25 Hydroxyvitamin D Deficiency and Its Relationship to Autoimmune Thyroid Disease in the Elderly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical and Biochemical Assessment
2.3. Definition of AT
2.4. Definition of Vitamin D Deficiency
2.5. Statistical Analysis
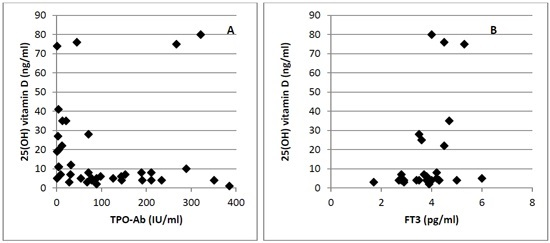
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Basit, S. Vitamin D in health and disease: A literature review. Br. J. Biomed. Sci. 2013, 70, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Mitri, J.; Mathieu, C.; Badenhoop, K.; Tamer, G.; Orio, F.; Mezza, T.; Vieth, R.; Colao, A.; Pittas, A. Mechanisms in endocrinology: Vitamin D as a potential contributor in endocrine health and disease. Eur. J. Endocrinol. 2014, 171, R101–R110. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P.; Pike, J.W.; O’Malley, B.W. The vitamin D receptor: A primitive steroid receptor related to thyroid hormone receptor. J. Steroid Biochem. 1988, 30, 41–46. [Google Scholar] [CrossRef]
- Tamer, G.; Arik, S.; Tamer, I.; Coksert, D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid 2011, 21, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Mazokopakis, E.E.; Kotsiris, D.A. Hashimoto’s autoimmune thyroiditis and vitamin D deficiency. Current aspects. Hell. J. Nucl. Med. 2014, 17, 37–40. [Google Scholar] [PubMed]
- Lacka, K.; Maciejewski, A. Vitamin D in the etiopathogenesis of autoimmune thyroiditis. Pol. Merkur. Lekarski 2013, 34, 281–285. [Google Scholar] [PubMed]
- Goswami, R.; Marwaha, R.K.; Gupta, N.; Tandon, N.; Sreenivas, V.; Tomar, N.; Ray, D.; Kanwar, R.; Agarwal, R. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: A community-based survey. Br. J. Nutr. 2009, 102, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Badenhoop, K.; Tijssen, J.G.P.; Wiersinga, W.M. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur. J. Endocrinol. 2012, 167, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Palomba, S.; Caggiano, M.; Tafuri, D.; Colao, A.; Orio, F. Low 25(OH) vitamin D levels are associated with autoimmune thyroid disease in polycystic ovary syndrome. Endocrine 2015. [Google Scholar] [CrossRef] [PubMed]
- Heijboer, A.C.; Blankenstein, M.A.; Kema, I.P.; Buijs, M.M. Accuracy of 6 routine 25-hydroxyvitamin D assays: Influence of vitamin D binding protein concentration. Clin. Chem. 2012, 58, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Mosekilde, L. Vitamin D and the elderly. Clin. Endocrinol. (Oxf.) 2005, 62, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Omdahl, J.L.; Garry, P.J.; Hunsaker, L.A.; Hunt, W.C.; Goodwin, J.S. Nutritional status in a healthy elderly population: Vitamin D. Am. J. Clin. Nutr. 1982, 36, 1225–1233. [Google Scholar] [PubMed]
- McKenna, M.J. Differences in vitamin D status between countries in young adults and the elderly. Am. J. Med. 1992, 93, 69–77. [Google Scholar] [CrossRef]
- Holick, M.F. Environmental factors that influence the cutaneous production of vitamin D. Am. J. Clin. Nutr. 1995, 61, 638S–645S. [Google Scholar] [PubMed]
- Muscogiuri, G.; Tirabassi, G.; Bizzaro, G.; Orio, F.; Paschou, S.A.; Vryonidou, A.; Balercia, G.; Shoenfeld, Y.; Colao, A. Vitamin D and thyroid disease: To D or not to D? Eur. J. Clin. Nutr. 2015, 69, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Etten, E.V.; Overbergh, L.; Mathieu, C. Vitamin D3 and the immune system: Maintaining the balance in health and disease. Nutr. Res. Rev. 2007, 20, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Karthaus, N.; van Spriel, A.B.; Looman, M.W.; Chen, S.; Spilgies, L.M.; Lieben, L.; Carmeliet, G.; Ansems, M.; Adema, G.J. Vitamin D controls murine and human plasmacytoid dendritic cell function. J. Investig. Dermatol. 2014, 134, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C. Vitamin D and the immune system: Getting it right. IBMS BoneKEy 2011, 8, 178–186. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Kurylowicz, A.; Ramos-Lopez, E.; Bednarczuk, T.; Badenhoop, K. Vitamin D-binding protein (DBP) gene polymorphism is associated with Graves’ disease and the vitamin D status in a Polish population study. Exp. Clin. Endocrinol. Diabetes 2006, 114, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Ban, Y.; Taniyama, M.; Katagiri, T. Vitamin D receptor initiation codon polymorphism in Japanese patients with Graves’ disease. Thyroid 2000, 10, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Li, H.; Chen, S.F.; Li, W.F.; Zhang, F.B. Polymorphisms in the vitamin D receptor gene and risk of autoimmune thyroid disease: A meta-analysis. Endocrine 2013, 43, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Pani, M.A.; Regulla, K.; Segni, M.; Krause, M.; Hofmann, S.; Hufner, M.; Herwig, J.; Pasquino, A.M.; Usadel, K.H.; Badenhoop, K. Vitamin D 1α-hydroxylase (CYP1α) polymorphism in Graves’ disease, Hashimoto’s Thyroiditis and type 1 diabetes mellitus. Eur. J. Endocrinol. 2002, 146, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, N.C.; Karbek, B.; Ucan, B.; Sahin, M.; Cakal, E.; Ozbek, M.; Delibasi, T. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr. Pract. 2013, 19, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Mackawy, A.M.; Al-Ayed, B.M.; Al-Rashidi, B.M. Vitamin d deficiency and its association with thyroid disease. Int. J. Health Sci. (Qassim) 2013, 7, 267–275. [Google Scholar] [CrossRef]

| Parameters | Total (n = 168) | Vitamin D Deficiency (25(OH) Vitamin D < 20 ng/mL) (n = 116) | Vitamin D Sufficiency (25(OH) Vitamin D ≥ 20 ng/mL) (n = 52) | p-Value |
|---|---|---|---|---|
| Age (years) | 81.6 ± 9.4 | 82.8 ± 8.4 | 78.8 ± 10.8 | 0.005 |
| 25(OH) vitamin D (ng/mL) | 16.4 ± 15.6 | 7.6 ± 4.0 | 33.3 ± 19.6 | <0.01 |
| BMI (kg/m2) | 29.8 ± 15.2 | 28.7 ± 8.6 | 32.4 ± 24.1 | 0.12 |
| Glycemia (mg/dL) | 102.4 ± 32.2 | 104.2 ± 33.2 | 101.5 ± 31.8 | 0.31 |
| Creatinine (mg/dL) | 1.1 ± 0.6 | 1.1 ± 0.7 | 0.9 ± 0.3 | 0.04 |
| AST (UI/L) | 19.9 ± 7.9 | 19.1 ± 6.4 | 21.7 ± 10.4 | 0.30 |
| ALT (UI/L) | 17.6 ± 11.7 | 16.9 ± 10.7 | 19.1 ± 13.7 | 0.13 |
| Total Cholesterol (mg/dL) | 173.8 ± 44.6 | 167.0 ± 41.9 | 176.9 ± 45.6 | 0.09 |
| Triglycerides (mg/dL) | 113.9 ± 7.8 | 104.7 ± 40.1 | 118.1 ± 82.1 | 0.13 |
| TSH (mcUI/mL) | 2.1 ± 0.9 | 2.1 ± 1.9 | 1.8 ± 1.3 | 0.14 |
| FT3 (pg/mL) | 3.3 ± 0.4 | 3.7 ± 1.0 | 4.1 ± 1.6 | 0.16 |
| FT4 (ng/dL) | 15.1 ± 4.7 | 15.3 ± 5.3 | 14.7 ± 3.1 | 0.33 |
| TPO-Ab (IU/mL) | 105 ± 45.9 | 117 ± 57.6 | 69.1 ± 19.8 | 0.01 |
| TG-Ab (IU/mL) | 121 ± 47.0 | 128 ± 55.7 | 101 ± 27.4 | 0.08 |
| AT prevalence (%) | 23 | 28 | 8 | 0.02 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscogiuri, G.; Mari, D.; Prolo, S.; Fatti, L.M.; Cantone, M.C.; Garagnani, P.; Arosio, B.; Di Somma, C.; Vitale, G. 25 Hydroxyvitamin D Deficiency and Its Relationship to Autoimmune Thyroid Disease in the Elderly. Int. J. Environ. Res. Public Health 2016, 13, 850. https://doi.org/10.3390/ijerph13090850
Muscogiuri G, Mari D, Prolo S, Fatti LM, Cantone MC, Garagnani P, Arosio B, Di Somma C, Vitale G. 25 Hydroxyvitamin D Deficiency and Its Relationship to Autoimmune Thyroid Disease in the Elderly. International Journal of Environmental Research and Public Health. 2016; 13(9):850. https://doi.org/10.3390/ijerph13090850
Chicago/Turabian StyleMuscogiuri, Giovanna, Daniela Mari, Silvia Prolo, Letizia M. Fatti, Maria Celeste Cantone, Paolo Garagnani, Beatrice Arosio, Carolina Di Somma, and Giovanni Vitale. 2016. "25 Hydroxyvitamin D Deficiency and Its Relationship to Autoimmune Thyroid Disease in the Elderly" International Journal of Environmental Research and Public Health 13, no. 9: 850. https://doi.org/10.3390/ijerph13090850
APA StyleMuscogiuri, G., Mari, D., Prolo, S., Fatti, L. M., Cantone, M. C., Garagnani, P., Arosio, B., Di Somma, C., & Vitale, G. (2016). 25 Hydroxyvitamin D Deficiency and Its Relationship to Autoimmune Thyroid Disease in the Elderly. International Journal of Environmental Research and Public Health, 13(9), 850. https://doi.org/10.3390/ijerph13090850