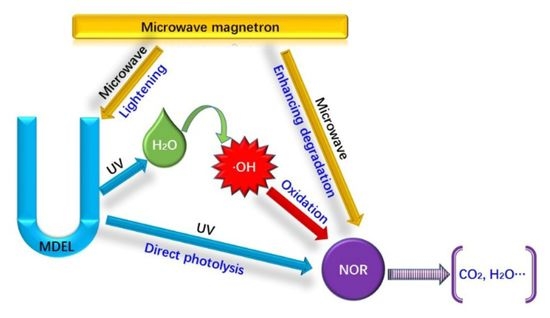
Microwave-Enhanced Photolysis of Norfloxacin: Kinetics, Matrix Effects, and Degradation Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Degradation of NOR in the MW/UV Process
2.3. Analytical Procedures
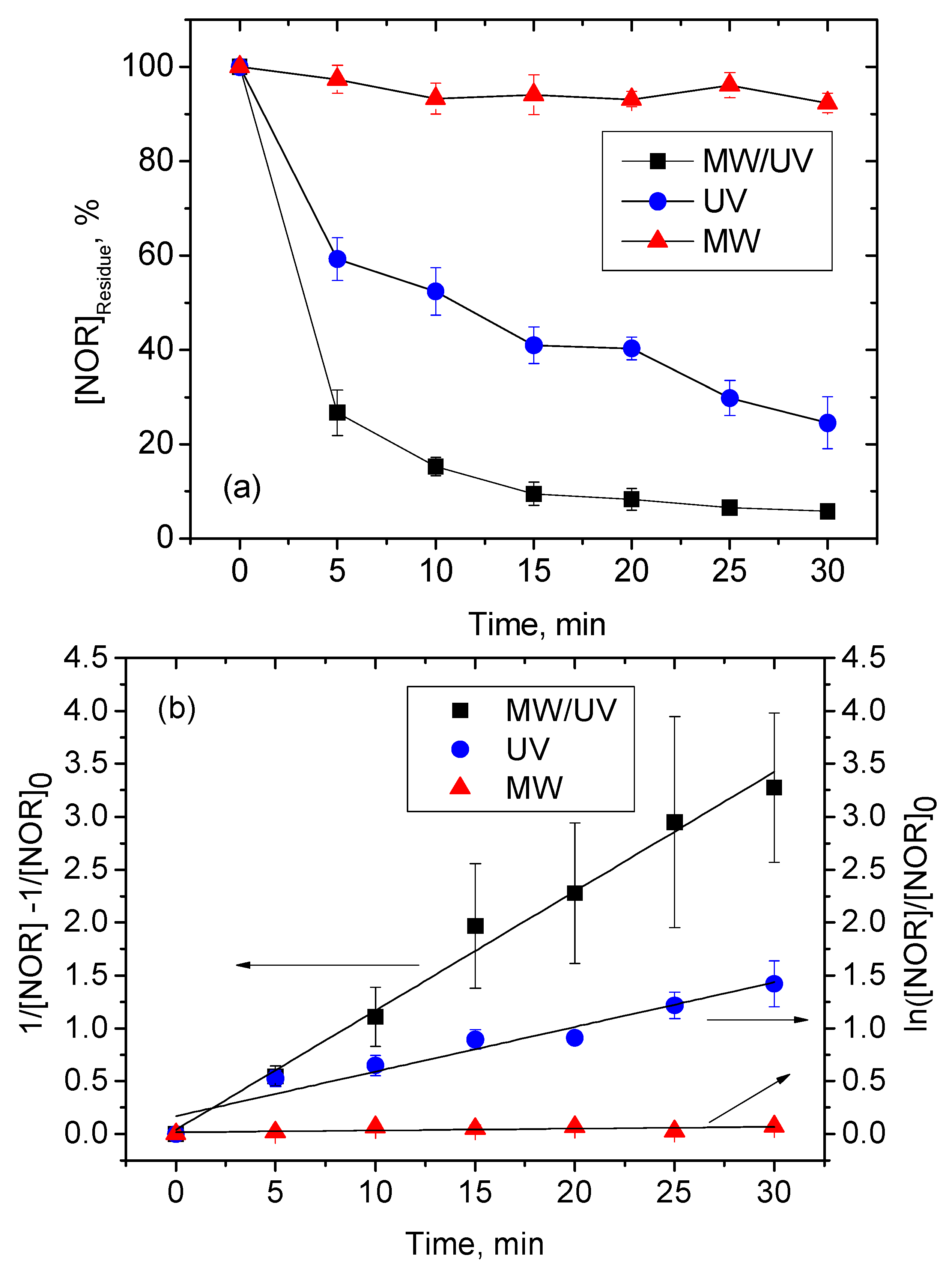
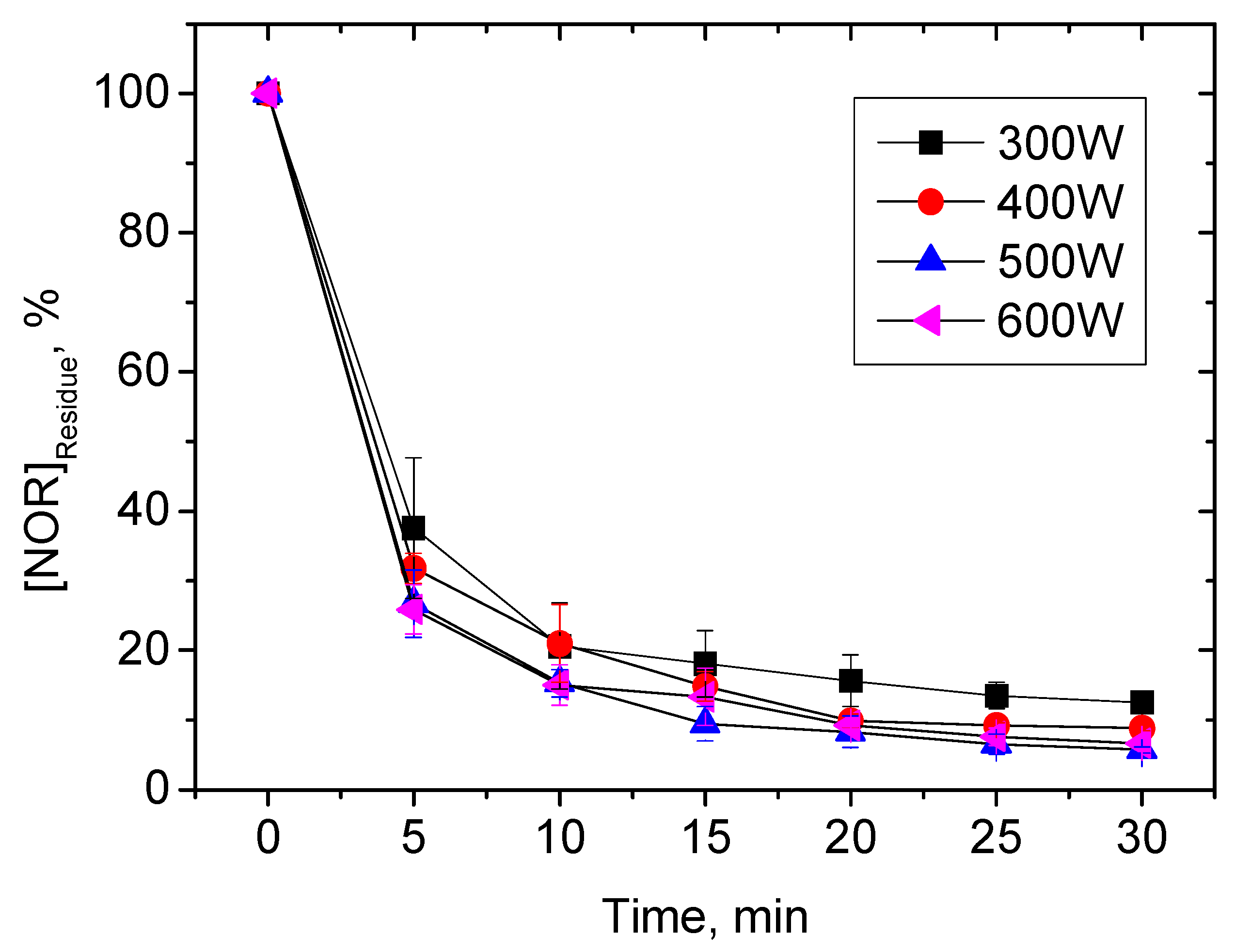
3. Results and Discussion
3.1. Kinetics
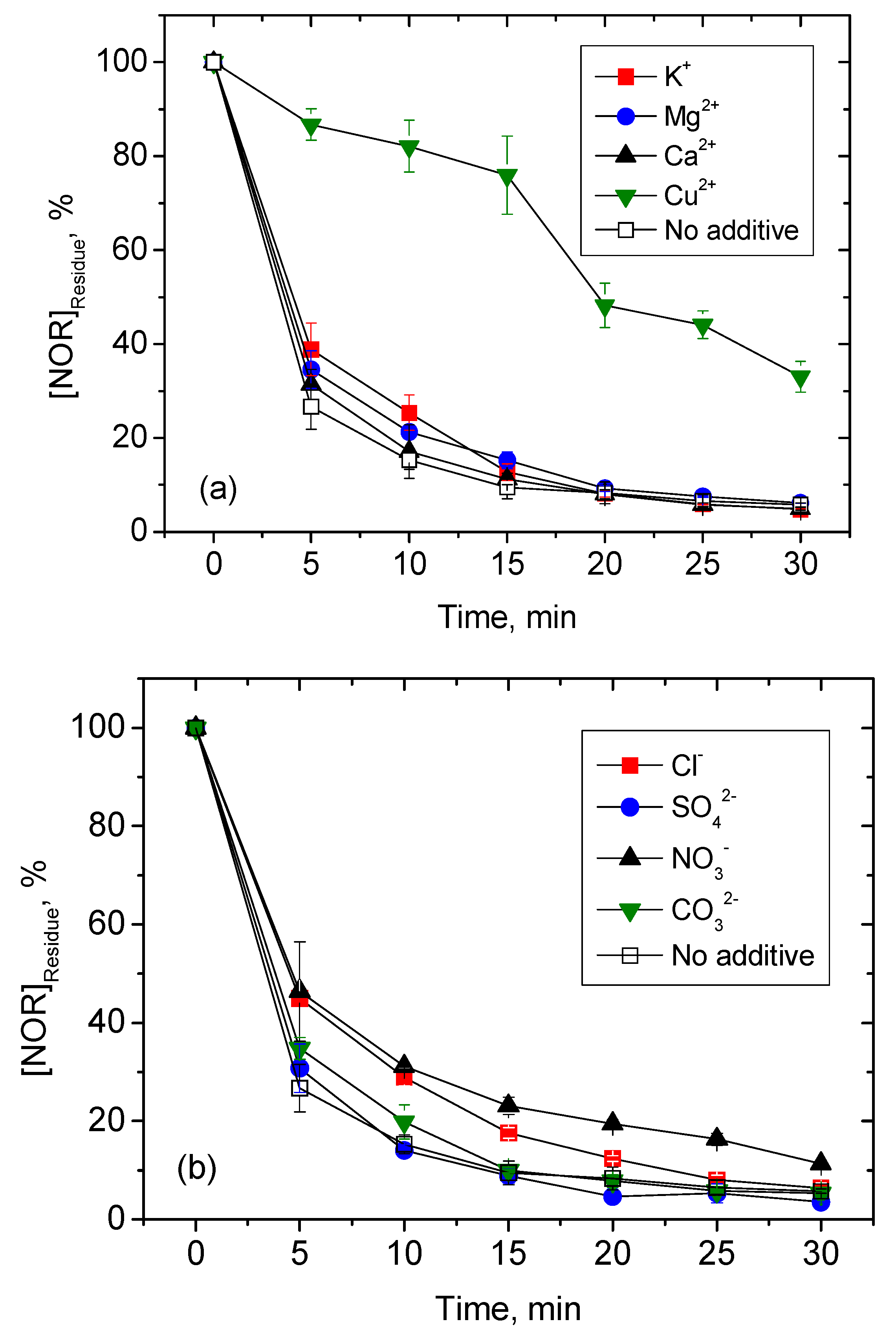
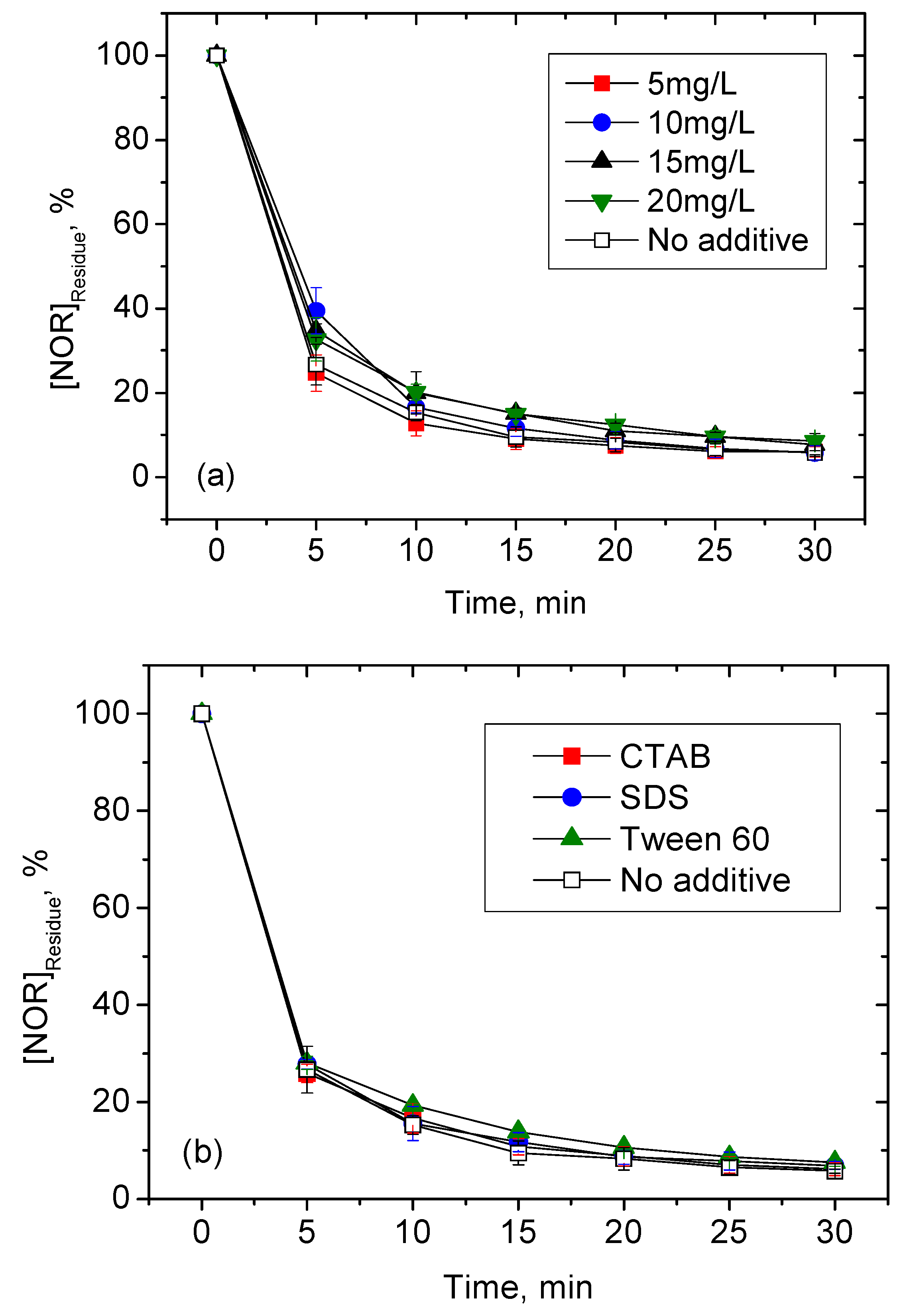
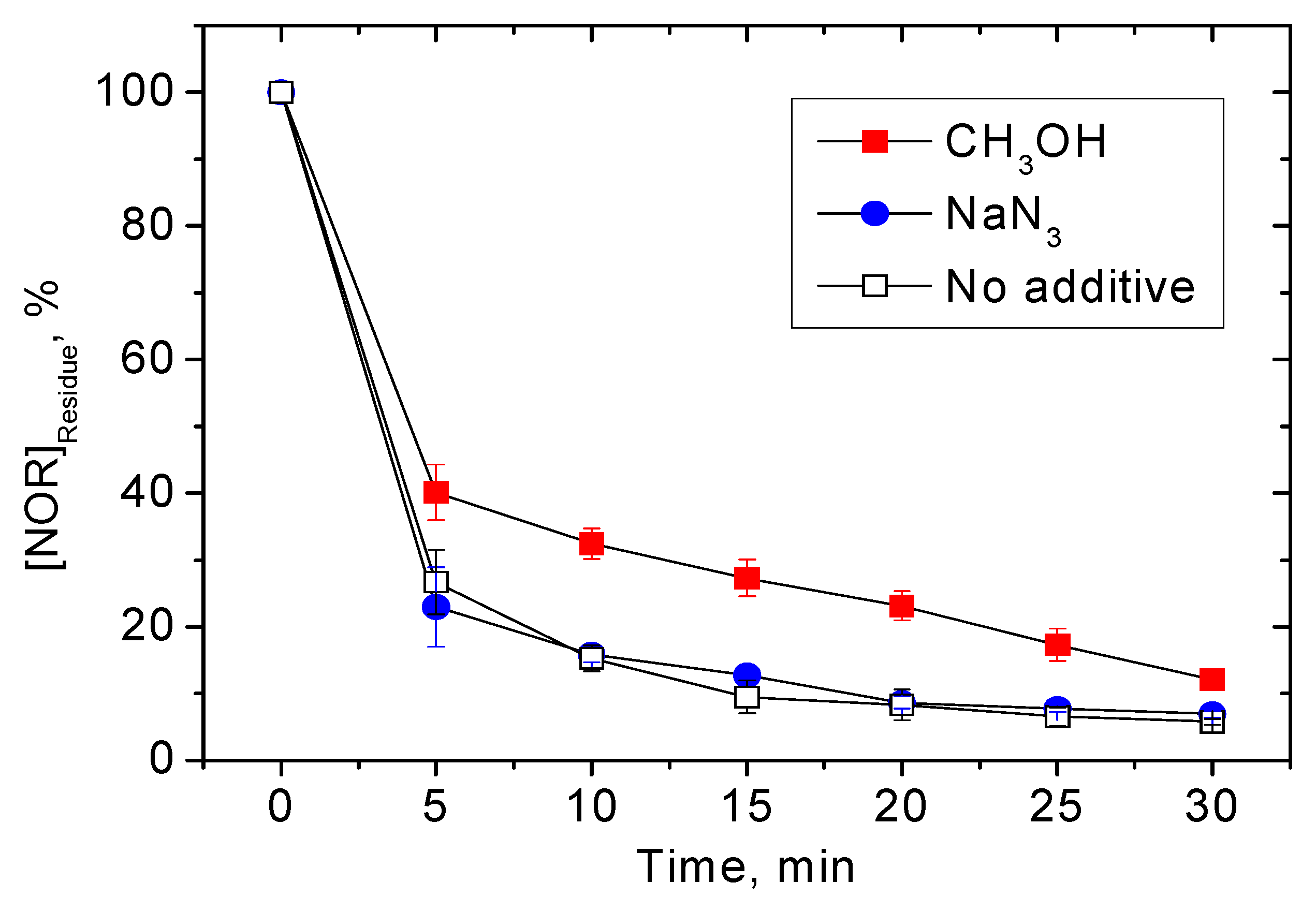
3.2. Effects of Different Water Constituents on NOR Degradation
3.2.1. Inorganic Ions
3.2.2. DOM and Surfactants
3.2.3. Real Water Samples
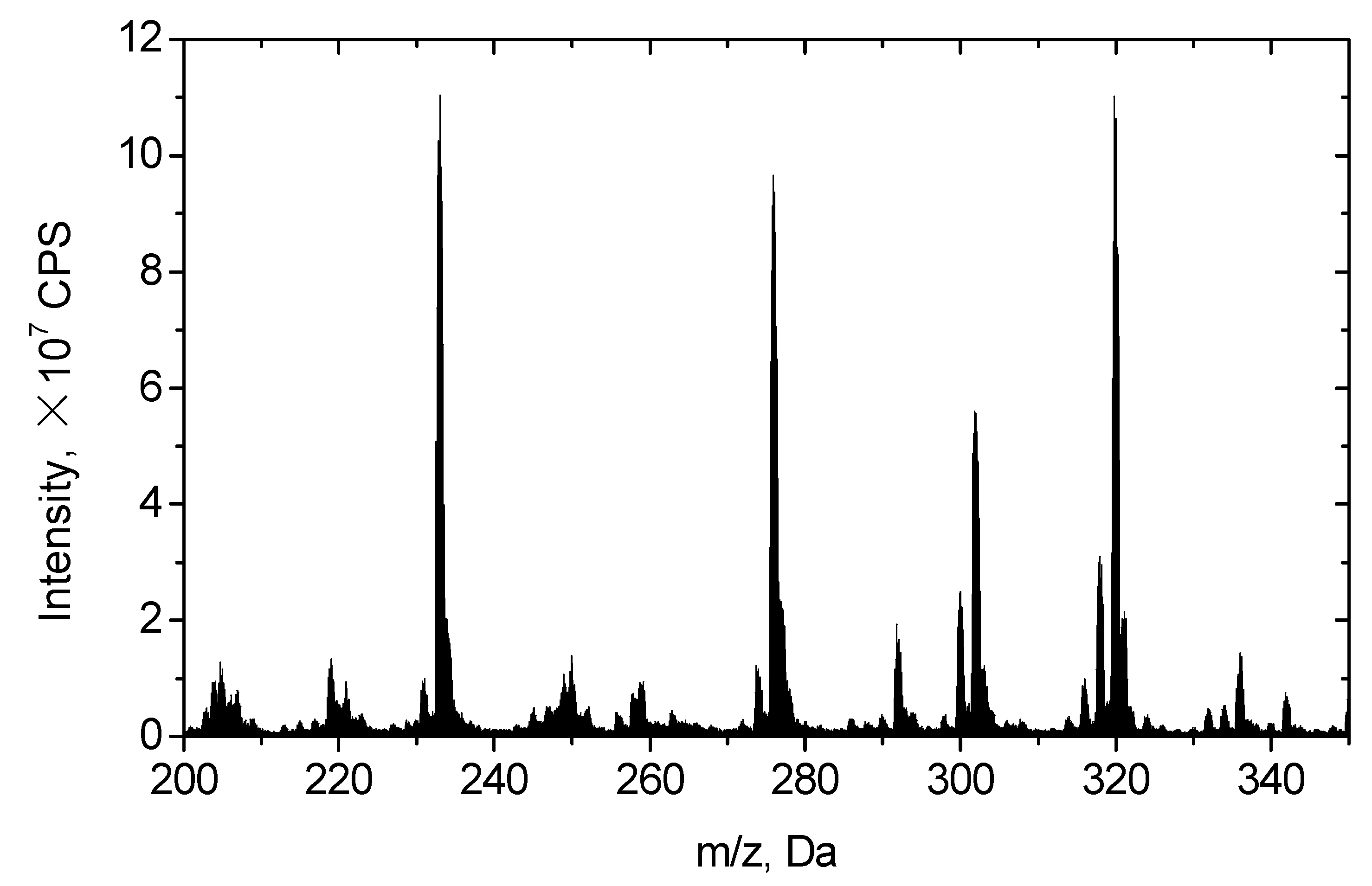
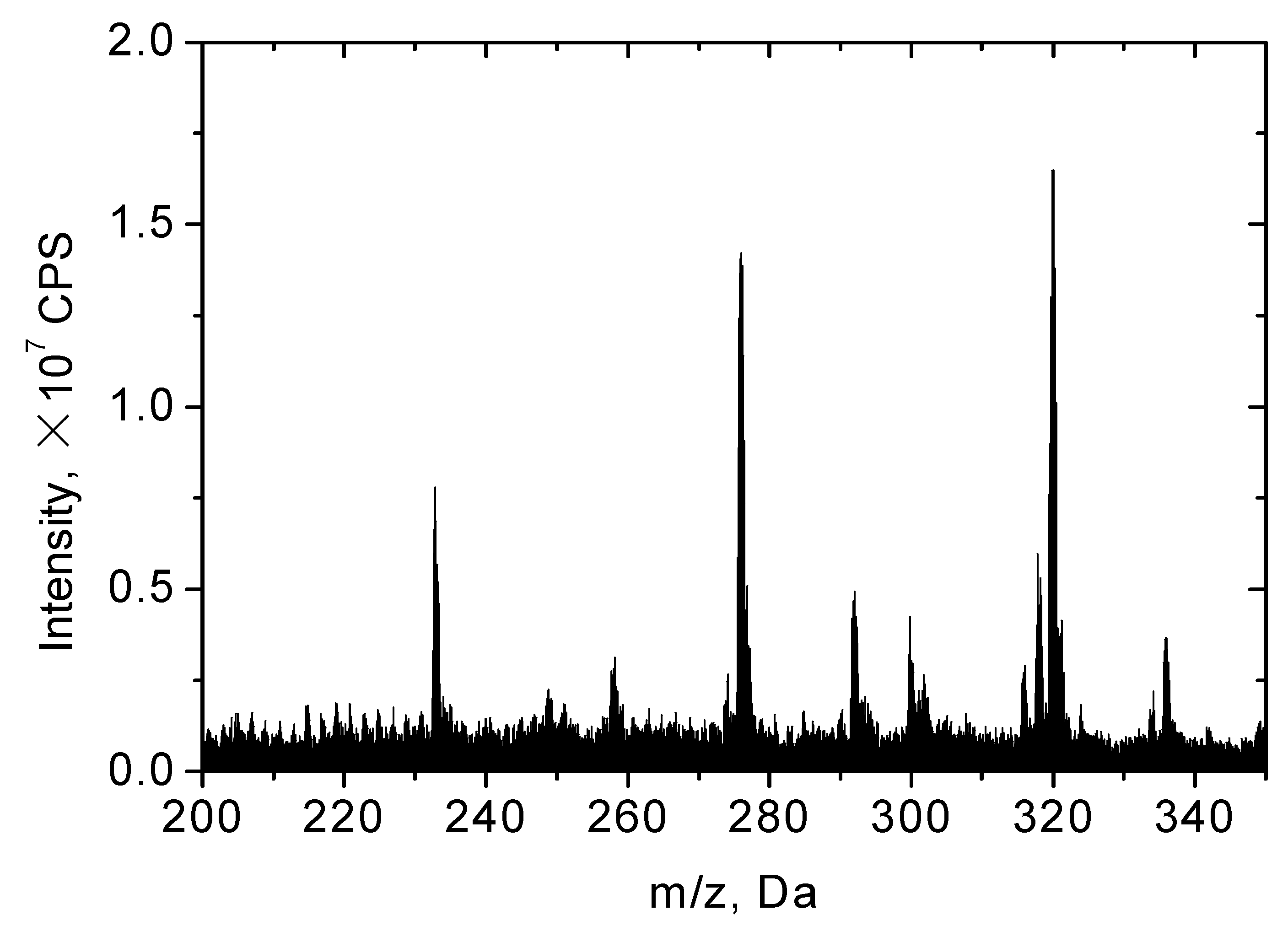
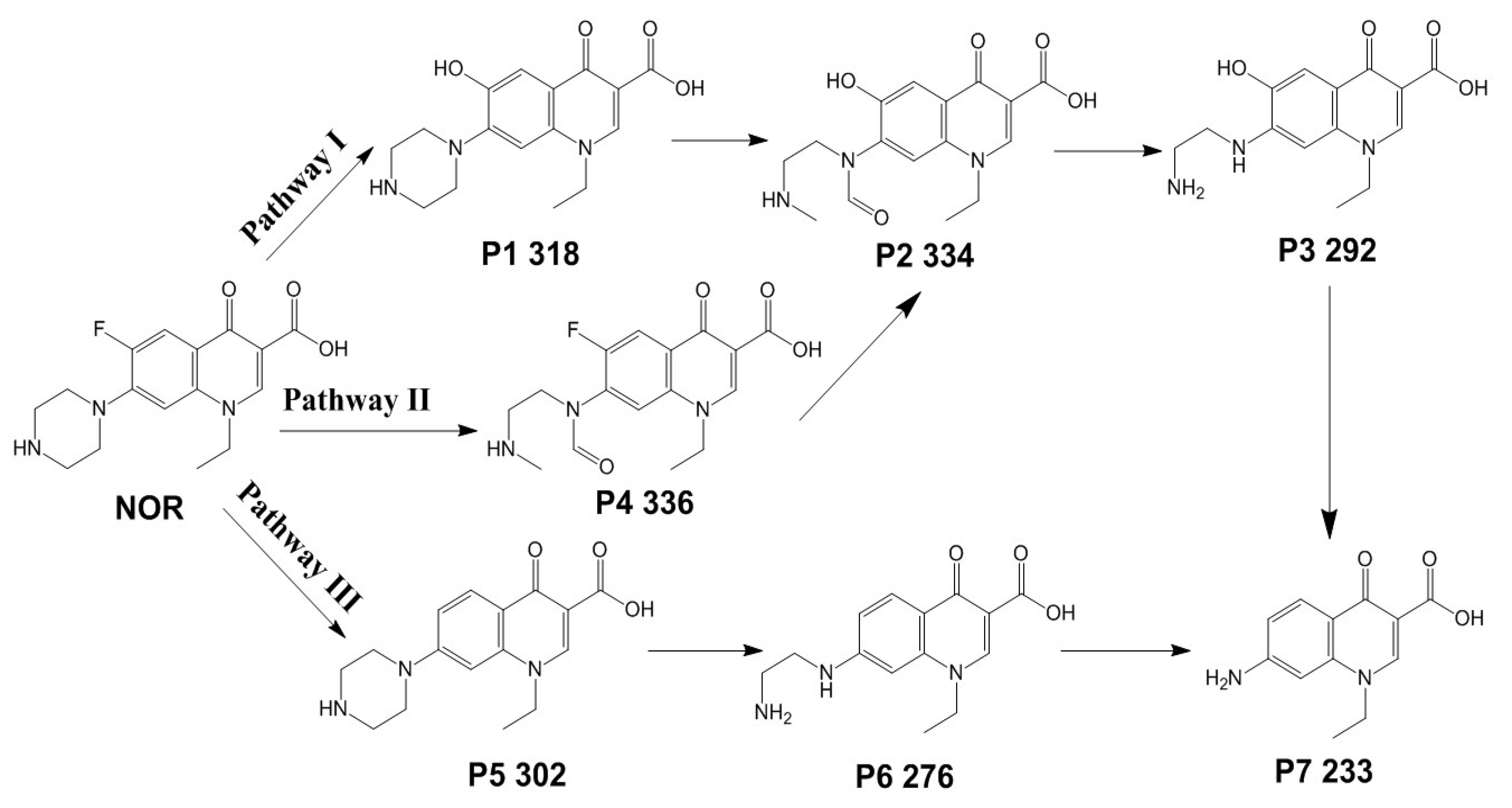
3.3. Reaction Pathways
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NOR | norfloxacin |
| MW/UV | microwave-enhanced photolysis process |
| UV | Photolysis process |
| MW | Microwave irradiation process |
| MDELs | Microwave discharged electrodeless lamps |
| DW | Deionized water |
| TW | Tap water |
| WW | Synthetic wastewater |
| RW | river water |
| SW | Sea water |
References
- Cizmas, L.; Sharma, V.K.; Gray, C.M.; McDonald, T.J. Pharmaceuticals and personal care products in waters: Occurrence, toxicity and risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.L.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Ecotoxic pharmaceuticals, personal care products, and other emerging contaminants: A review of environmental, receptor-mediated, developmental, and epigenetic toxicity with discussion of proposed toxicity to humans. Crit. Environ. Sci. Technol. 2016, 46, 336–381. [Google Scholar] [CrossRef]
- Christou, A.; Aguera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schroder, P.; et al. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes—A review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; He, Y.L.; Huang, C.H. Oxidation of fluoroquinolone antibiotics and structurally related amines by chlorine dioxide: Reaction kinetics, product and pathway evaluation. Water Res. 2010, 44, 5989–5998. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.W.; Minh, T.; Murphy, M.B.; Lam, J.C.; So, M.K.; Martin, M.; Lam, P.K.; Richardson, B.J. Distribution, fate and risk assessment of antibiotics in sewage treatment plants in Hong Kong, South China. Environ. Int. 2012, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Nanaboina, V.; Korshin, G.V.; Jiang, W. Spectroscopic study of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed in ozonated wastewater. Water Res. 2012, 46, 5235–5246. [Google Scholar] [CrossRef] [PubMed]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere. 2007, 68, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Feng, S.; Zhang, X.; Li, Y.; Zhang, X. Adsorption of norfloxacin onto titanium oxide: Effect of drug carrier and dissolved humic acid. Sci. Total Environ. 2012, 438, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Sun, D.; Gao, J.; Zhao, Q.; Wang, X.; Teng, F.; Quan, X.; Chen, J. Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aquoues solution. J. Hazard. Mater. 2013, 244, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Wammer, K.H.; Korte, A.R.; Lundeen, R.A.; Sundberg, J.E.; McNeill, K.; Arnold, W.A. Direct photochemistry of three fluoroquinolone antibacterials: Norfloxacin, ofloxacin, and enrofloxacin. Water Res. 2013, 47, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Moreau-Guigon, E.; Labadie, P.; Alliot, F.; Teil, M.-J.; Blanchard, M.; Chevreuil, M. Occurrence of antibiotics in rural catchments. Chemosphere 2017, 168, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.; Zhang, Q.; Vyawahare, S.; Rogers, E.; Rosenberg, S.M.; Austin, R.H. Emergence of antibiotic resistance from multinucleated bacterial filaments. Proc. Natl. Acad. Sci. USA 2015, 112, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Rutgersson, C.; Fick, J.; Marathe, N.; Kristiansson, E.; Janzon, A.; Angelin, M.; Johansson, A.; Shouche, Y.; Flach, C.F.; Larsson, D.G.J. Fluoroquinolones and qnr genes in sediment, water, soil, and human fecal flora in an environment polluted by manufacturing discharges. Environ. Sci. Technol. 2014, 48, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Gao, H.Y.; He, J.J.; Yang, P.; Wang, D.S.; Ma, T.; Xia, H.; Xu, X.Z. Removal of norfloxacin using coupled synthesized nanoscale zero-valent iron (nZVI) with H2O2 system: Optimization of operating conditions and degradation pathway. Sep. Purif. Technol. 2017, 172, 158–167. [Google Scholar] [CrossRef]
- Kümmerer, K.; Al-Ahmad, A.; Mersch-Sundermann, V. Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere 2000, 40, 701–710. [Google Scholar] [CrossRef]
- Feng, M.; Cizmas, L.; Wang, Z.; Sharma, V.K. Synergistic effect of aqueous removal of fluoroquinolones by a combined use of peroxymonosulfate and ferrate(VI). Chemosphere 2017, 177, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.Z.; Busetti, F.; Langsa, M.; Croué, J.-P. Roles of singlet oxygen and dissolved organic matter in self-sensitized photo-oxidation of antibiotic norfloxacin under sunlight irradiation. Water Res. 2016, 106, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Bano, R.; Musharraf, S.G.; Sheraz, M.A.; Ahmed, S.; Tahir, H.; ul Arfeen, Q.; Bhatti, M.S.; Shad, Z.; Hussain, S.F. Photodegradation of norfloxacin in aqueous and organic solvents: A kinetic study. J. Photochem. Photobiol. A Chem. 2015, 302, 1–10. [Google Scholar] [CrossRef]
- Liang, C.; Zhao, H.M.; Deng, M.J.; Quan, X.; Chen, S.; Wang, H. Impact of dissolved organic matter on the photolysis of the ionizable antibiotic norfloxacin. J. Environ. Sci. 2015, 27, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Chu, W. H2O2 assisted degradation of antibiotic norfloxacin over simulated solar light mediated Bi2WO6: Kinetics and reaction pathway. Chem. Eng. J. 2016, 296, 310–318. [Google Scholar] [CrossRef]
- Autin, O.; Hart, J.; Jarvis, P.; MacAdam, J.; Parsons, S.A.; Jefferson, B. The impact of background organic matter and alkalinity on the degradation of the pesticide metaldehyde by two advanced oxidation processes: UV/H2O2 and UV/TiO2. Water Res. 2013, 47, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, S.J.; Seo, S.G.; Jung, S.C. Assessment of microwave/UV/O3 in the photo-catalytic degradation of bromothymol blue in aqueous nano TiO2 particles dispersions. Nanoscale Res. Lett. 2010, 5, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Cai, J.H.; Cui, M.C.; Khim, J. Sonophotolytic diethyl phthalate (DEP) degradation with UVC or VUV irradiation. Ultrason. Sonochem. 2012, 19, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Minatodani, Y.; Tsutsumi, H.; Uchida, H.; Abe, M.; Serpone, N. Influence of lattice distortion and oxygen vacancies on the UV-driven/microwave-assisted TiO2 photocatalysis. J. Photochem. Photobiol. A Chem. 2013, 265, 20–28. [Google Scholar] [CrossRef]
- Horikoshi, S.; Tsuchida, A.; Sakai, H.; Abe, M.; Serpone, N. Microwave discharge electrodeless lamps (MDELs). VI. Performance evaluation of a novel microwave discharge granulated electrodeless lamp (MDGEL)-photoassisted defluorination of perfluoroalkoxy acids in aqueous media. J. Photochem. Photobiol. A Chem. 2011, 222, 97–104. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, T.T.; Zheng, L.Q.; Yu, J. Photocatalytic degradation of hydrogen sulfide using TiO2 film under microwave electrodeless discharge lamp irradiation. Chem. Eng. J. 2013, 225, 9–15. [Google Scholar] [CrossRef]
- Žabová, H.; Církva, V.; Hájek, M. Microwave photocatalysis II: Novel continuous-flowmicrowave photo-catalytic experimental set-up with titania-coated mercury electrodeless discharge lamps. J. Chem. Technol. Biotechnol. 2009, 84, 1125. [Google Scholar] [CrossRef]
- Zhang, X.W.; Sun, D.D.; Li, G.T.; Wang, Y.Z. Investigation of the roles of active oxygen species in photodegradation of azo dye AO7 in TiO2 photocatalysis illuminated by microwave electrodeless lamp. J. Photochem. Photobiol. A Chem. 2008, 199, 311–315. [Google Scholar] [CrossRef]
- Liao, W.C.; Zheng, T.; Wang, P.; Tu, S.S.; Pan, W.Q. Microwave-Assisted Photocatalytic Degradation of Dimethyl Phthalate Over a Novel ZrOx Catalyst. Environ. Eng. Sci. 2010, 27, 1001–1007. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Coupled microwave/photoassisted methods for environmental remediation. Molecules 2014, 19, 18102–18128. [Google Scholar] [CrossRef] [PubMed]
- Žabová, H.; Církva, V. Microwave photocatalysis III. Transition metal on-doped TiO2 thin films on mercury electrodeless discharge lamps: Preparation, characterization and their effect on the photocatalytic degradation of mono-chloroacetic acid and Rhodamine B. J. Chem. Technol. Biotechnol. 2009, 84, 1624–1630. [Google Scholar] [CrossRef]
- Ju, Y.M.; Qiao, J.Q.; Peng, X.C.; Xu, Z.C.; Fang, J.D.; Yang, S.G.; Sun, C. Photodegradation of malachite green using UV-vis light from two microwave-powered electrodeless discharge lamps (MPEDL-2): Further investigation on products, dominant routes and mechanism. Chem. Eng. J. 2013, 221, 353–362. [Google Scholar] [CrossRef]
- Hong, J.; Han, B.; Yuan, N.N.; Gu, J.L. The roles of active species in photo-decomposition of organic compounds by microwave powered electrodeless discharge lamps. J. Environ. Sci. 2015, 33, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Tsuchida, A.; Shinomiya, T.; Serpone, N. Microwave discharge electrodeless lamps (MDELs). Part IX. A novel MDEL photoreactor for the photolytic and chemical oxidation treatment of contaminated wastewaters. Photochem. Photobiol. Sci. 2015, 14, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Nishimura, T.; Tsutsumi, H.; Serpone, N. Microwave discharge electrodeless lamps. Part VIII: Continuous on-site solar energy remediation of contaminated water. Chem. Eng. Technol. 2016, 39, 102–107. [Google Scholar] [CrossRef]
- Ai, Z.H.; Yang, P.; Lu, X.H. Comparison of the direct photolysis and photocatalytic degradation of 4-chlorophenol in a microwave-assisted UV system. Fresenius Environ. Bull. 2004, 13, 550–554. [Google Scholar]
- Zhao, Y.J.; Chen, Q.; Hou, H.Q.; He, J.A. Photolysis of gaseous butyl acetate using built-in microwave discharge electrodeless lamps. J. Hazard. Mater. 2011, 186, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, B.; He, L.A.; Hou, H.Q.; Zhang, R.X. Photolysis of H2S with interior microwave discharge electrodeless lamps. Chin. Chem. Lett. 2010, 21, 968–972. [Google Scholar] [CrossRef]
- Wang, L.Y.; Meng, D.; Li, L. Thermal or nonthermal microwave effects—The mechanism of microwave heating. Chem. Bull. 2013, 76, 698–703. [Google Scholar]
- Loupy, A.; Maurel, F.; Sabatié-Gogová, A. Improvements in Diels-Alder cycloadditions with some acetylenic compounds under solvent-free microwave-assisted conditions: Experimental results and theoretical approaches. Tetrahedron. 2004, 60, 1683–1691. [Google Scholar] [CrossRef]
- Liu, J.X.; Jin, Y.L.; Han, Q.H.; Cang, D.Q. Non-thermal effect of desulfurization and denitration of solid metallurgical waste in the field of microwave. J. Wuhan Univ. Sci. Technol. 2009, 32, 131–133. [Google Scholar]
- Feng, M.B.; Wang, X.H.; Chen, J.; Qu, R.J.; Sui, Y.X.; Cizmas, L.; Wang, Z.Y.; Sharma, V.K. Degradation of fluoroquinolone antibiotics by ferrate (VI): Effects of water constituents and oxidized products. Water Res. 2016, 103, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.K.; Chen, J.W.; Zhang, S.Y.; Cai, X.Y.; Wang, Z.; Wang, C.L. Photodegration of fluoroquinolone antibiotic gatifloxacin in aqueous solution. Chin. Sci. Bull. 2010, 55, 1495–1500. [Google Scholar] [CrossRef]
- Walse, S.S.; Morgan, S.L.; Kong, L.; Ferry, J.L. Role of dissolved organic matter, nitrate and bicarbonate in the photolysis of aqueous fipronil. Environ. Sci. Technol. 2004, 38, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, J.F.; Wang, W.L. Photolysis of enrofloxacin in aqueous systems under simulated sunlight irradiation: Kinetics, mechanism and toxicity of photolysis products. Chemosphere 2011, 85, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Brezonik, P.L.; Brekken, J.F. Nitrate-induced photolysis in natural waters: Control on concentrations of hydroxyl radical photo-intermediates by natural scavenging agents. Environ. Sci. Technol. 1998, 32, 3004–3010. [Google Scholar] [CrossRef]
- Murtaza, S.; Javed, A.K.; Luqman, A.S.; Noor, S.S.; Hasan, M.K.; Faiza, R.; Abdur, R.K.; Asad, M.K. Degradation of quinolone antibiotic, norfloxacin, in aqueous solution using gamma-ray irradiation. Environ. Sci. Pollut. Res. 2016, 23, 13155–13168. [Google Scholar]
- Zhu, L.Y.; Santiago-Schübel, B.; Xiao, H.X.; Hollert, H.; Kueppers, S. Electrochemical oxidation of fluoroquinolone antibiotics: Mechanism, residual antibacterial activity and toxicity change. Water Res. 2016, 102, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Kleiser, G.; Frimmel, F.H. Removal of precursors for disinfection by-products (DBPs)-differences between ozone- and OH-radical-induced oxidation. Sci. Total Environ. 2000, 256, 1–9. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, C.J.; Zhang, G.; Zhou, Q. Degradation of Perfluorooctanoic Acid by UV/Chloride Process. Chem. J. Chin. Univ. 2016, 37, 1499–1508. [Google Scholar]



| Parameter | DW | TW | RW | SW | WW |
|---|---|---|---|---|---|
| pH | 7.2 | 6.7 | 7.6 | 6.6 | 8.0 |
| Conductivity (μs/cm) | 0.25 | 71.9 | 1627 | 1.07 × 105 | 1700 |
| Turbidity (NTU) | 0.46 | 0.77 | 4.61 | 21.40 | 1.65 |
| DO (mg/L) | 87.2 | 82.6 | 77.1 | 80.5 | 1.5 |
| TN (mg/L) | 0.29 | 0.29 | 1.93 | 2.00 | 10.00 |
| COD (mg/L) | 0 | 47 | 334 | 1869 | 100 |
| Scan Range (m/z) | Drying Gas Temperature | Drying Gas Flow Rate |
| 50–350 | 300 °C | 8 L/min |
| Fragmentor | Nebulizer Pressure | Capillary Voltage |
| 150 V | 35 psi | 4000 V |
| Source | III-Type Sum of SQUARE | df | Mean Square | F | Sig. | Partial Eta Square | Non Central Parameter | Observed Power b |
|---|---|---|---|---|---|---|---|---|
| Calibration model | 86,276.499 a | 20 | 4313.825 | 448.834 | 0.000 | 0.995 | 8976.674 | 1.000 |
| Intercept | 200,831.323 | 1 | 20,0831.323 | 20,895.577 | 0.000 | 0.998 | 20,895.577 | 1.000 |
| V11 c | 22,262.405 | 6 | 3710.401 | 386.050 | 0.000 | 0.982 | 2316.301 | 1.000 |
| V12 c | 53,814.143 | 2 | 26,907.071 | 2799.557 | 0.000 | 0.993 | 5599.115 | 1.000 |
| V11*V12 d | 10,199.951 | 12 | 849.996 | 88.438 | 0.000 | 0.962 | 1061.258 | 1.000 |
| Error | 403.670 | 42 | 9.611 | 0.000 | ||||
| Total | 287,511.492 | 63 | ||||||
| Calibrated total | 86,680.168 | 62 |
| Process | kobs | (min) |
|---|---|---|
| MW/UV | (1.13 ± 0.31) × 10−1 mg−1 min−1 | 1.77 a |
| UV | (4.22 ± 0.56) × 10−2 min−1 | 16.43 b |
| MW | (1.73 ± 0.21) × 10−3 min−1 | 400.66 b |
| Intermediates | Molecular Ion, [M + H]+ | Chemical Structure | Fragments (m/z) |
|---|---|---|---|
| NOR | 320 |  | |
| P1 | 318 |  | 300.2, 257, 231.1 |
| P2 | 334.1 |  | 316.2, 245 |
| P3 | 292 |  | 221.1 |
| P4 | 336 |  | 318.2, 275, 247, 233, 221.1, 205 |
| P5 | 301.8 |  | 231 |
| P6 | 275.9 |  | 233.1, 205.1 |
| P7 | 232.7 |  | 203.2, 176.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.; Sharma, V.K.; Xu, S.; Li, Q.; Wang, L. Microwave-Enhanced Photolysis of Norfloxacin: Kinetics, Matrix Effects, and Degradation Pathways. Int. J. Environ. Res. Public Health 2017, 14, 1564. https://doi.org/10.3390/ijerph14121564
Liao W, Sharma VK, Xu S, Li Q, Wang L. Microwave-Enhanced Photolysis of Norfloxacin: Kinetics, Matrix Effects, and Degradation Pathways. International Journal of Environmental Research and Public Health. 2017; 14(12):1564. https://doi.org/10.3390/ijerph14121564
Chicago/Turabian StyleLiao, Wenchao, Virender K. Sharma, Su Xu, Qingsong Li, and Lei Wang. 2017. "Microwave-Enhanced Photolysis of Norfloxacin: Kinetics, Matrix Effects, and Degradation Pathways" International Journal of Environmental Research and Public Health 14, no. 12: 1564. https://doi.org/10.3390/ijerph14121564