Antibiotic Resistance of Bacteria Involved in Urinary Infections in Brazil: A Cross-Sectional and Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
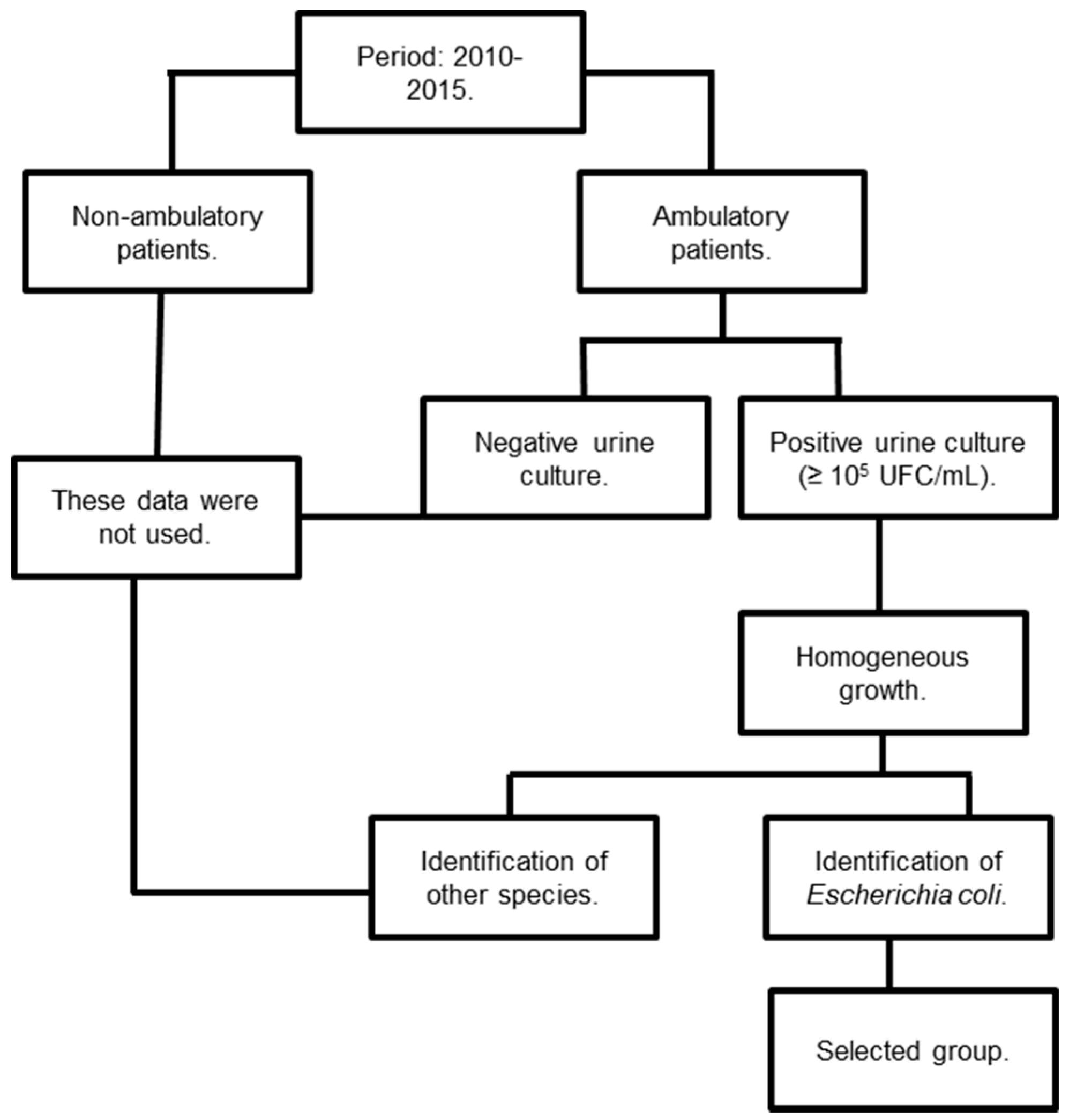
2.1. Experimental Design
2.2. Inclusion and Exclusion Criteria
2.3. Bacterial Identification
2.4. Antimicrobial Susceptibility Testing
2.5. Commercial Values
2.6. Statistical Analysis
3. Results
3.1. Efficacy of Antibiotics Against Patient Uroculture-Derived E. coli Samples
3.2. Relative Frequency of Antibiotics Used for Empirical Treatment of Urinary Infections
3.3. Temporal Variation in Antimicrobial Resistance in Brazil
3.4. Variation in the Cost of Drugs Used to Treat Genitourinary Infections Caused by E. coli
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dalbosco, V.; Srougi, M.; Dall’Ogilo, M. Infecções do trato urinário. Rev. Bras. Med. 2003, 60, 320–322. [Google Scholar]
- Zhanel, G.G.; Hisanaga, T.L.; Laing, N.M.; DeCorby, M.R.; Nichol, K.A.; Palatnik, L.P.; Johnson, J.; Noreddin, A.; Harding, G.K.; Nicolle, L.E.; et al. Antibiotic resistance in outpatient urinary isolates: Final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int. J. Antimicrob. Agents 2005, 26, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- World Health Organization. Tuberculosis. Saudi Med. J. 2013, 34, 1205–1207. [Google Scholar]
- Cherkaoui, A.; Emonet, S.; Renzi, G.; Riat, A.; Greub, G.; Schrenzel, J. ESBL and carbapenemases in Enterobacteriaceae. Rev. Med. Suisse 2014, 10, 2142–2148. [Google Scholar] [PubMed]
- Trang, N.H.T.; Nga, T.V.T.; Campbell, J.I.; Hiep, N.T.; Farrar, J.; Baker, S.; Duy, P.T. The characterization of ESBL genes in Escherichia coli and Klebsiella pneumoniae causing nosocomial infections in Vietnam. J. Infect. Dev. Ctries. 2013, 7, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Coque, T.; Baquero, F.; Canton, R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Eur. Surveill. 2008, 13, 5437–5453. [Google Scholar]
- Higgins, C.F. Multiple molecular mechanisms for multidrug resistance transporters. Nature 2007, 446, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Krizova, L.; Nemec, A. A 63 kb genomic resistance island found in a multidrug-resistant Acinetobacter baumannii isolate of European clone I from 1977. J. Antimicrob. Chemother. 2010, 65, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sharma, M.; Chaudhary, U. Biofilm and multidrug resistance in uropathogenic Escherichia coli. Pathog. Glob. Health 2015, 109, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Okeke, I.N.; Lamikanra, A.; Edelman, R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg. Infect. Dis. 1999, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S. Factors affecting the cost effectiveness of antibiotics. Chemother. Res. Pract. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Barriere, S.L. Clinical, economic and societal impact of antibiotic resistance. Expert Opin. Pharmacother. 2015, 16, 151–153. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Twelfth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Arango, H.G. Bioestatística teórica e computacional. In Bioestatística Teórica e Computacional; Guanabara Koogan: Rio de Janeiro, Brazil, 2001. [Google Scholar]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Publ. Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef] [PubMed]
- Linhares, I.; Raposo, T.; Rodrigues, A.; Almeida, A. Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: A ten-year surveillance study (2000–2009). BMC Infect. Dis. 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, L.; Trautner, B.W.; Gupta, K. Diagnosis and management of urinary tract infections in the outpatient setting: A review. JAMA 2014, 312, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Ikeda, M.; Nakamura, A.; Kodera, S. Efficacy of empirical therapy with non-carbapenems for urinary tract infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Int. J. Infect. Dis. 2014, 29, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Seija, V.; Frantchez, V.; Ventura, V.; Pintos, M.; González, M. Factores asociados al desarrollo de infección urinaria de origen comunitario causada por Escherichia coli resistente a fluoroquinolonas. Rev. Chilena Infectol. 2014, 31, 400–405. [Google Scholar] [PubMed]
- Ironmonger, D.; Edeghere, O.; Bains, A.; Loy, R.; Woodford, N.; Hawkey, P.M. Surveillance of antibiotic susceptibility of urinary tract pathogens for a population of 5.6 million over 4 years. J. Antimicrob. Chemother. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.L.; MacGowan, A.P.; Bowker, K.E.; Elder, F.; Beck, C.R.; McNulty, C. Prevalence of antibiotic resistance in Escherichia coli isolated from urine samples routinely referred by general practitioners in a large urban centre in south-west England. J. Antimicrob. Chemother. 2015, 70, 2167–2169. [Google Scholar] [CrossRef] [PubMed]
- Hooton, T.M.; Besser, R.; Foxman, B.; Fritsche, T.R.; Nicolle, L.E. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: A proposed approach to empirical therapy. Clin. Infect. Dis. 2004, 39, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Rattanaumpawan, P. Risk Factors for and Impact of Ambulatory Urinary Tract Infections Caused by High MIC-Fluoroquinolone Susceptible E. coli in Women. Ph.D. Dissertation, University of Pennsylvania, Philadelphia, PA, USA, 2013. [Google Scholar]
- Evans, R.S.; Larsen, R.A.; Burke, J.P.; Gardner, R.M.; Meier, F.A.; Jacobson, J.A.; Conti, M.T.; Jacobson, J.T.; Hulse, R.K. Computer surveillance of hospital-acquired infections and antibiotic use. JAMA 1986, 256, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Tängdén, T.; Giske, C.G. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: Clinical perspectives on detection, treatment and infection control. J. Intern. Med. 2015, 277, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Harris, P.N.; Roberts, M.J.; Tambyah, P.A.; Schembri, M.A.; Pezzani, M.D.; Williamson, D.A.; Paterson, D.L. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat. Rev. Urol. 2015, 12, 570–584. [Google Scholar] [CrossRef] [PubMed]
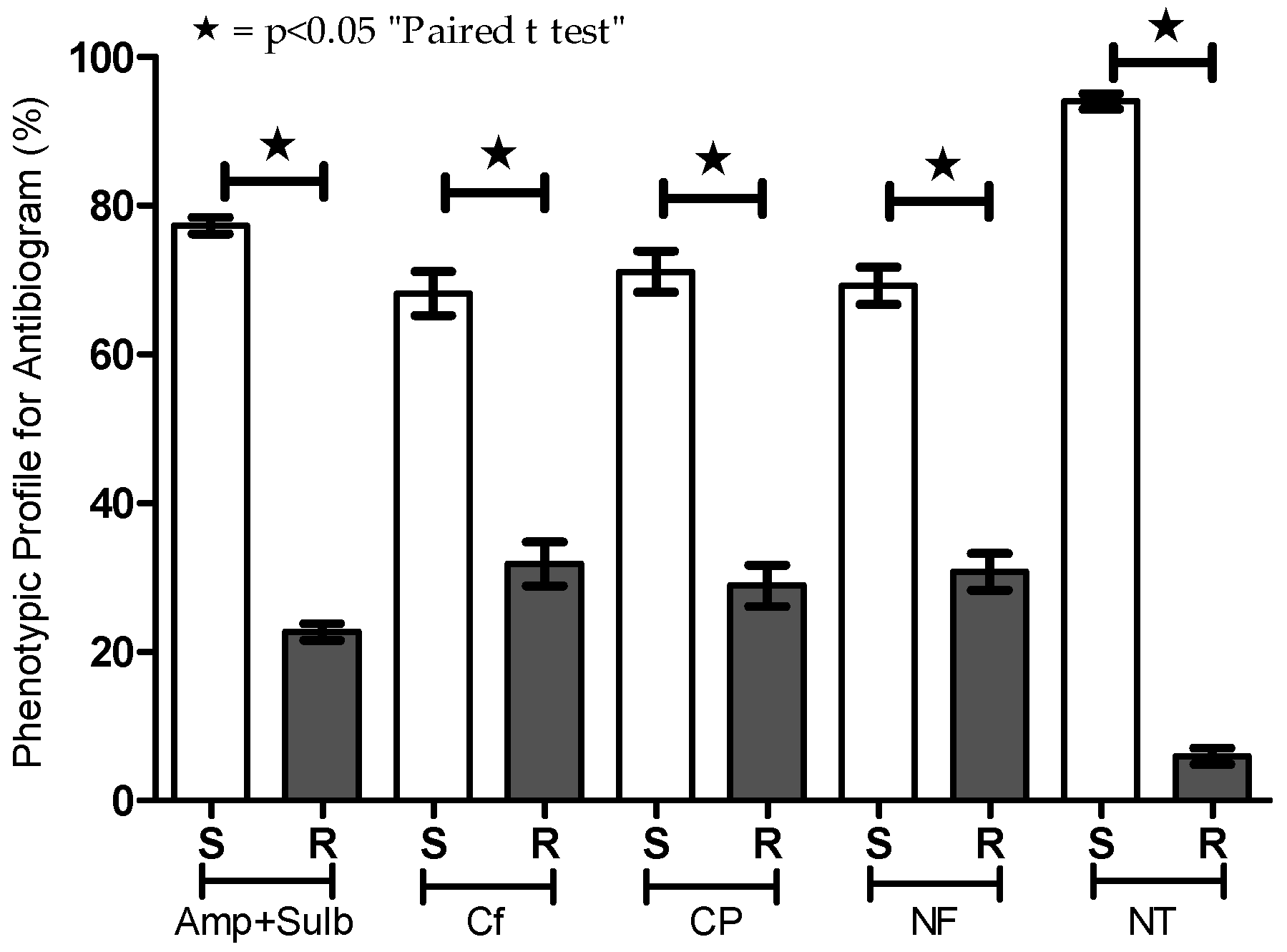
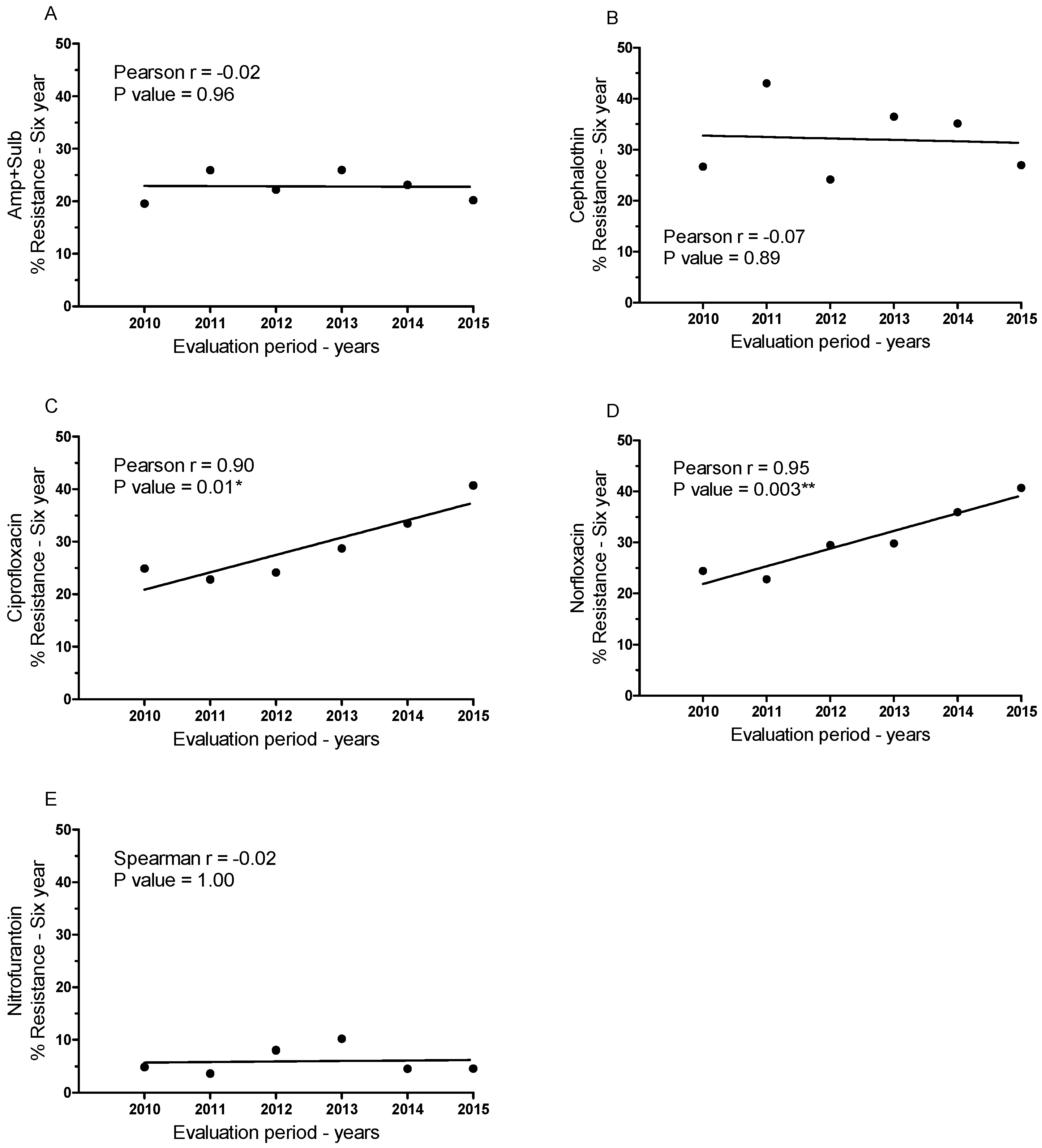
| Year | Amp + Sulb | Cephalothin | Ciprofloxacin | Norfloxacin | Nitrofurantoin | N | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | S | R | S | R | ||
| 2010 | 181 | 44 | 165 | 60 | 169 | 56 | 170 | 55 | 214 | 11 | 225 |
| 2011 | 143 | 50 | 110 | 83 | 149 | 44 | 149 | 44 | 186 | 7 | 193 |
| 2012 | 203 | 58 | 198 | 63 | 198 | 63 | 184 | 77 | 240 | 21 | 261 |
| 2013 | 268 | 94 | 230 | 132 | 258 | 104 | 254 | 108 | 325 | 37 | 362 |
| 2014 | 196 | 56 | 167 | 85 | 171 | 81 | 165 | 87 | 241 | 11 | 242 |
| 2015 | 296 | 75 | 271 | 100 | 220 | 151 | 220 | 151 | 354 | 17 | 371 |
| Total | 1654 | ||||||||||
| Years | (∑RFS)/(∑RFRes) | Ratio of Relative Frequency (S/Res)/Antibiotic | ||||
|---|---|---|---|---|---|---|
| Amp + Sulb | Cephalothin | Ciprofloxacin | Norfloxacin | Nitrofurantoin | ||
| 2010 | 1 | 1.03 | 0.69 | 0.77 | 0.77 | 4.94 |
| 2011 | 1 | 0.90 | 0.42 | 1.03 | 1.03 | 7.47 |
| 2012 | 1 | 0.89 | 0.82 | 0.82 | 0.82 | 2.79 |
| 2013 | 1 | 1.01 | 0.61 | 0.88 | 0.88 | 2.96 |
| 2014 | 1 | 1.17 | 0.67 | 0.70 | 0.70 | 6.41 |
| 2015 | 1 | 1.43 | 0.98 | 0.53 | 0.53 | 7.56 |
| Ẋ ± Std. Error | - | 1.07 ± 0.08 a | 0.70 ± 0.07 b | 0.79 ± 0.07 a,b | 0.79 ± 0.07 a,b | 5.35 ± 0.87 c |
| Fluoroquinolones | Resistance to Fluoroquinolones (%) | |||||
|---|---|---|---|---|---|---|
| 50 | 60 | 70 | 80 | 90 | 100 | |
| Norfloxacin (Year) | 2017 | 2020 | 2023 | 2026 | 2029 | 2032 |
| Ciprofloxacin (Year) | 2018 | 2021 | 2024 | 2027 | 2030 | 2033 |
| Product (Manufacturer) | Presentation | MPC (R$) | Mean ± SE (MPC) | Mean ± SE (GE-Brand) | |||
|---|---|---|---|---|---|---|---|
| 12% | 17% | 18% | 19% | ||||
| Sulbamox BD® (Farmasa) | 875 + 125 mg—14 cpr | 72.00 | 76.34 | 77.27 | 78.22 | 75.96 ± 1.37 | 84.60 ± 8.64 |
| Amoxicilina + clav K (Eurof.) | 875 + 125 mg—14 cpr | 88.38 | 93.71 | 94.85 | 96.01 | 93.24 ± 1.69 | |
| Cephalothin (Ariston) | 1g inj ct 50 un | 231.33 | 245.28 | 248.26 | 251.31 | 244.00 ± 4.41 | 244.00 |
| Cephalothin (Novafarma) | 1g inj ct 50 un | 231.3 | 245.23 | 248.23 | 251.28 | 244.00 ± 4.41 | |
| Cipro® (Bayer) | 500 mg—14 cpr | 218.74 | 231.93 | 234.75 | 237.64 | 230.80 ± 4.17 | 136.50 ± 94.28 |
| Clor. Ciprofloxacin (Medley) | 500 mg—14 cpr | 40.04 | 42.46 | 42.97 | 43.5 | 42.24 ± 0.76 | |
| Floxacin® (Merck Sharp) | 400 mg—14 cpr | 30.25 | 32.07 | 32.46 | 32.86 | 31.91 ± 0.58 | 31.92 ± 0.01 |
| Norfloxacin (Medley) | 400 mg—14 cpr | 30.26 | 32.09 | 32.48 | 32.88 | 31.93 ± 0.58 | |
| Macrodantina® (Sc.P) | 100 mg—28 cpr | 8.99 | 9.54 | 9.65 | 9.77 | 9.49 ± 0.17 | 7.83 ± 1.66 |
| Nitrofurantoin (Teuto) | 100 mg—28 cpr | 5.85 | 6.2 | 6.27 | 6.35 | 6.17 ± 0.11 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, W.F.; Miguel, C.B.; Nogueira, A.P.O.; Ueira-Vieira, C.; Paulino, T.D.P.; Soares, S.D.C.; De Resende, E.A.M.R.; Lazo-Chica, J.E.; Araújo, M.C.; Oliveira, C.J. Antibiotic Resistance of Bacteria Involved in Urinary Infections in Brazil: A Cross-Sectional and Retrospective Study. Int. J. Environ. Res. Public Health 2016, 13, 918. https://doi.org/10.3390/ijerph13090918
Rodrigues WF, Miguel CB, Nogueira APO, Ueira-Vieira C, Paulino TDP, Soares SDC, De Resende EAMR, Lazo-Chica JE, Araújo MC, Oliveira CJ. Antibiotic Resistance of Bacteria Involved in Urinary Infections in Brazil: A Cross-Sectional and Retrospective Study. International Journal of Environmental Research and Public Health. 2016; 13(9):918. https://doi.org/10.3390/ijerph13090918
Chicago/Turabian StyleRodrigues, Wellington Francisco, Camila Botelho Miguel, Ana Paula Oliveira Nogueira, Carlos Ueira-Vieira, Tony De Paiva Paulino, Siomar De Castro Soares, Elisabete Aparecida Mantovani Rodrigues De Resende, Javier Emilio Lazo-Chica, Marcelo Costa Araújo, and Carlo José Oliveira. 2016. "Antibiotic Resistance of Bacteria Involved in Urinary Infections in Brazil: A Cross-Sectional and Retrospective Study" International Journal of Environmental Research and Public Health 13, no. 9: 918. https://doi.org/10.3390/ijerph13090918






