Addressing Emerging Risks: Scientific and Regulatory Challenges Associated with Environmentally Persistent Free Radicals
Abstract
:1. Introduction
2. Epidemiological Studies of Hazardous Waste Incineration and Other Thermal Processes
3. The Case for Environmentally Persistent Free Radicals
4. Mechanisms for EPFR Formation
5. EPFR-Induced Production of Reaction Oxygen Species (ROS) and Their Potential Health Effects
6. Exposure Models of Toxicity—Challenges and Unresolved Questions
6.1. Particle Aggregation
6.2. Particle Storage
6.3. Use of Controls
6.4. Mixtures
7. EPFRs and the Regulatory Framework
7.1. Incineration of Hazardous Materials
7.2. Clean Air Act (CAA) Regulation of Particulate Matter
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CAA | Clean Air Act |
| EPFR | Environmentally persistent free radical |
| EPR | Electron paramagnetic resonance |
| DEP | Diesel exhaust particles |
| GEP | Gasoline exhaust particles |
| PE | Poly(ethylene) |
| PET | Poly (ethylene terephthalate) |
| PM | Particulate matter |
| PM10 | Particulate matter (diameter < 10 μm) |
| PM2.5 | Particulate matter (diameter < 2.5 μm) |
| PP | Poly(propylene) |
| PS | Poly(styrene) |
| PVC | Poly(vinylchloride) |
| ROS | Reactive oxygen species |
| TSP | Total suspended particulates |
References
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and Asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Kloog, I.; Nordio, F.; Zanobetti, A.; Coull, B.A.; Koutrakis, P.; Schwartz, J.D. Short term effects of particle exposure on hospital admissions in the mid-atlantic states: A population estimate. PLoS ONE 2014, 9, E88578. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Chen, L.C.; Gordon, T.; Ito, K.; Thurston, G.D. National Particle Component Toxicity (Npact) Initiative: Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components; Hei Publications: Cambridge, MA, USA, 2013; pp. 5–13. [Google Scholar]
- Nganai, S.; Lomnicki, S.M.; Dellinger, B. Formation of Pcdd/Fs from the Copper Oxide-Mediated Pyrolysis and Oxidation of 1,2-Dichlorobenzene. Environ. Sci. Technol. 2011, 45, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Nganai, S.; Dellinger, B.; Lomnicki, S. Pcdd/pcdf ratio in the precursor formation model over cuo surface. Environ. Sci. Technol. 2014, 48, 13864–13870. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, M.; Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Mechanisms for formation, chlorination, dechlorination and destruction of polychlorinated Dibenzo-P-Dioxins and dibenzofurans (Pcdd/Fs). Prog. Energy Combust. 2009, 35, 245–274. [Google Scholar] [CrossRef]
- Lomnicki, S.; Dellinger, B. A detailed mechanism of the surface-mediated formation of pcdd/f from the oxidation of 2-chlorophenol on cuo/silica surface. J. Phys. Chem. 2003, 107, 4387–4395. [Google Scholar] [CrossRef]
- Lomnicki, S.; Truong, H.; Vejerano, E.; Dellinger, B. Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ. Sci. Technol. 2008, 42, 4982–4988. [Google Scholar] [CrossRef] [PubMed]
- Vejerano, E.; Lomnicki, S.; Dellinger, B. Lifetime of combustion-generated environmentally persistent free radicals on zn(ii)o and other transition metal oxides. J. Environ. Monit. 2012, 14, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Gao, J.; Liu, C.; Dionysiou, D.D.; Wang, Y.; Zhou, D. Key role of persistent free radicals in hydrogen peroxide activation by biochar: Implications to organic contaminant degradation. Environ. Sci. Technol. 2014, 48, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Koshland, C.P.; Yano, J.; Yachandra, V.K.; Yu, I.T.; Lee, S.C.; Lucas, D. Carbon-centered free radicals in particulate matter emissions from wood and coal combustion. Energy Fuels 2009, 23, 2523–2526. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Bakeas, E.B.; Vlahogianni, T. Electron paramagnetic resonance study of the generation of reactive oxygen species catalyzed by transition metals and quinoid redox cycling by inhalable ambient particulate matter. Redox Rep. Commun. Free Radic. Res. 2005, 10, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Iliopoulos, N.; Gotsis, G.; Fiotakis, K. Persistent free radicals, heavy metals and pahs generated in particulate soot emissions and residue ash from controlled combustion of common types of plastic. J. Hazard. Mater. 2008, 156, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gehling, W.; Dellinger, B. Environmentally persistent free radicals and their lifetimes in PM2.5. Environ. Sci. Technol. 2013, 47, 8172–8178. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlahogianni, T.; Papadimitriou, V.; Pantikaki, V. Determination of selective quinones and quinoid radicals in airborne particulate matter and vehicular exhaust particles. Environ. Chem. 2006, 3, 118–123. [Google Scholar] [CrossRef]
- Pan, C.J.; Schmitz, D.A.; Cho, A.K.; Froines, J.; Fukuto, J.M. Inherent redox properties of diesel exhaust particles: Catalysis of the generation of reactive oxygen species by biological reductants. Toxicol. Sci. 2004, 81, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, A.L.; Cook, R.L.; Dellinger, B.; Lomnicki, S.M.; Donnelly, K.C.; Kelley, M.A.; Cosgriff, D. Assessment of environmentally persistent free radicals in soils and sediments from three superfund sites. Environ. Sci. Process. Impacts 2014, 16, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, A.L.; Gehling, W.; Lomnicki, S.; Cook, R.; Dellinger, B. Detection of environmentally persistent free radicals at a superfund wood treating site. Environ. Sci. Technol. 2011, 45, 6356–6365. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, B.; Pryor, W.A.; Cueto, R.; Squadrito, G.; Deutsch, W.A. The role of combustion-generated radicals in the toxicity of PM2.5. Proc. Combust. Inst. 2000, 28, 2675–2681. [Google Scholar] [CrossRef]
- Kiruri, L.W.; Dellinger, B.; Lomnicki, S. Tar balls from deep water horizon oil spill: Environmentally persistent free radicals (epfr) formation during crude weathering. Environ. Sci. Technol. 2013, 47, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Weinmayr, G.; Romeo, E.; De Sario, M.; Weiland, S.K.; Forastiere, F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: A systematic review and meta-analysis. Environ. Health Perspect. 2010, 118, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.A.; Demers, P.A.; Karr, C.J.; Koehoorn, M.; Lencar, C.; Tamburic, L.; Brauer, M. Effect of early life exposure to air pollution on development of childhood asthma. Environ. Health Perspect. 2010, 118, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.H.; Chen, T.; Liu, M.M.; Wang, D.; Ma, Y.N.; Ren, W.H.; Lee, Y.L.; Zhao, Y.D.; He, Q.C. Gender differences and effect of air pollution on asthma in children with and without allergic predisposition: Northeast chinese children health study. PLoS ONE 2011, 6, E22470. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.K.; Galanter, J.M.; Roth, L.A.; Oh, S.S.; Thakur, N.; Nguyen, E.A.; Thyne, S.; Farber, H.J.; Serebrisky, D.; Kumar, R.; et al. Early-life air pollution and asthma risk in minority children. The gala II and sage ii studies. Am. J. Respir. Crit. Care Med. 2013, 188, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.B.; Ljungman, P.L.; Wilker, E.H.; Gold, D.R.; Schwartz, J.D.; Koutrakis, P.; Washko, G.R.; O’connor, G.T.; Mittleman, M.A. Short-term exposure to air pollution and lung function in the framingham heart study. Am. J. Respir. Crit. Care Med. 2013, 188, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution—epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; De Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 european cohorts from the escape project. BMJ 2014, 348, F7412. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Burnett, R.T.; Kwong, J.C.; Villeneuve, P.J.; Goldberg, M.S.; Brook, R.D.; Van Donkelaar, A.; Jerrett, M.; Martin, R.V.; Kopp, A.; et al. Spatial association between ambient fine particulate matter and incident hypertension. Circulation 2014, 129, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Cass, G.R. Comments on sources, atmospheric levels and characterization of airborne particulate matter. Inhal. Toxicol. 1995, 7, 765–768. [Google Scholar] [CrossRef]
- Cass, G.R.; Hughes, L.A.; Bhave, P.; Kleeman, M.J.; Allen, J.O.; Salmon, L.G. The chemical composition of atmospheric ultrafine particles. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2000, 358, 2567–2580. [Google Scholar] [CrossRef]
- Taylor, E.T.; Nakai, S. Prevalence of acute respiratory infections in women and children in western sierra leone due to smoke from wood and charcoal stoves. Int. J. Environ. Res. Public Health 2012, 9, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zou, Y.; Li, X.; Chen, S.; Zhao, Z.; He, F.; Zou, W.; Luo, Q.; Li, W.; Pan, Y.; et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: A 9-year prospective cohort study. PLoS Med. 2014, 11, E1001621. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, D.P.; Gotto, A.M., Jr. Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am. J. Pathol. 2013, 182, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Holvoet, P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J. Cell. Mol. Med. 2010, 14, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.E.; Garshick, E.; Smith, T.J.; Davis, M.E.; Laden, F. Ischaemic heart disease mortality and years of work in trucking industry workers. Occup. Environ. Med. 2013, 70, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos Paulino, S.; Oliveira, R.L.; Loyola, J.; Minho, A.S.; Arbilla, G.; Quiterio, S.L.; Escaleira, V. Trace metals in pm10 and PM2.5 samples collected in a highly industrialized chemical/petrochemical area and its urbanized surroundings. Bull. Environ. Contam. Toxicol. 2014, 92, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Liati, A.; Schreiber, D.; Dimopoulos Eggenschwiler, P.; Arroyo Rojas Dasilva, Y. Metal Particle emissions in the exhaust stream of diesel engines: An electron microscope study. Environ. Sci. Technol. 2013, 47, 14495–14501. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, A.M.; Hart, J.E.; Cavallari, J.M.; Smith, T.J.; Dockery, D.W.; Coull, B.A.; Garshick, E.; Laden, F. Traffic-related exposures and biomarkers of systemic inflammation, endothelial activation and oxidative stress: A panel study in the us trucking industry. Environ. Health A Glob. Access Sci. Source 2013, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Bertazzi, P.A.; Cantone, L.; Pignatelli, P.; Angelici, L.; Bollati, V.; Bonzini, M.; Carugno, M.; Mannucci, P.M.; Violi, F. Does enhancement of oxidative stress markers mediate health effects of ambient air particles? Antioxid. Redox Signal. 2014, 21, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liberda, E.N.; Qu, S.; Guo, X.; Li, X.; Zhang, J.; Meng, J.; Yan, B.; Li, N.; Zhong, M.; et al. The role of metal components in the cardiovascular effects of PM2.5. PLoS ONE 2013, 8, E83782. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Beelen, R.; Stafoggia, M.; Raaschou-Nielsen, O.; Andersen, Z.J.; Hoffmann, B.; Fischer, P.; Houthuijs, D.; Nieuwenhuijsen, M.; Weinmayr, G.; et al. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 european cohorts: Results from the escape and transphorm projects. Environ. Int. 2014, 66, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Harris, M.; Sie, L.; Malig, B.; Broadwin, R.; Green, R. Effects of fine particulate matter and its constituents on low birth weight among full-term infants in California. Environ. Res. 2014, 128, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, B.; Lomnicki, S.; Khachatryan, L.; Maskos, Z.; Hall, R.W.; Adounkpe, J.; Mcferrin, C.; Truong, H. Formation and stabilization of persistent free radicals. Proc. Combust. Inst. Int. Symp. Combust. 2007, 31, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Uebersfeld, J.; Etienne, A.; Combrisson, J. Paramagnetic resonance, a new property of coal-like materials. Nature 1954, 174, 614. [Google Scholar] [CrossRef]
- Lyons, M.J.; Gibson, J.F.; Ingram, D.J. Free-radicals produced in cigarette smoke. Nature 1958, 181, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Church, D.F.; Pryor, W.A. Free-Radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Y.; Stone, K.; Pryor, W.A. Detection of free radicals in aqueous extracts of cigarette tar by electron spin resonance. Free Radic. Biol. Med. 1995, 19, 161–167. [Google Scholar] [CrossRef]
- Stone, K.; Bermudez, E.; Zang, L.Y.; Carter, K.M.; Queenan, K.E.; Pryor, W.A. The ESR properties, DNA nicking, and dna association of aged solutions of catechol versus aqueous extracts of tar from cigarette smoke. Arch. Biochem. Biophys. 1995, 319, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.M.; Chedekel, M.R.; Risby, T.H. Electron paramagnetic resonance spectrometry of diesel particulate matter. Environ. Int. 1982, 7, 325–329. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahoyianni, T.; Fiotakis, K. Comparative study of the formation of oxidative damage marker 8-hydroxy-2′-deoxyguanosine (8-ohdg) adduct from the nucleoside 2′-deoxyguanosine by transition metals and suspensions of particulate matter in relation to metal content and redox reactivity. Free Radic. Res. 2005, 39, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Cueto, R.; Dellinger, B.; Pryor, W.A. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic. Biol. Med. 2001, 31, 1132–1138. [Google Scholar] [CrossRef]
- Dellinger, B.; Taylor, P.H.; Tirey, D.A.; Lee, C.C. Pathways of formation of chlorinated pics from the thermal-degradation of simple chlorinated hydrocarbons. J. Hazard. Mater. 1989, 22, 175–186. [Google Scholar] [CrossRef]
- Wehrmeier, A.; Lenoir, D.; Sidhu, S.S.; Taylor, P.H.; Rubey, W.A.; Kettrup, A.; Dellinger, B. Role of copper species in chlorination and condensation reactions of acetylene. Environ. Sci. Technol. 1998, 32, 2741–2748. [Google Scholar] [CrossRef]
- Taylor, P.H.; Dellinger, B. Pyrolysis and molecular growth of chlorinated hydrocarbons. J. Anal. Appl. Pyrol. 1999, 49, 9–29. [Google Scholar] [CrossRef]
- Hu, C.-W.; Chao, M.-R.; Wu, K.-Y.; Chang-Chen, G.-P.; Lee, W.-J.; Chang, L.W.; Lee, W.-S. Characterization of airborne particulate metals in the surroundings of a municipal waste incinerator in Taiwan. Atmos. Environ. 2003, 37, 2852–2582. [Google Scholar] [CrossRef]
- Oh, J.F.; Kim, E.J.; Chang, Y.S. Distribution of pcdd/fs with atmospheric particle size. Organohalogen Compd. 2001, 52, 487–490. [Google Scholar]
- Pio, C.; Alves, C.; Carvalho, A.; Santos, C. Size distribution characteristics of organic species in atmospheric particulate matter from finnish and german rural sites with variable anthropogenic influence. Environ. Eng. Sci. 2006, 23, 933–941. [Google Scholar] [CrossRef]
- Alderman, S.L.; Farquar, G.R.; Poliakoff, E.D.; Dellinger, B. An infrared and x-ray spectroscopic study of the reactions of 2-chlorophenol, 1,2-dichlorobenzene, and chlorobenzene with model cuo/silica fly ash surfaces. Environ. Sci. Technol. 2005, 39, 7396–7401. [Google Scholar] [CrossRef] [PubMed]
- Sussan, T.E.; Gajghate, S.; Thimmulappa, R.K.; Ma, J.; Kim, J.H.; Sudini, K.; Consolini, N.; Cormier, S.A.; Lomnicki, S.; Hasan, F.; et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS ONE 2015, 10, E0116861. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wagner, B.A.; Lehmler, H.J.; Buettner, G.R. Semiquinone radicals from oxygenated polychlorinated biphenyls: Electron paramagnetic resonance studies. Chem. Res. Toxicol. 2008, 21, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, L.; Dellinger, B. Environmentally persistent free radicals (epfrs)-2. are free hydroxyl radicals generated in aqueous solutions? Environ. Sci. Technol. 2011, 45, 9232–9239. [Google Scholar] [CrossRef] [PubMed]
- Sedman, R.M.; Polisini, J.M.; Esparza, J.R. The evaluation of stack metal emissions from hazardous waste incinerators: Assessing human exposure through noninhalation pathways. Environ. Health Perspect. 1994, 102 (Suppl. 2), 105–112. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, S.; Lomnicki, S.; Mcavey, K.M.; Cole, R.B.; Dellinger, B.; Cormier, S.A. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part. Fibre Toxicol. 2009, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, B.; Pryor, W.A.; Cueto, R.; Squadrito, G.L.; Hegde, V.; Deutsch, W.A. Role of free radicals in the toxicity of airborne fine particulate matter. Chem. Res. Toxicol. 2001, 14, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.A.; Hebert, V.Y.; Thibeaux, T.M.; Orchard, M.A.; Hasan, F.; Cormier, S.A.; Thevenot, P.T.; Lomnicki, S.M.; Varner, K.J.; Dellinger, B.; et al. Model combustion-generated particulate matter containing persistent free radicals redox cycle to produce reactive oxygen species. Chem. Res. Toxicol. 2013, 26, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Alaghmand, M.; Blough, N.V. Source-dependent variation in hydroxyl radical production by airborne particulate matter. Environ. Sci. Technol. 2007, 41, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Distefano, E.; Eiguren-Fernandez, A.; Delfino, R.J.; Sioutas, C.; Froines, J.R.; Cho, A.K. Determination of metal-based hydroxyl radical generating capacity of ambient and diesel exhaust particles. Inhal. Toxicol. 2009, 21, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.; Maidt, L.; Poyer, L. Superoxide dismutase and fenton chemistry. reaction of ferric-edta complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(ii). Biochem. J. 1990, 269, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Salika, A.; Theodoropoulou, A. Generation of hydroxyl radicals by urban suspended particulate air matter. the role of iron ions. Atmos. Environ. 2000, 34, 2379–2386. [Google Scholar] [CrossRef]
- Gehling, W.; Khachatryan, L.; Dellinger, B. Hydroxyl radical generation from environmentally persistent free radicals (epfrs) in PM2.5. Environ. Sci. Technol. 2014, 48, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, L.; Vejerano, E.; Lomnicki, S.; Dellinger, B. Environmentally persistent free radicals (epfrs). 1. generation of reactive oxygen species in aqueous solutions. Environ. Sci. Technol. 2011, 45, 8559–8566. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Charrier, J.G.; Kodani, S.D.; Vogel, C.F.; Kado, S.Y.; Anderson, D.S.; Anastasio, C.; Van Winkle, L.S. Combustion-derived flame generated ultrafine soot generates reactive oxygen species and activates NRF2 antioxidants differently in neonatal and adult rat lungs. Part. Fibre Toxicol. 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Visalli, G.; Munao, F.; Baluce, B.; La Maestra, S.; Primerano, P.; Corigliano, F.; De Flora, S. Oxidative damage in human epithelial alveolar cells exposed in vitro to oil fly ash transition metals. Int. J. Hyg. Environ. Health 2009, 212, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.T.; Saravia, J.; Jin, N.; Giaimo, J.D.; Chustz, R.E.; Mahne, S.; Kelley, M.A.; Hebert, V.Y.; Dellinger, B.; Dugas, T.R.; et al. Radical-containing ultrafine particulate matter initiates epithelial-to-mesenchymal transitions in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2013, 48, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Thevenot, P.; Saravia, J.; Ahlert, T.; Cormier, S.A. Radical-containing particles activate dendritic cells and enhance th17 inflammation in a mouse model of Asthma. Am. J. Respir. Cell Mol. Biol. 2011, 45, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, S.; Saravia, J.; Thevenot, P.; Ahlert, T.; Lominiki, S.; Dellinger, B.; Cormier, S.A. Environmentally persistent free radicals induce airway hyperresponsiveness in neonatal rat lungs. Part. Fiber Toxicol. 2011, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Saravia, J.; You, D.; Shrestha, B.; Jaligama, S.; Hebert, V.Y.; Dugas, T.R.; Cormier, S.A. Exposure to combustion generated environmentally persistent free radicals enhances severity of influenza virus infection. Part. Fiber Toxicol. 2014, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Saravia, J.; You, D.; Thevenot, P.; Lee, G.I.; Shrestha, B.; Lomnicki, S.; Cormier, S.A. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal Immunol. 2014, 7, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Lord, K.; Moll, D.; Lindsey, J.K.; Mahne, S.; Raman, G.; Dugas, T.; Cormier, S.; Troxlair, D.; Lomnicki, S.; Dellinger, B.; et al. Environmentally persistent free radicals decrease cardiac function before and after ischemia/reperfusion injury in vivo. J. Recept. Signal Transduct. Res. 2011, 31, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Burn, B.R.; Varner, K.J. Environmentally persistent free radicals compromise left ventricular function during ischemia/reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H998–H1006. [Google Scholar] [CrossRef] [PubMed]
- Mahne, S.; Chuang, G.C.; Pankey, E.; Kiruri, L.; Kadowitz, P.J.; Dellinger, B.; Varner, K.J. Environmentally persistent free radicals decrease cardiac function and increase pulmonary artery pressure. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1135–H1142. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Current in vitro methods in nanoparticle risk assessment: Limitations and challenges. Eur. J. Pharm. Biopharm. 2009, 72, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Metal ion-catalyzed oxidation of proteins: Biochemical mechanism and biological consequences. Free Radic. Biol. Med. 1990, 9, 315–325. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 1st ed.; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Shang, Y.; Zhu, T.; Lenz, A.G.; Frankenberger, B.; Tian, F.; Chen, C.; Stoeger, T. Reduced in vitro toxicity of fine particulate matter collected during the 2008 summer olympic games in beijing: The roles of chemical and biological components. Toxicol. In Vitro 2013, 27, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kwon, J.W.; Seo, J.H.; Kim, H.B.; Lee, S.Y.; Park, K.S.; Yu, J.; Kim, H.C.; Leem, J.H.; Sakong, J.; et al. Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Ann. Allergy Asthma Immunol. 2011, 107, 214–219, E211. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.; Lomnicki, S.; Dellinger, B. Potential for misidentification of environmentally persistent free radicals as molecular pollutants in particulate matter. Environ. Sci. Technol. 2010, 44, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Aspects of Air Pollution with Particulate Matter, Ozone and Nitrogen Dioxide; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- World Health Organization. Health Aspects of Air Pollution; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Kingdon, J. Agendas, Alternatives, And Public Policies, 2nd ed.; Harper-Collins Press: New York, NY, USA, 1995. [Google Scholar]
- Office Of Technology Assessment. Environmental Policy Tools: A User’s Guide; Ota: Washington, DC, USA, 1995. [Google Scholar]
- Resvesz, R.L.; Stevins, R.N. Environmental Law and Policy; Harvard University, John F. Kennedy School of Government: Cambridge, MA, USA, 2004. [Google Scholar]
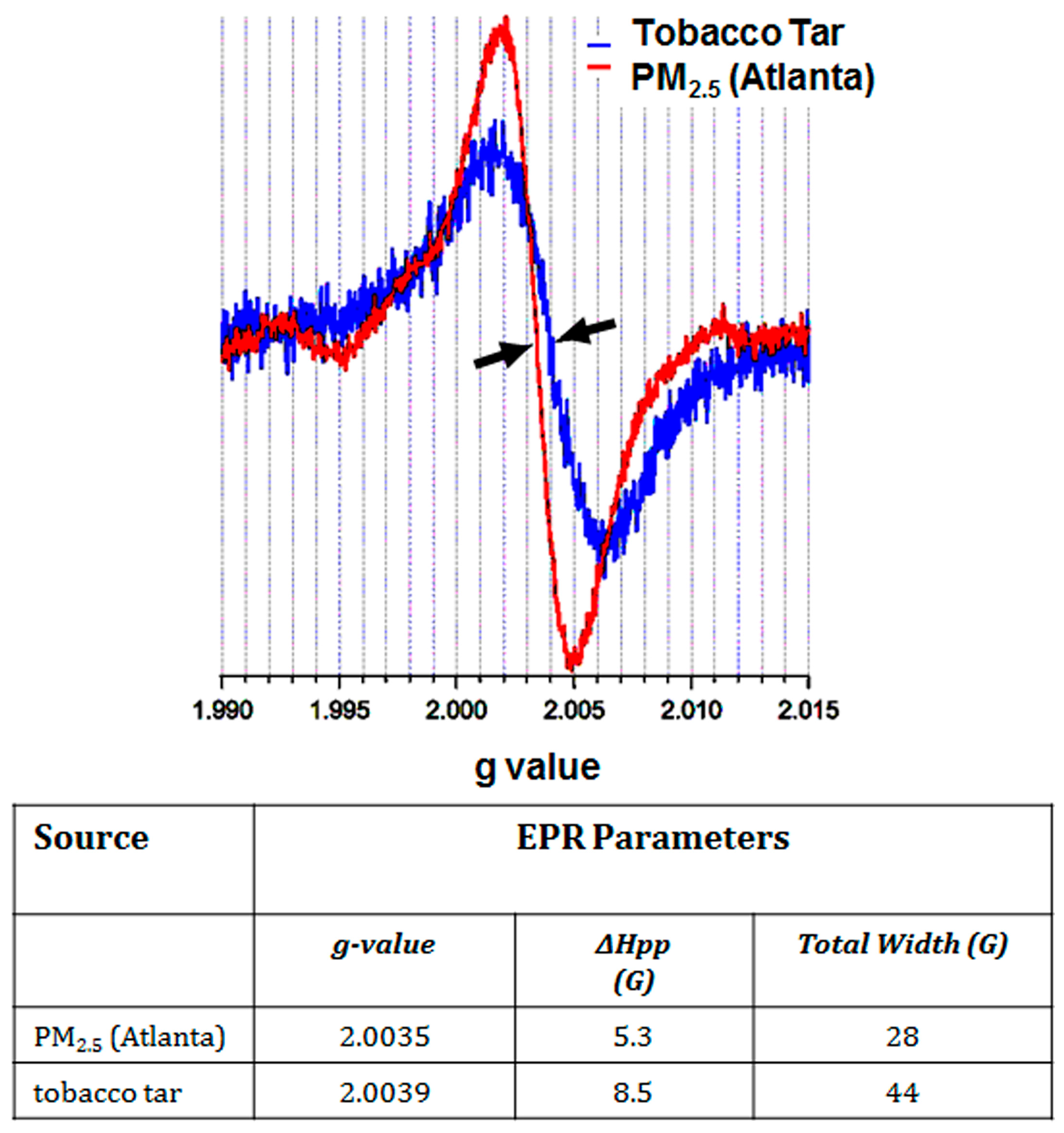
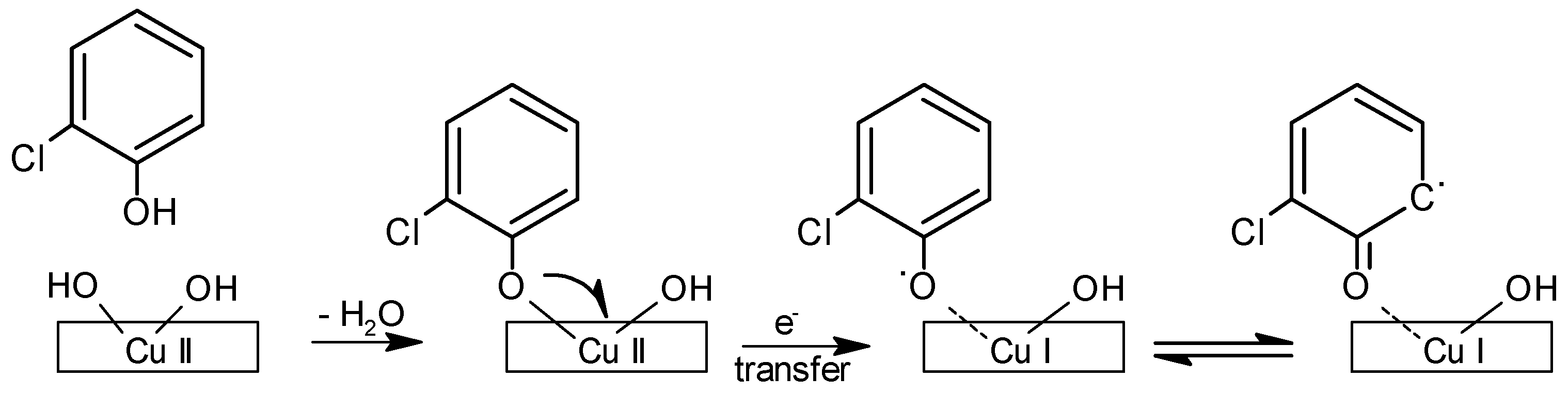
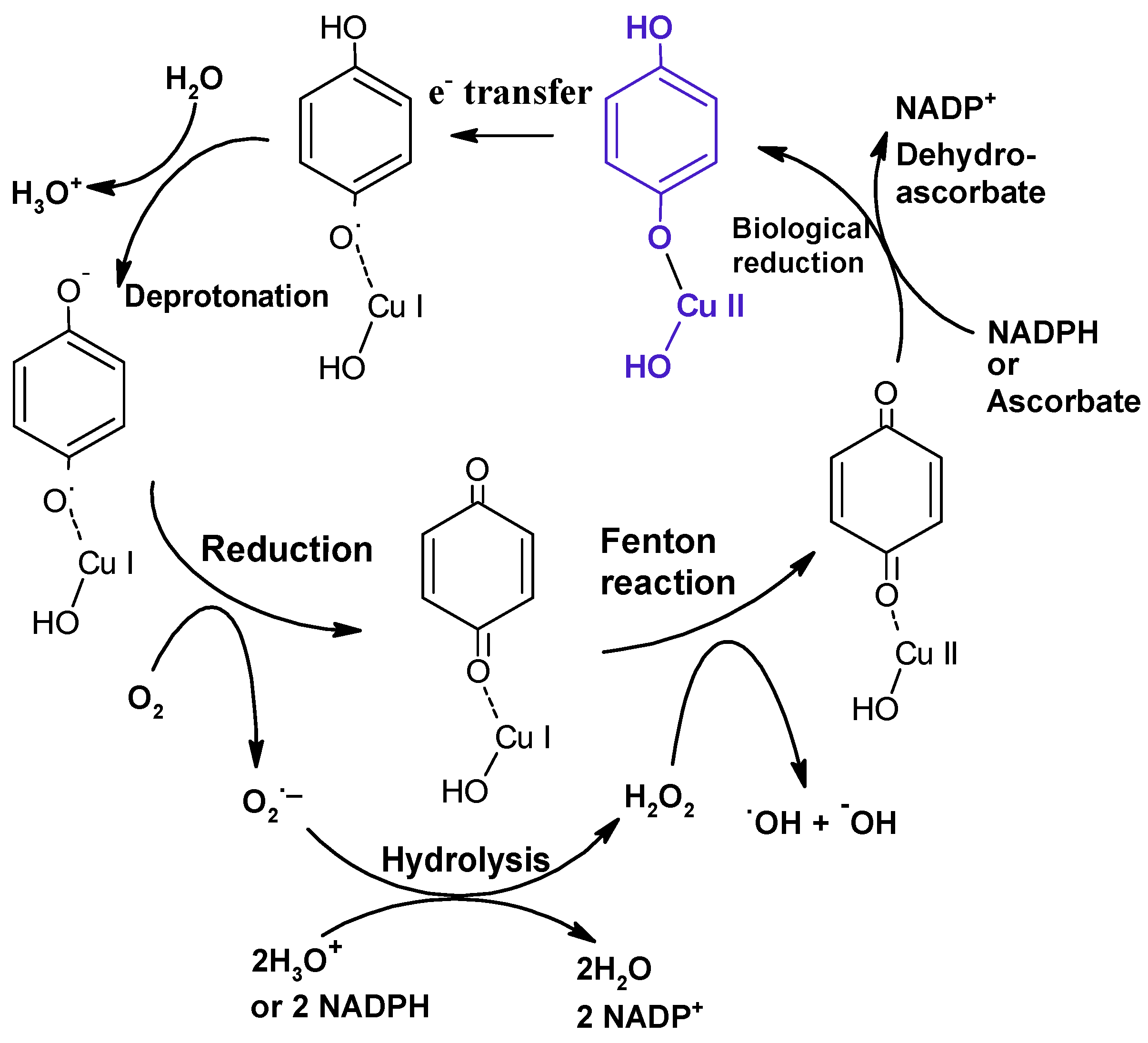
| Source Material | EPR Signal (g Value) | (Free Radical) (Spins/g) | Reference |
|---|---|---|---|
| Wood (fatwood, pine wood) Coal (bituminous and anthracite) | 2.0029–2.0035 | 2.3 × 1017–1.2 × 1018 | [12] |
| Biochar (pine needles, wheat straw and maize straw | 2.0028–2.0037 | 1.96–30.2 × 1018 | [11] |
| DEP, GEP, woodsmoke, cigarette tar, and airborne PM 1 | 2.0025–2.0040 | 1015–1017 | [13] |
| TSP (Athens), Urban street dusts, DEP, GEP | 2.0036 (single, broad signal) | [16] | |
| DEP | ~2.0 | [17] | |
| Polymer: PS, PVC, PE, PP, PET | 2.0028–2.004 | 2 × 1012–8 × 1013 | [14] |
| Source | Finding | References |
|---|---|---|
| TSP (Athens); Urban street dusts; DEP; GEP | PM generates hydroxyl radical in aqueous suspension. Hydroxyl radical formation was linked with redox-active metal content. | [72] |
| Biochar | Biochar contains persistent free radicals evident by EPR. Biochar can activate H2O2 to produce hydroxyl radical. | [11] |
| DEP; Coal fly ash | Suspensions of DEP and coal fly ash produce hydroxyl radical. Metal ions and superoxide implicated in its production. Neither kaolinite nor silica produce ·OH. | [69] |
| Ambient air PM (California); DEP | In the presence of ascorbate, ambient air PM and DEP both generate ·OH. ·OH production is correlated with Cu content | [70] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugas, T.R.; Lomnicki, S.; Cormier, S.A.; Dellinger, B.; Reams, M. Addressing Emerging Risks: Scientific and Regulatory Challenges Associated with Environmentally Persistent Free Radicals. Int. J. Environ. Res. Public Health 2016, 13, 573. https://doi.org/10.3390/ijerph13060573
Dugas TR, Lomnicki S, Cormier SA, Dellinger B, Reams M. Addressing Emerging Risks: Scientific and Regulatory Challenges Associated with Environmentally Persistent Free Radicals. International Journal of Environmental Research and Public Health. 2016; 13(6):573. https://doi.org/10.3390/ijerph13060573
Chicago/Turabian StyleDugas, Tammy R., Slawomir Lomnicki, Stephania A. Cormier, Barry Dellinger, and Margaret Reams. 2016. "Addressing Emerging Risks: Scientific and Regulatory Challenges Associated with Environmentally Persistent Free Radicals" International Journal of Environmental Research and Public Health 13, no. 6: 573. https://doi.org/10.3390/ijerph13060573
APA StyleDugas, T. R., Lomnicki, S., Cormier, S. A., Dellinger, B., & Reams, M. (2016). Addressing Emerging Risks: Scientific and Regulatory Challenges Associated with Environmentally Persistent Free Radicals. International Journal of Environmental Research and Public Health, 13(6), 573. https://doi.org/10.3390/ijerph13060573






