High Laccase Expression by Trametes versicolor in a Simulated Textile Effluent with Different Carbon Sources and PHs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungus and Medium Composition
2.2. Culture Conditions
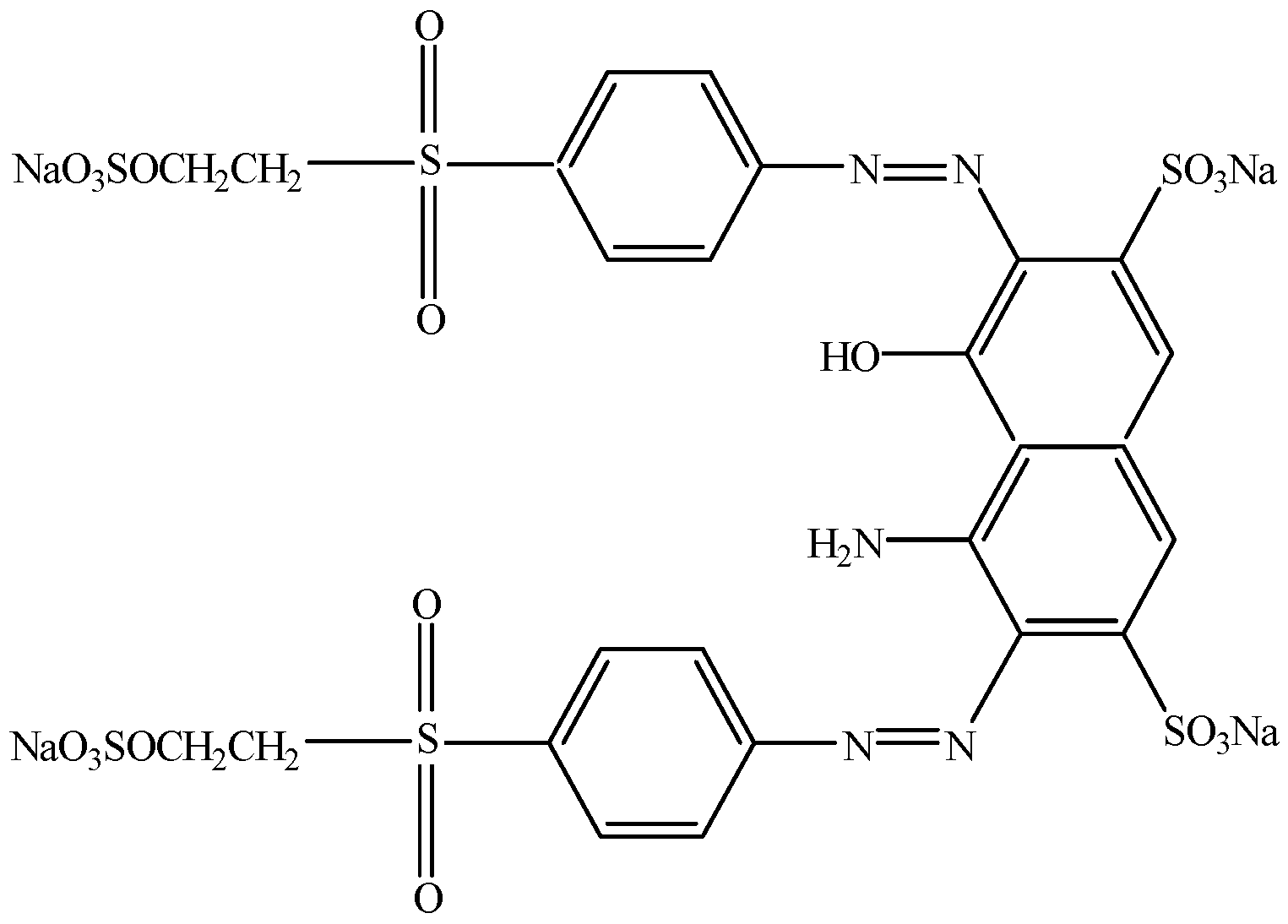
2.2.1. Decolourisation of RB5 in a Fixed-Bed Bioreactor
2.2.2. Carbon Source Monitoring
2.3. Influence of Different Carbon Sources on Expression of Lcc Gene
2.3.1. Genomic DNA Isolation, Sequencing and Sequence Analysis
2.3.2. RNA Extraction and RT-PCR
2.4. Analytical Methods
2.5. Enzymatic Assays
3. Results and Discussion
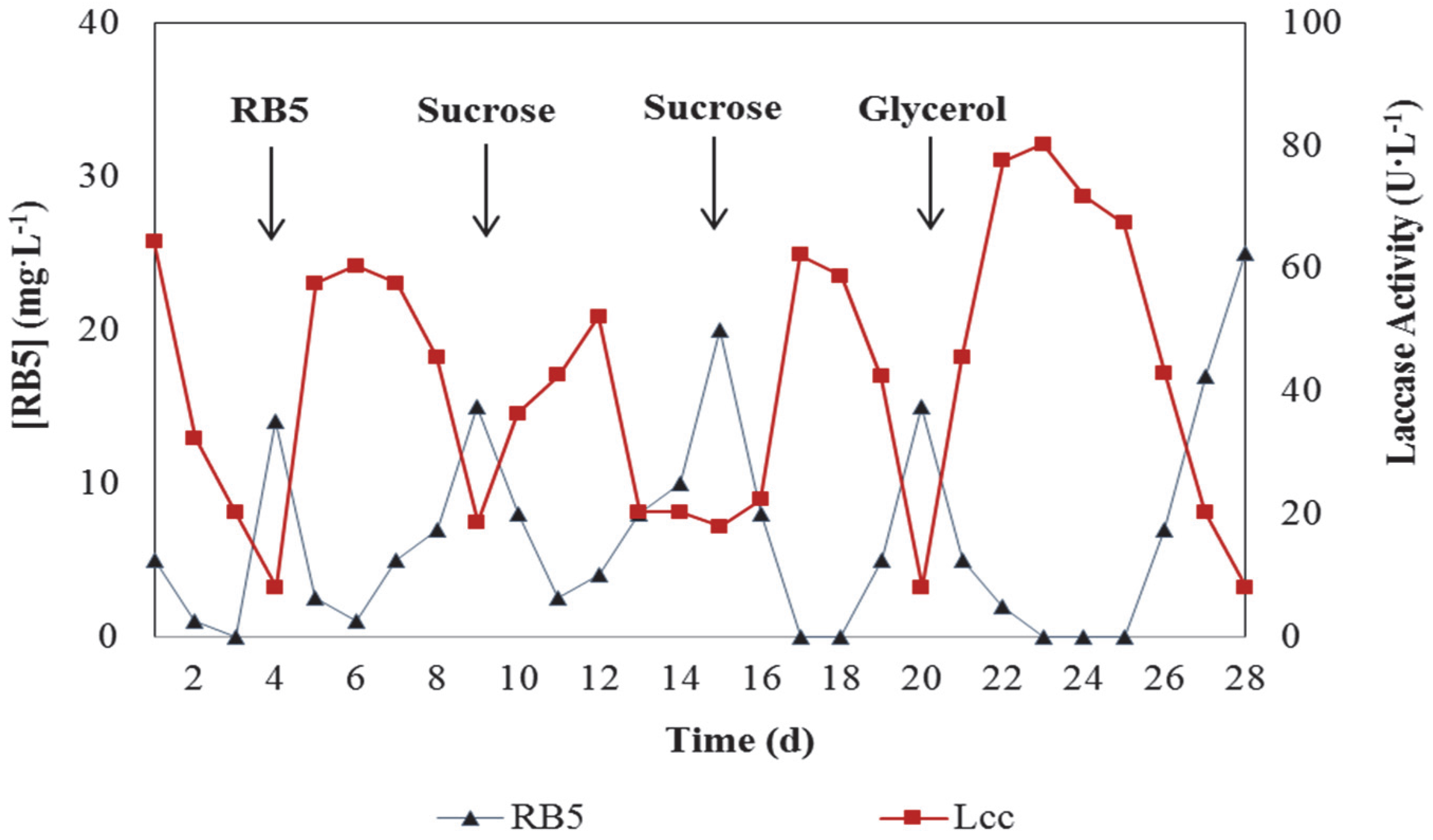
3.1. Decolourisation of RB5
3.2. Gene Expression of lcc2 Gene
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kocyigit, A.; Pazarbasi, M.B.; Yasa, Y.; Ozdemir, G.; Karaboz, I. Production of laccase from Trametes trogii TEM H2: A newly isolated white-rot fungus by air sampling. J. Basic Microbiol. 2012, 52, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Cost analysis in laccase production. J. Environ. Manag. 2011, 92, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jahan, M.S.; Liu, S.; Miao, Q.; Ni, Y. Lignin removal enhancement from prehydrolysis liquor of kraft-based dissolving pulp production by laccase-induced polymerization. Bioresour. Technol. 2014, 164, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kaur, K.; Puri, S.; Sharma, P. Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl. Microbiol. Biotechnol. 2015, 99, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, C.A.; Santos, C.; Kozakiewicz, Z.; Lima, N. White-rot fungi capable of decolourising textile dyes under alkaline conditions. Folia Microbiol. 2013, 58, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, C.; Lima, L.; Santos, C.; Lima, N. Effect of different carbon sources on decolourisation of an industrial textile dye under alkaline-saline conditions. Curr. Microbiol. 2014, 68, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, R.; Belbahri, L.; Woodward, S.; Ellouz, M.; Dhouib, A.; Sayadi, S.; Mechichi, T. Decolourization and detoxification of textile industry wastewater by the laccase-mediator system. J. Hazard. Mater. 2010, 175, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Teng, Z.; Ding, S. Heterologous expression and characterization of a novel laccase isoenzyme with dyes decolorization potential from Coprinus comatus. Mol. Biol. Rep. 2013, 40, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.J.; Zhu, F.; Du, G.Q.; Zhang, H.X.; Wang, T.B.; Ng, T.B. Purification a laccase exhibiting dye decolorizing ability from an edible mushroom Russula virescens. Int. Biodeter. Biodegr. 2013, 82, 33–39. [Google Scholar] [CrossRef]
- Ling, Z.R.; Wang, S.S.; Zhu, M.J.; Ning, Y.J.; Wang, S.N.; Li, B.; Yang, A.Z.; Zhang, G.Q.; Zhao, X.M. An extracellular laccase with potent dye decolorizing ability from white rot fungus Trametes sp. LAC-01. Int. J. Biol. Macromol. 2015, 81, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Chairin, T.; Nitheranont, T.; Watanabe, A.; Asada, Y.; Khanongnuch, C.; Lumyong, S. Biodegradation of bisphenol A and decolorization of synthetic dyes by laccase from white-rot fungus, Trametes polyzona. Appl. Biochem. Biotechnol. 2013, 169, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Roriz, M.S.; Osma, J.F.; Teixeira, J.A.; Couto, S.R. Application of response surface methodological approach to optimise Reactive Black 5 decolouration by crude laccase from Trametes pubescens. J. Hazard. Mater. 2009, 169, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Enayatzamir, K.; Alikhani, H.A.; Couto, S.R. Simultaneous production of laccase and decolouration of the diazo dye Reactive Black 5 in fixed-bed bioreactor. J. Hazard. Mater. 2009, 164, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.S.; Anand, A.P.; Muthukrishnaraj, A.; Thilakavathi, R.; Balasubramanian, N. In situ electro-catalytic treatment of a Reactive Golden Yellow HER synthetic dye effluent. J. Environ. Chem. Eng. 2013, 1, 2–8. [Google Scholar] [CrossRef]
- Daâssi, D.; Zouari-Mechichi, H.; Prieto, A.; Martínez, M.J.; Nasri, M.; Mechichi, T. Purification and biochemical characterization of a new alkali-stable laccase from Trametes sp. isolated in Tunisia: Role of the enzyme in olive mill waste water treatment. World J. Microbiol. Biotech. 2013, 29, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Torres-Salas, P.; Mate, D.M.; Ghazi, I.; Plou, F.J.; Ballesteros, A.O.; Alcalde, M. Widening the pH activity profile of a fungal laccase by directed evolution. ChemBioChem 2013, 14, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.; He, F.; Zhang, X.; Yang, Y. Characterization of a yeast recombinant laccase rLAC-EN3-1 and its application in decolorizing synthetic dye with the coexistence of metal ions and organic solvents. Biochem. Eng. J. 2015, 93, 63–72. [Google Scholar] [CrossRef]
- Ma, L.; Zhuo, R.; Liu, H.; Yu, D.; Jiang, M.; Zhang, X.; Yang, Y. Efficient decolorization and detoxification of the sulfonated azo dye Reactive Orange 16 and simulated textile wastewater containing Reactive Orange 16 by the white-rot fungus Ganoderma sp. En3 isolated from the forest of Tzu-chin Mountain in China. Biochem. Eng. J. 2014, 82, 1–9. [Google Scholar] [CrossRef]
- Myasoedova, N.M.; Gasanov, N.B.; Chernykh, A.M.; Kolomytseva, M.P.; Golovleva, L.A. Selective regulation of laccase isoform production by the Lentinus strigosus 1566 fungus. Appl. Biochem. Microbiol. 2015, 51, 222–229. [Google Scholar] [CrossRef]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, U.; Frömmel, S.; Bley, T.; Müller, M.; Frankenfeld, K.; Miethe, P. Solid-state fermentation of lignocellulotic materials for the production of enzymes by the white-rot fungus Trametes hirsuta in a modular bioreactor. Eng. Life Sci. 2011, 11, 395–401. [Google Scholar] [CrossRef]
- Tišma, M.; Žnidaršič-Plazl, P.; Vasić-Rački, Đ.; Zelić, B. Optimization of laccase production by Trametes versicolor cultivated on industrial waste. Appl. Biochem. Biotechnol. 2012, 166, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.; Ma, L.; Fan, F.; Gong, Y.; Wan, X.; Jiang, M.; Zhang, X.; Yang, Y. Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp. En3 and cloning and functional analysis of its laccase gene. J. Hazard. Mater. 2011, 192, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Parenti, A.; Muguerza, E.; Iroz, A.R.; Omarini, A.; Conde, E.; Alfaro, E.; Castanera, R.; Santoyo, F.; Ramírez, L.; Pisabarro, A.G. Induction of laccase activity in the white rot fungus Pleurotus ostreatus using water polluted with wheat straw extracts. Bioresour. Technol. 2013, 133, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Passarini, M.R.Z.; Ottoni, C.A.; Santos, C.; Lima, N.; Sette, L.D. Induction, expression and characterisation of laccase genes from the marine-derived fungal strains Nigrospora sp. CBMAI 1328 and Arthopyrenia sp. CBMAI 1330. AMB Express 2015, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Couto, S.; Rodrígues, A.; Paterson, R.R.M.; Lima, N.; Teixeira, J.A. Laccase activity from the fungus Trametes hirsuta using an air-lift bioreactor. Lett. Appl. Microbiol. 2006, 42, 612–616. [Google Scholar]
- Baccar, R.; Blánquez, P.; Bouzida, J.; Feki, M.; Attiya, H.; Sarrà, M. Decolorization of a tannery dye: From fungal screening to bioreactor application. Biochem. Eng. J. 2011, 56, 184–189. [Google Scholar] [CrossRef]
- Pocedič, J.; Knotec, O.; Šíma, J.; Hasal, P. Rotating biological contactor and its application for decolorization of textile dyes by Irpex lacteus. Chem. Eng. Trans. 2010, 20, 67–72. [Google Scholar]
- Raeder, U.; Broda, P. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1985, 1, 17–20. [Google Scholar] [CrossRef]
- Cassland, P.; Jönsson, L.J. Characterization of a gene encoding Trametes versicolor laccase A and improved heterologous expression in Saccharomyces cerevisiae by decreased cultivation temperature. Appl. Microbiol. Biotechnol. 1999, 52, 393–400. [Google Scholar] [CrossRef] [PubMed]
- BLAST—Basic Local Alignment Search Tool. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 7 June 2016).
- ClustalW2. Available online: www.ebi.ac.uk/Tools/msa/clustalw2/ (accessed on 7 June 2016).
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Imagej-1-44f. Available online: http://www.macosxfreeware.com/imagej-1-44f-scriptable-java-app-for-scientific-image-processinganalysis/ (accessed on 7 June 2016).
- Martins, M.A.M.; Lima, N.; Silvestre, A.J.D.; Queiroz, M.J. Comparative studies of fungal degradation of single or mixed bioaccessible reactive azo dyes. Chemosphere 2003, 52, 967–973. [Google Scholar] [CrossRef]
- Kanwal, H.K.; Reddy, M.S. Effect of carbon, nitrogen sources and inducers on ligninolytic enzyme production by Morchella crassipes. World J. Microbiol. Biotechnol. 2011, 27, 687–691. [Google Scholar] [CrossRef]
- Kumar, V.V.; Sathyaselvabala, V.; Premkumar, M.P.; Vidyadevi, T.; Sivanesan, S. Biochemical characterization of three phase partitioned laccase and its application in decolorization and degradation of synthetic dyes. J. Mol. Catal. B Enzym. 2012, 74, 63–72. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Metreveli, E.; Elisashvili, V. Modulation of Cerrena unicolor laccase and manganese peroxidase production. SpringerPlus 2014, 3, 463. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, H.L.; Liu, G.S.; Li, X.; Yao, J.M. The effect of carbon source succession on laccase activity in the co-culture process of Ganoderma lucidum and a yeast. Enzyme Microb. Technol. 2011, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pakshirajan, K.; Kheria, S. Continuous treatment of coloured industry wastewater using immobilized Phanerochaete chrysosporium in a rotating biological contactor reactor. J. Environ. Manag. 2012, 101, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Borchert, M.; Libra, J.A. Decolorization of reactive dyes by the white rot fungus Trametes versicolor in sequencing batch reactors. Biotechnol. Bioeng. 2001, 75, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Niebisch, C.H.; Malinowski, A.K.; Schadeck, R.; Mitchell, D.A.; Kava-Cordeiro, V.; Paba, J. Decolorization and biodegradation of reactive blue 220 textile dye by Lentinus crinitus extracellular extract. J. Hazard. Mater. 2010, 180, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Zouari-Mechichi, H.; Mechichi, T.; Dhouib, A.; Sayadi, S.; Martínez, A.T.; Martínez, M.J. Laccase purification and characterization from, Trametes trogii isolated in, Tunisia: Decolorization of textile dyes by the purified enzyme. Enzyme Microb. Technol. 2006, 39, 141–148. [Google Scholar] [CrossRef]
- Michniewicz, A.; Ullrich, R.; Ledakowicz, S.; Hofrichter, M. The white-rot fungus, Cerrena unicolor strain 137 produces two laccase isoforms with different physicochemical and catalytic properties. Appl. Microbiol. Biotechnol. 2006, 69, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Ye, M.; Lu, Y.; Zhang, X.; Li, G. Improving the decolorization for textile dyes of a metagenome-derived alkaline laccase by directed evolution. Appl. Microbiol. Biotechnol. 2011, 91, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Andriani, A.; Tachibana, S.; Itoh, K. Effects of saline-alkaline stress on benzo[a]pyrene biotransformation and ligninolytic enzyme expression by, Bjerkandera adusta, SM46. World J. Microbiol. Biotechnol. 2016, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.B.; Song, C.M.; Zhang, N.; Zhou, W.; Xu, C.W.; Zhou, L.X.; Zhao, H.; Cai, Y.J.; Liao, X.R. Overexpression, characterization, and dye-decolorizing ability of a thermostable, pH-stable, and organic solvent-tolerant laccase from, Bacillus pumilus, W3. J. Mol. Catal. B Enzym. 2014, 101, 1–6. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottoni, C.; Simões, M.F.; Fernandes, S.; Santos, C.R.; Lima, N. High Laccase Expression by Trametes versicolor in a Simulated Textile Effluent with Different Carbon Sources and PHs. Int. J. Environ. Res. Public Health 2016, 13, 778. https://doi.org/10.3390/ijerph13080778
Ottoni C, Simões MF, Fernandes S, Santos CR, Lima N. High Laccase Expression by Trametes versicolor in a Simulated Textile Effluent with Different Carbon Sources and PHs. International Journal of Environmental Research and Public Health. 2016; 13(8):778. https://doi.org/10.3390/ijerph13080778
Chicago/Turabian StyleOttoni, Cristiane, Marta F. Simões, Sara Fernandes, Cledir R. Santos, and Nelson Lima. 2016. "High Laccase Expression by Trametes versicolor in a Simulated Textile Effluent with Different Carbon Sources and PHs" International Journal of Environmental Research and Public Health 13, no. 8: 778. https://doi.org/10.3390/ijerph13080778
APA StyleOttoni, C., Simões, M. F., Fernandes, S., Santos, C. R., & Lima, N. (2016). High Laccase Expression by Trametes versicolor in a Simulated Textile Effluent with Different Carbon Sources and PHs. International Journal of Environmental Research and Public Health, 13(8), 778. https://doi.org/10.3390/ijerph13080778







