Expression of Hepcidin and Ferroportin in the Placenta, and Ferritin and Transferrin Receptor 1 Levels in Maternal and Umbilical Cord Blood in Pregnant Women with and without Gestational Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement and Collection of Samples
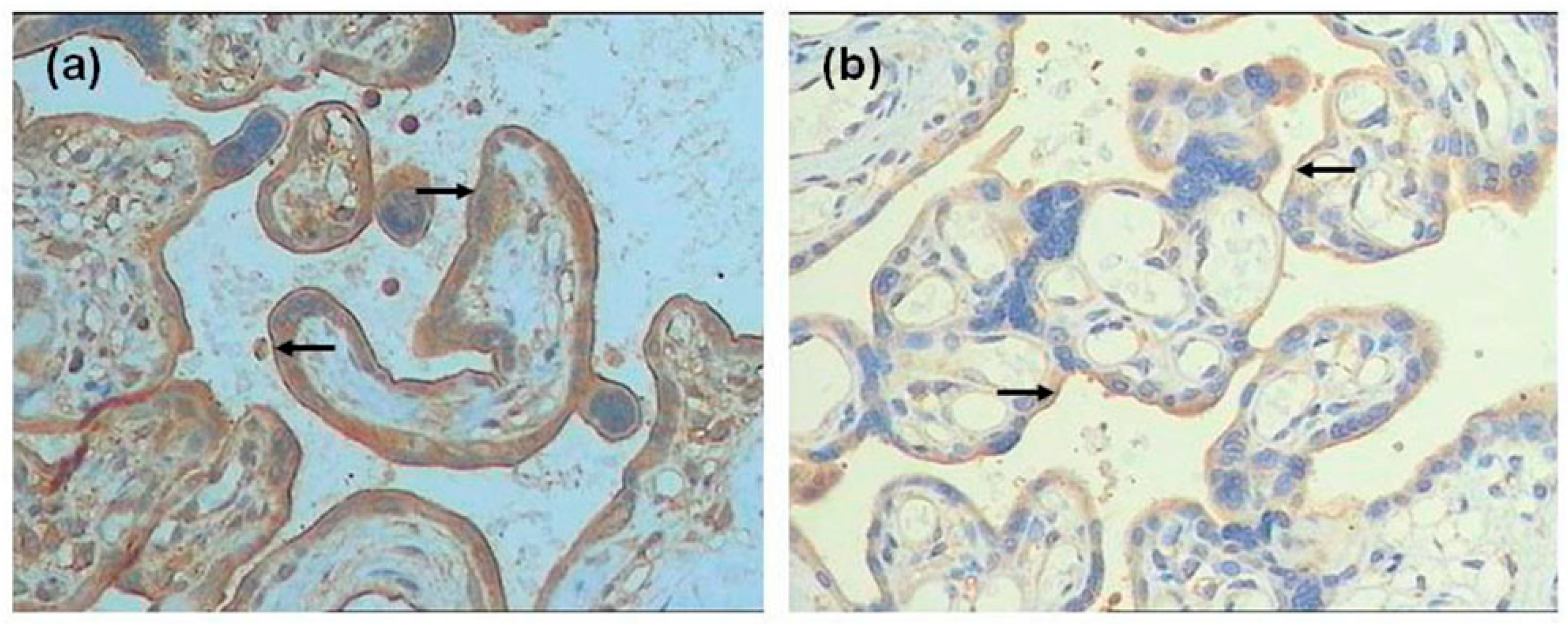
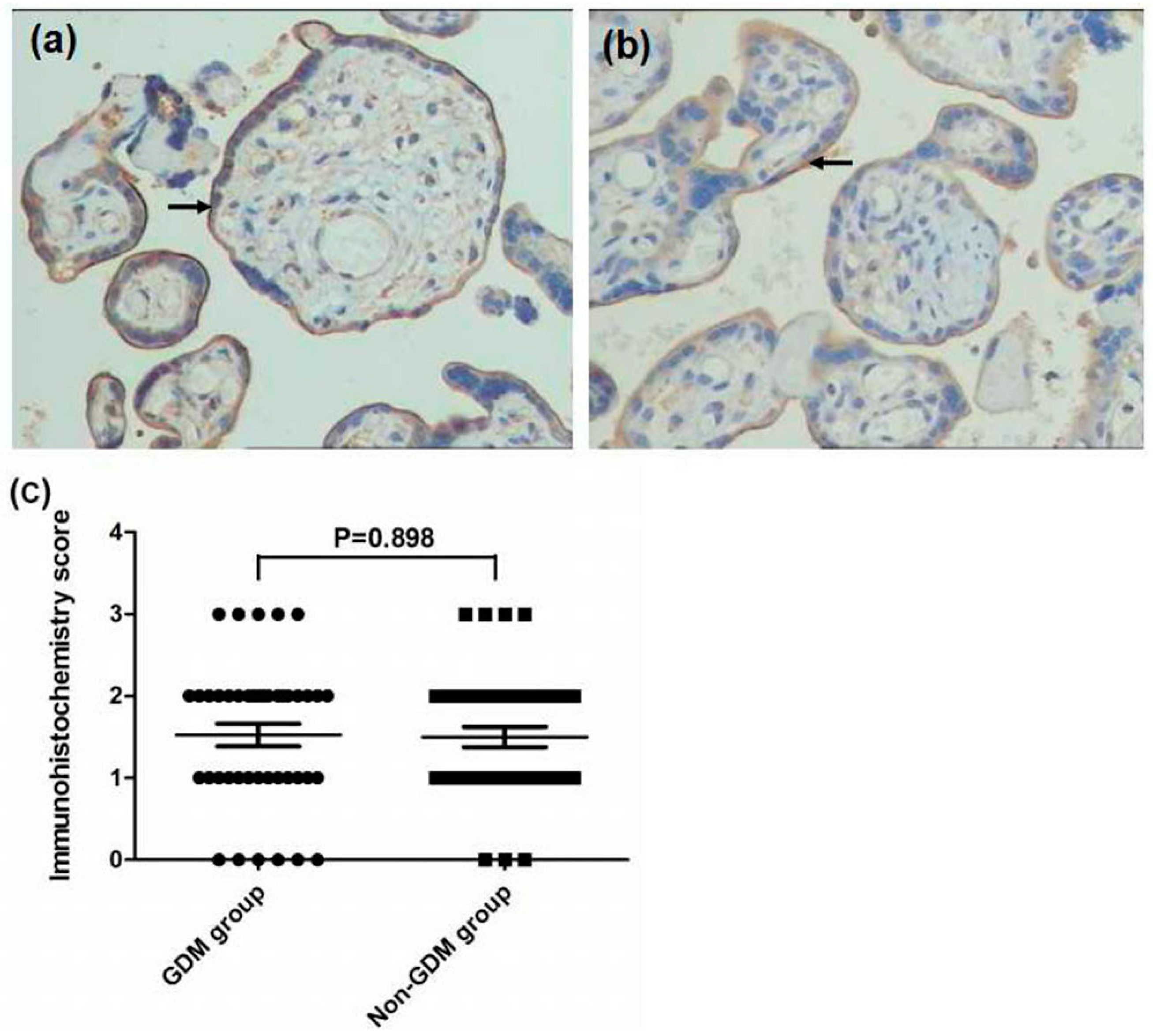
2.3. Immunohistochemistry
2.4. Immunohistochemical Evaluation
2.5. Statistical Analysis
3. Results
3.1. Sample Clinical Characteristics
3.2. Maternal and Cord Blood Iron Metabolism Biochemistry
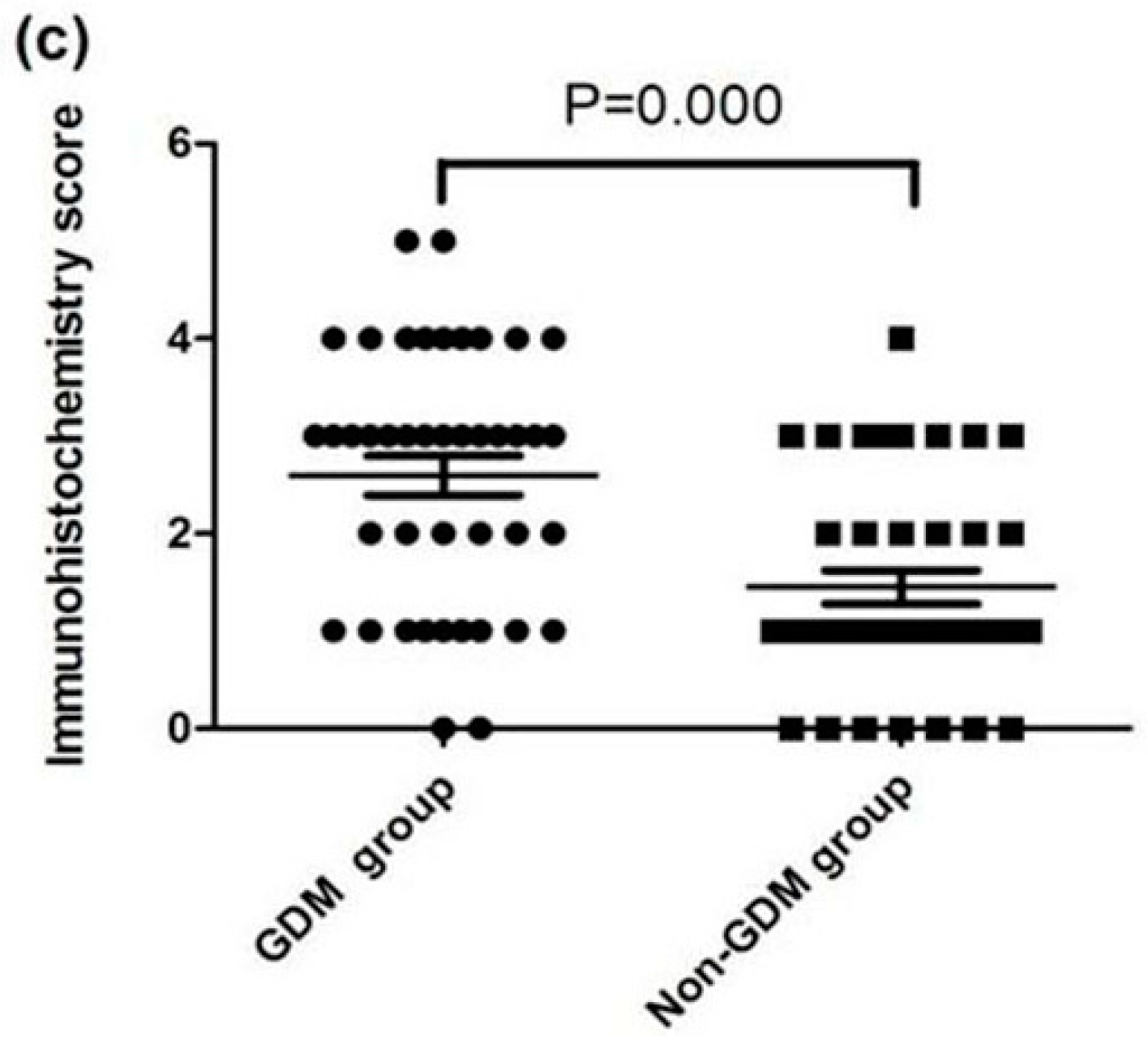
3.3. Expression of FPN, Hep, and TfR1 in the Placenta
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GDM | Gestational Diabetes |
| STfR | Soluble transferrin receptor (sTfR) |
| SF | Serum ferritin |
| FPN | Ferroportin |
| NGT | Normal glucose tolerance |
| STB | Syncytiotrophoblast |
| HCT | Hematocrit |
| OGTT | Oral glucose tolerance test |
| HEP | Hepcidin |
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar]
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004, 27 (Suppl. 1), 88–90. [Google Scholar]
- Zhang, F.; Dong, L.; Zhang, C.P.; Li, B.; Wen, J.; Gao, W.; Sun, S.; Lv, F.; Tian, H.; Tuomilehto, J.; et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabetic Med. 2011, 28, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Ziaee, A.; Feizi, A.; Mousavinasab, N.; Anjomshoaa, A.; Mokhtari, P. Serum ferritin concentration in gestational diabetes mellitus and risk of subsequent development of early postpartum diabetes mellitus. Diabetes Metab. Syndr. Obes. 2010, 3, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Soubasi, V.; Petridou, S.; Sarafidis, K.; Tsantali, C.; Diamanti, E.; Buonocore, G.; Drossou-Agakidou, V. Association of increased maternal ferritin levels with gestational diabetes and intra-uterine growth retardation. Diabetes Metab. 2010, 36, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Koga, M. Indicators of glycemic control in patients with gestational diabetes mellitus and pregnant women with diabetes mellitus. World J. Diabetes 2015, 6, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Stotland, N.E.; Caughey, A.B.; Breed, E.M.; Escobar, G.J. Risk factors and obstetric complications associated with macrosomia. Int. J. Gynecol. Obstet. 2004, 87, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Carrapato, M.R.G. The offspring of gestational diabetes. J. Perinat. Med. 2003, 31, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Khatun, N.; Latif, S.A.; Uddin, M.M. Infant outcomes of gestational diabetes mellitus. Mymensingh Med. J. 2005, 14, 29–31. [Google Scholar] [PubMed]
- Wright, C.S.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Taveras, E.M.; Gillman, M.W.; Oken, E. Intrauterine Exposure to Gestational Diabetes, Child Adiposity, and Blood Pressure. Am. J. Hypertens. 2009, 22, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Dkv, P. Iron Levels Increased in Serum from Gestational Diabetes Mellitus Mothers in Coastal Area of Andhra Pradesh. J. Diabetes Metab. 2013, 4, 1–5. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; López-Bermejo, A.; Ricart, W. Iron Stores, Blood Donation, and Insulin Sensitivity and Secretion. Clin. Chem. 2005, 51, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Manson, J.E.; Meigs, J.B.; Ma, J.; Rifai, N.; Hu, F.B. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004, 291, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Zein, S.; Rachidi, S.; Awada, S.; Osman, M.; Al-Hajje, A.; Shami, N.; Sharara, I.; Cheikh-Ali, K.; Salameh, P.; Hininger-Favier, I. High iron level in early pregnancy increased glucose intolerance. J. Trace Elem. Med. Biol. 2015, 30, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Edison, E.S.; Bajel, A.; Chandy, M. Iron homeostasis: New players, newer insights. Eur. J. Haematol. 2008, 81, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A.; Dannenberg, A.J.; Rush, D.; Woerner, B.M.; Khan, K.N.; Masferrer, J.; Koki, A.T. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 2000, 89, 2637–2645. [Google Scholar] [CrossRef]
- Sun, L.; Franco, O.H.; Hu, F.B.; Cai, L.; Yu, Z.; Li, H.; Ye, X.; Qi, Q.; Wang, J.; Pan, A.; et al. Ferritin Concentrations, Metabolic Syndrome, and Type 2 Diabetes in Middle-Aged and Elderly Chinese. J. Clin. Endocrinol. Metab. 2008, 93, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Jehn, M.L.; Guallar, E.; Clark, J.M.; Couper, D.; Duncan, B.B.; Ballantyne, C.M.; Hoogeveen, R.C.; Harris, Z.L.; Pankow, J.S. A Prospective Study of Plasma Ferritin Level and Incident Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol. 2007, 165, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Lao, T.T.; Chan, L.Y.; Tam, K.F.; Ho, L.F. Maternal hemoglobin and risk of gestational diabetes mellitus in Chinese women. Obstet. Gynecol. 2002, 99, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.N.; Basirat, Z.; Omidvar, S.; Sharbatdaran, M.; Tilaki, K.H.; Pouramir, M. Comparison of the serum iron, ferritin levels and total iron-binding capacity between pregnant women with and without gestational diabetes. J. Nat. Sci. Biol. Med. 2013, 4, 302–305. [Google Scholar] [PubMed]
- Helin, A.; Kinnunen, T.I.; Raitanen, J.; Ahonen, S.; Virtanen, S.M.; Luoto, R. Iron intake, haemoglobin and risk of gestational diabetes: A prospective cohort study. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.; Ellinger, I. Endocytic and Transcytotic Processes in Villous Syncytiotrophoblast: Role in Nutrient Transport to the Human Fetus. Traffic 2004, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Berti, C.; Mando, C.; Parisi, F. Placental iron transport and maternal absorption. Ann. Nutr. Metab. 2011, 59, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Leibold, E.A.; Harris, Z.L.; Wobken, J.D.; Clarke, S.; Zumbrennen, K.B.; Elisenstein, R.S.; Georgieff, M.K. Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R894–R901. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.O.; Zavaleta, N.; Abrams, S.A.; Caulfield, L.E. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am. J. Clin. Nutr. 2003, 77, 924–930. [Google Scholar] [PubMed]
- Afkhami-Ardekani, M.; Rashidi, M. Iron status in women with and without gestational diabetes mellitus. J. Diabetes Its Complicat. 2009, 23, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scholl, T.O.; Stein, T.P. Association of Elevated Serum Ferritin Levels and the Risk of Gestational Diabetes Mellitus in Pregnant Women: The Camden Study. Diabetes Care 2006, 29, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Agger, A.O.; Nielsen, O.J. Iron status markers and serum erythropoietin in 120 mothers and newborn infants: Effect of iron supplementation in normal pregnancy. Acta Obstet. Gynecol. Scand. 1994, 73, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta 2012, 1823, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Bastin, J.; Drakesmith, H.; Rees, M.; Sargent, I.; Townsend, A. Localisation of proteins of iron metabolism in the human placenta and liver. Br. J. Haematol. 2006, 134, 532–543. [Google Scholar] [CrossRef] [PubMed]

| Clinical Features | GDM (n = 42) | Non GDM (n = 40) | p Value |
|---|---|---|---|
| Maternal age (year) | 30.52 ± 3.81 | 29.39 ± 3.76 | 0.090 |
| BMI * | 27.10 ± 2.39 | 25.96 ± 2.07 | 0.032 |
| Newborn birth weight (kg) | 3.25 ± 0.39 | 3.10 ± 0.34 | 0.083 |
| Wk of gestation (week) | 38.71 ± 1.0 | 37.70 ± 2.86 | 0.071 |
| OGTT fasting (mg/dL) | |||
| 1-h * | 10.30 ± 1.11 | 8.92 ± 0.94 | 0.000 |
| 2-h * | 9.21 ± 1.22 | 7.71 ± 0.69 | 0.000 |
| Group | GDM (n = 42) | Control (n = 40) | p Value |
|---|---|---|---|
| Maternal Hb (g/dL) | 116.20 ± 7.0 | 117.76 ± 8.31 | 0.391 |
| Umbilical cord Hb (g/dL) | 158.14 ± 9.54 | 154.76 ± 11.51 | 0.182 |
| Maternal blood sTfR (nM) | 31.82 ± 11.51 | 22.80 ± 8.30 | 0.003 a |
| Cord blood sTfR (nM) | 34.63 ± 8.33 | 32.60 ± 6.00 | 0.364 |
| Maternal SF (ng/mL) | 21.71 (13.86–42.62) Δ | 30.90 (25.00–39.00) Δ | 0.118 |
| Umbilical cord SF (ng/mL) | 172.75 (112.08–192.03) Δ | 111.6 (68.29–150.95) Δ | 0.003 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, A.; Zhao, J.; Lu, M.; Gu, Y.; Zhu, Y.; Chen, D.; Fu, J. Expression of Hepcidin and Ferroportin in the Placenta, and Ferritin and Transferrin Receptor 1 Levels in Maternal and Umbilical Cord Blood in Pregnant Women with and without Gestational Diabetes. Int. J. Environ. Res. Public Health 2016, 13, 766. https://doi.org/10.3390/ijerph13080766
Yang A, Zhao J, Lu M, Gu Y, Zhu Y, Chen D, Fu J. Expression of Hepcidin and Ferroportin in the Placenta, and Ferritin and Transferrin Receptor 1 Levels in Maternal and Umbilical Cord Blood in Pregnant Women with and without Gestational Diabetes. International Journal of Environmental Research and Public Health. 2016; 13(8):766. https://doi.org/10.3390/ijerph13080766
Chicago/Turabian StyleYang, Anqiang, Jun Zhao, Minhua Lu, Ying Gu, Yunlong Zhu, Daozhen Chen, and Jinyan Fu. 2016. "Expression of Hepcidin and Ferroportin in the Placenta, and Ferritin and Transferrin Receptor 1 Levels in Maternal and Umbilical Cord Blood in Pregnant Women with and without Gestational Diabetes" International Journal of Environmental Research and Public Health 13, no. 8: 766. https://doi.org/10.3390/ijerph13080766





