willson, D.; Boothman, D.A. Low dose IR-induced IGF-1-sCLU expression: A p53-repressed expression cascade that interferes with TGFbeta1 signaling to confer a pro-survival bystander effect. Oncogene 2013, 32, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Widel, M.; Przybuszewski, W.M.; Cieslar-Pobuda, A.; Saenko, Y.V.; Rzeszowska-Wolny, J. Bystander normal human fibroblasts reduce damage response in radiation targeted cancer cells through intercellular ROS level modulation. Mutat. Res. 2012, 731, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.K.; Stuzrt, J.; Chuang, Y.Y.; Little, J.B.; Yuan, Z.M. Low-dose radiation-induced senescent stromal fibroblasts render nearby breast cancer cells radioresistant. Radiat. Res. 2009, 172, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Wang, F.; Wei, G.; Wang, B.; Yang, J.Y.; Huang, Y.Z.; Zhang, L.; Zheng, F.; Guo, L.Y.; Wang, J.N.; et al. S-phase kinase-associated protein 2 knockdown blocks colorectal cancer growth via regulation of both p27 and p16 expression. Cancer Gene Ther. 2013, 20, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Jiang, X.; Liu, F.; Qiao, H.; Zhou, B.; Zhai, B.; Zhang, L.; Zhang, X.; Han, L.; Jiang, H.; et al. Downregulation of Skp2 inhibits the growth and metastasis of gastric cancer cells in vitro and in vivo. Tumour Biol. 2013, 34, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Wu, Y.P.; Ye, B.; Lin, D.C.; Feng, Y.B.; Zhang, Z.Q.; Xu, X.; Han, Y.L.; Cai, Y.; Dong, J.T.; et al. Suppression of anoikis by SKP2 amplification and overexpression promotes metastasis of esophageal squamous cell carcinoma. Mol. Cancer Res. 2009, 7, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Moro, L.; Arbini, A.A.; Marra, E.; Greco, M. Up-regulation of SKP2 after prostate cancer cell adhesion to basement membranes results in BRCA2 degradation and cell proliferation. J. Biol. Chem. 2006, 281, 22100–22107. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, X.L.; Huang, W.H.; Chen, C.F.; Bai, J.W.; Zhang, G.J. Cytoplasmic SKP2 expression is associated with p-Akt1 and predicts poor prognosis in human breast carcinomas. PLoS ONE 2012, 7, e52675. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Liu, Y.; Guo, J.W.; Liu, J.H.; Zuo, L.F. Relation of overexpression of S phase kinase-associated protein 2 with reduced expression of p27 and PTEN in human gastric carcinoma. World J. Gastroenterol. 2005, 11, 6716–6721. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Liu, W.; Doihara, H.; Wan, Y. Regulation of SKP2-p27 axis by the Cdh1/anaphase-promoting complex pathway in colorectal tumorigenesis. Am. J. Pathol. 2008, 173, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Tian, L.L.; Tian, J.; Jiang, X.Y. Overexpression of SKP2 promotes the radiation resistance of esophageal squamous cell carcinoma. Radiat. Res. 2012, 177, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, E.; Iliakis, G. Induction and repair of DNA double strand breaks: The increasing spectrum of non-homologous end joining pathways. Mutat. Res. 2011, 711, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Vispe, S.; Cazaux, C.; Lesca, C.; Defais, M. Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Res. 1998, 26, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- Galanos, P.; Vougas, K.; Walter, D.; Polyzos, A.; Maya-Mendoza, A.; Haagensen, E.J.; Kokkalis, A.; Roumelioti, F.M.; Gagos, S.; Tzetis, M.; et al. Chronic p53-independent p21 expression causes genomic instability by deregulating repication licensing. Nat. Cell Biol. 2016, 18, 777–789. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-C.; Zhang, T.-J.; Guo, Z.-J.; Xiao, C.-Y.; Ding, X.-W.; Fang, F.; Sheng, W.-T.; Shu, X.; Li, J. Overexpression of SKP2 Inhibits the Radiation-Induced Bystander Effects of Esophageal Carcinoma. Int. J. Environ. Res. Public Health 2017, 14, 155. https://doi.org/10.3390/ijerph14020155
Wang X-C, Zhang T-J, Guo Z-J, Xiao C-Y, Ding X-W, Fang F, Sheng W-T, Shu X, Li J. Overexpression of SKP2 Inhibits the Radiation-Induced Bystander Effects of Esophageal Carcinoma. International Journal of Environmental Research and Public Health. 2017; 14(2):155. https://doi.org/10.3390/ijerph14020155
Chicago/Turabian StyleWang, Xiao-Chun, Tie-Jun Zhang, Zi-Jian Guo, Chang-Yan Xiao, Xiao-Wen Ding, Fang Fang, Wen-Tao Sheng, Xu Shu, and Jue Li. 2017. "Overexpression of SKP2 Inhibits the Radiation-Induced Bystander Effects of Esophageal Carcinoma" International Journal of Environmental Research and Public Health 14, no. 2: 155. https://doi.org/10.3390/ijerph14020155




