Prevalence of Depression among Rural Residents with Diabetes Mellitus: A Cross-Sectional Study from Northeast China
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection and Measurements
2.3. Definitions
2.4. Statistical Analysis
3. Result
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q.; et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qian, D.; Chen, J.; Hu, D.; Hou, M.; Chen, S.; Wang, P. Prevalence, awareness, treatment and control of diabetes mellitus in rural China: Results from Shandong Province. Diabet. Med. 2015, 33, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guan, H.; Zheng, L.; Li, Z.; Guo, X.; Yang, H.; Yu, S.; Sun, G.; Li, W.; Hu, W.; et al. Prevalence and awareness of diabetes mellitus among a rural population in China: Results from Liaoning Province. Diabet. Med. 2015, 32, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C. Epidemiological study of mental disorders in 12 regions of China: Methodology and data analysis. Zhonghua Shen Jing Jing Shen Ke Za Zhi 1986, 19, 65–69. [Google Scholar] [PubMed]
- Wang, J.; Wang, D.; Shen, Y. Epidemiological survey on affective disorder in 7 areas of China. J. Psychiatry 1998, 2, 75–78. (In Chinese) [Google Scholar]
- Gupta, S.; Goren, A.; Dong, P.; Liu, D. Prevalence, awareness, and burden of major depressive disorder in urban China. Expert Rev. Pharmacoecon. Outcomes Res. 2015, 23, 1–15. [Google Scholar] [CrossRef]
- De Groot, M.; Doyle, T.; Averyt, J.; Risaliti, C.; Shubroo, J. Depressive symptoms and type 2 diabetes mellitus in rural Appalachia: An 18-month follow-up study. Int. J. Psychiatry Med. 2015, 48, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Reynolds, C.R. Depression among older adults with diabetes mellitus. Clin. Geriatr. Med. 2015, 31, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ford, E.S.; Strine, T.W.; Mokdad, A.H. Prevalence of depression among U.S. adults with diabetes: Findings from the 2006 behavioral risk factor surveillance system. Diabetes Care 2008, 31, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Stone, M.A.; Peters, J.L.; Davies, M.J.; Khunti, K. The prevalence of co-morbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2006, 23, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Sweileh, W.M.; Abu-Hadeed, H.M.; Al-Jabi, S.W.; Zyoud, S.H. Prevalence of depression among people with type 2 diabetes mellitus: A cross sectional study in Palestine. BMC Public Health 2014, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; WHO: Geneva, Switzerland, 2006; pp. 1–3. [Google Scholar]
- Genuth, S.; Alberti, K.G.; Bennett, P.; Buse, J.; Defronzo, R.; Kahn, R.; Kitzmiller, J.; Knowler, W.C.; Lebovitz, H.; Lernmark, A.; et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003, 26, 3160–3167. [Google Scholar] [PubMed]
- Lowe, B.; Kroenke, K.; Herzog, W.; Grafe, K. Measuring depression outcome with a brief self-report instrument: Sensitivity to change of the Patient Health Questionnaire (PHQ-9). J. Affect. Disord. 2004, 81, 61–66. [Google Scholar] [CrossRef]
- Pignone, M.P.; Gaynes, B.N.; Rushton, J.L.; Burchell, C.M.; Orleans, C.T.; Mulrow, C.D.; Lohr, K.N. Screening for depression in adults: A summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern Med. 2002, 136, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J. Diagnostic and statistical manual of mental disorders (4th ed., text revision) (DsM-IV-TR). Br. J. Psychiatry 2001, 179, 85–85. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Manea, L.; Gilbody, S.; McMillan, D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. CMAJ 2012, 184, E191–E196. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Tuomilehto, J.; Silventoinen, K.; Barengo, N.; Jousilahti, P. Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur. Heart J. 2004, 25, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- El, M.A. Prevalence and Predictors of Depression among Type 2 Diabetes Mellitus Outpatients in Eastern Province, Saudi Arabia. Int. J. Health Sci. (Qassim) 2015, 9, 119–126. [Google Scholar]
- Alonso-Moran, E.; Satylganova, A.; Orueta, J.F.; Nuno-Solinis, R. Prevalence of depression in adults with type 2 diabetes in the Basque Country: Relationship with glycaemic control and health care costs. BMC Public Health 2014, 14, 769. [Google Scholar] [CrossRef] [PubMed]
- Papelbaum, M.; Moreira, R.O.; Coutinho, W.; Kupfer, R.; Zagury, L.; Freitas, S.; Appolinario, J.C. Depression, glycemic control and type 2 diabetes. Diabetol. Metab. Syndr. 2011, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Katon, W.; Russo, J.; Lin, E.H.; Heckbert, S.R.; Karter, A.J.; Williams, L.H.; Ciechanowski, P.; Ludman, E.; Von Korff, M. Diabetes and poor disease control: Is comorbid depression associated with poor medication adherence or lack of treatment intensification? Psychosom. Med. 2009, 71, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, J.T.; Kravitz, H.M. Mood and menopause: Findings from the Study of Women's Health across the Nation (SWAN) over 10 years. Obstet. Gynecol. Clin. North Am. 2011, 38, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Al-Amer, R.M.; Sobeh, M.M.; Zayed, A.A.; Al-Domi, H.A. Depression among adults with diabetes in Jordan: Risk factors and relationship to blood sugar control. J. Diabetes Complications 2011, 25, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Bensbaa, S.; Agerd, L.; Boujraf, S.; Araab, C.; Aalouane, R.; Rammouz, I.; Ajdi, F. Clinical assessment of depression and type 2 diabetes in Morocco: Economical and social components. J. Neurosci. Rural Pract. 2014, 5, 250–253. [Google Scholar] [PubMed]
- Dismuke, C.E.; Egede, L.E. Association between major depression, depressive symptoms and personal income in US adults with diabetes. Gen. Hosp. Psychiatry 2010, 32, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Niraula, K.; Kohrt, B.A.; Flora, M.S.; Thapa, N.; Mumu, S.J.; Pathak, R.; Stray-Pedersen, B.; Ghimire, P.; Regmi, B.; Macfarlane, E.K.; et al. Prevalence of depression and associated risk factors among persons with type-2 diabetes mellitus without a prior psychiatric history: A cross-sectional study in clinical settings in urban Nepal. BMC Psychiatry 2013, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.V.; Smith, G.D. Patterns, distribution, and determinants of under- and overnutrition: A population-based study of women in India. Am. J. Clin. Nutr. 2006, 84, 633–640. [Google Scholar] [PubMed]
- Chang, J.J.; Salas, J.; Habicht, K.; Pien, G.W.; Stamatakis, K.A.; Brownson, R.C. The association of sleep duration and depressive symptoms in rural communities of Missouri, Tennessee, and Arkansas. J. Rural Health 2012, 28, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Nanri, A.; Kochi, T.; Tsuruoka, H.; Pham, N.M.; Kabe, I.; Matsuda, S.; Mizoue, T. Bedtime and sleep duration in relation to depressive symptoms among Japanese workers. J. Occup. Health 2013, 55, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, K.A.; Punjabi, N.M. Long sleep duration: A risk to health or a marker of risk? Sleep Med. Rev. 2007, 11, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Kripke, D.F.; Brunner, R.; Freeman, R.; Hendrix, S.L.; Jackson, R.D.; Masaki, K.; Carter, R.A. Sleep complaints of postmenopausal women. Clin. J. Womens. Health 2001, 1, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Boden, J.M.; Fergusson, D.M. Alcohol and depression. Addiction 2011, 106, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Conner, K.R.; Pinquart, M.; Gamble, S.A. Meta-analysis of depression and substance use among individuals with alcohol use disorders. J. Subst. Abuse Treat. 2009, 37, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Baggio, S.; Iglesias, K.; Studer, J.; Dupuis, M.; Daeppen, J.B.; Gmel, G. Is the relationship between major depressive disorder and self-reported alcohol use disorder an artificial one? Alcohol Alcohol. 2015, 50, 195–199. [Google Scholar] [CrossRef] [PubMed]
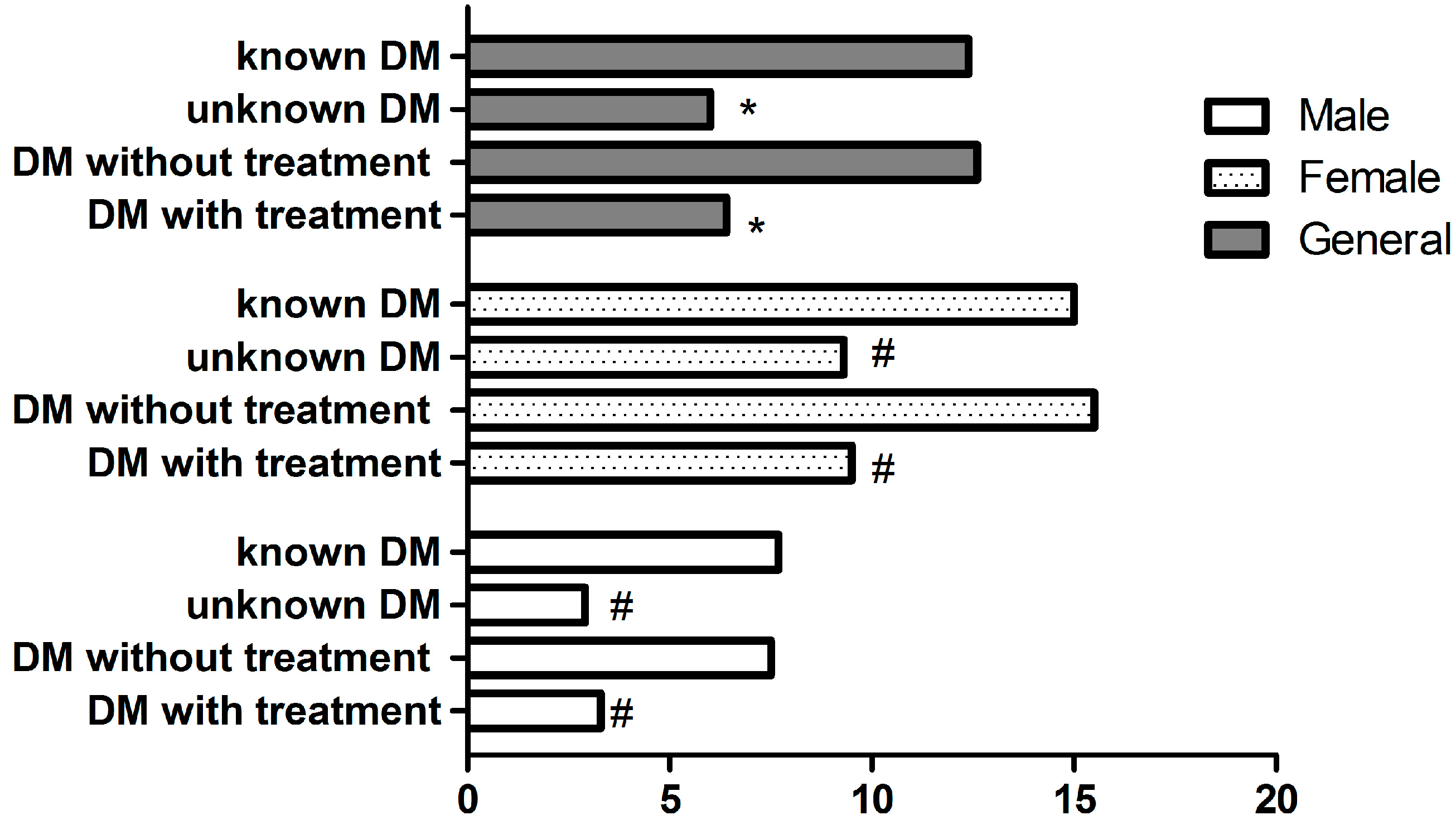
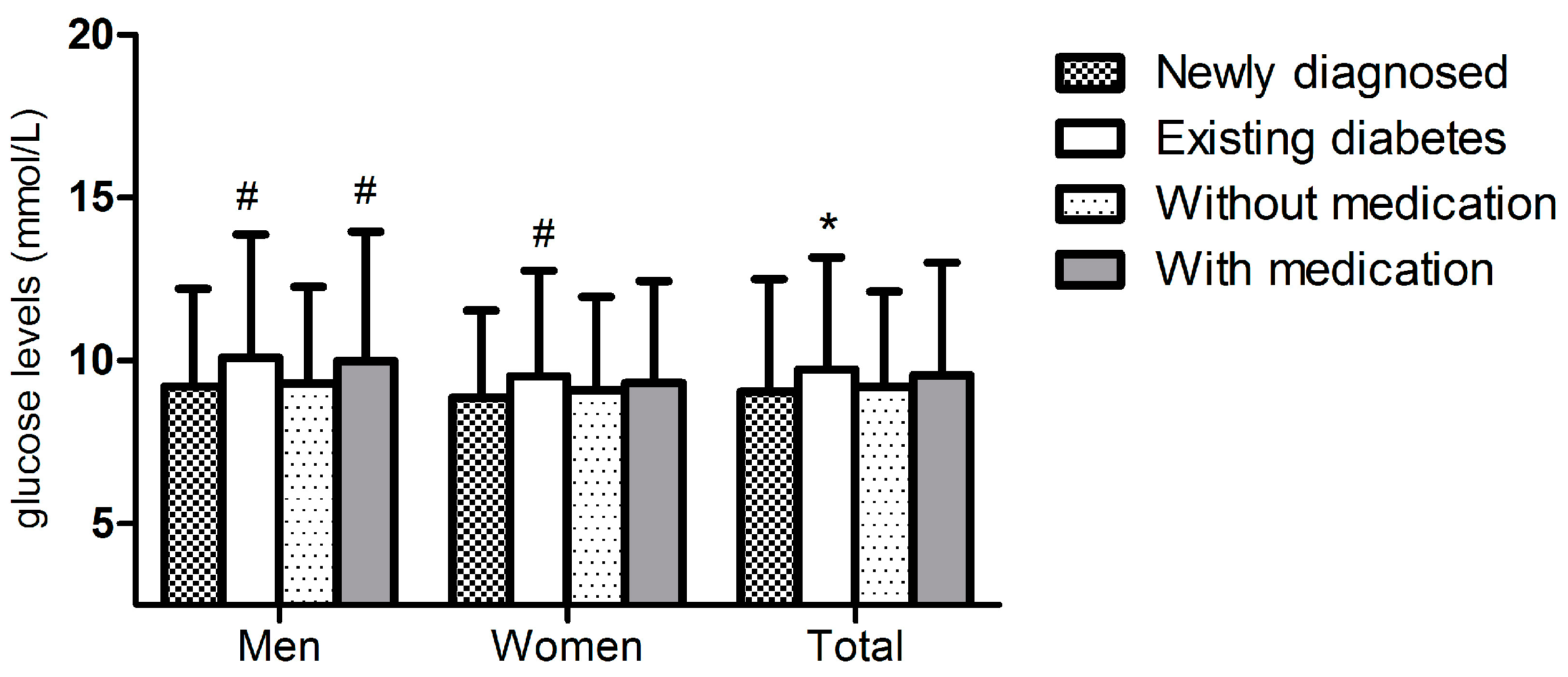
| Variables | Total n = 1187 | PHQ-9 Depressive Score | Unadjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| <10 n = 1083 | ≥10 n = 104 | ||||
| Age group (years) | |||||
| 35–44 | 127 (10.7) | 122 (96.1) | 5 (3.9) | Reference | |
| 45–54 | 334 (28.1) | 306 (91.6) | 28 (8.4) | 2.233 (0.843, 5.916) | 0.106 |
| 55–64 | 470 (39.6) | 432 (91.9) | 38 (8.1) | 2.146 (0.827, 5.571) | 0.117 |
| ≥65 | 256 (21.6) | 223 (87.1) | 33 (12.9) | 3.611 (1.374, 9.488) | 0.009 |
| Gender | |||||
| Male | 526 (44.3) | 502 (95.4) | 24 (4.6) | Reference | |
| Female | 661 (55.7) | 581 (87.9) | 80 (12.1) | 2.880 (1.797, 4.615) | <0.001 |
| Educational status | |||||
| Primary school or below | 696 (58.6) | 625 (89.8) | 71 (10.2) | Reference | |
| Middle school | 392 (33.0) | 362 (92.3) | 30 (7.7) | 0.730 (0.467, 1.140) | 0.166 |
| High school or above | 99 (8.3) | 96 (97.0) | 3 (3.0) | 0.275 (0.085, 0.891) | 0.031 |
| Ethnicity | |||||
| Han | 1123 (94.6) | 1026 (91.4) | 97 (8.6) | Reference | |
| Others a | 64 (5.4) | 57 (89.1) | 7 (10.9) | 0.770 (0.342, 1.734) | 0.528 |
| Marital status | |||||
| Married | 1068 (90.0) | 979 (91.7) | 89 (8.3) | Reference | |
| Others | 119 (10.0) | 104 (87.4) | 15 (12.6) | 1.587 (0.885, 2.843) | 0.121 |
| Physical activity | |||||
| Light | 472 (39.8) | 417 (88.3) | 55 (11.7) | Reference | |
| Moderate | 642 (54.1) | 598 (93.1) | 44 (6.9) | 0.558 (0.368,0.845) | 0.006 |
| Severe | 73 (6.1) | 68 (93.2) | 5 (6.8) | 0.557 (0.215, 1.443) | 0.228 |
| Annual income (CNY/year) | |||||
| ≤5000 | 167 (14.1) | 139 (83.2) | 28 (16.8) | Reference | |
| 5000–20,000 | 642 (54.1) | 587 (91.4) | 55 (8.6) | 0.465 (0.285, 0.760) | 0.002 |
| >20,000 | 378 (31.8) | 357 (94.4) | 21 (5.6) | 0.292 (0.160, 0.531) | <0.001 |
| Sleep duration (h/d) | |||||
| ≤7 | 613 (51.6) | 546 (89.1) | 67 (10.9) | Reference | |
| 7–8 | 303 (25.5) | 289 (95.4) | 14 (4.6) | 0.395 (0.218, 0.715) | 0.002 |
| 8–9 | 168 (14.2) | 155 (92.3) | 13 (7.7) | 0.683 (0.368, 1.271) | 0.229 |
| >9 | 103 (8.7) | 93 (90.3) | 10 (9.7) | 0.876 (0.435, 1.764) | 0.711 |
| Current smoking status | |||||
| No | 821 (69.2) | 741 (90.3) | 80 (9.7) | Reference | |
| Yes | 366 (30.8) | 342 (93.4) | 24 (6.6) | 0.650 (0.405, 1.044) | 0.075 |
| Current drinking status | |||||
| No | 930 (78.3) | 833 (89.6) | 97 (10.4) | Reference | |
| Yes | 257 (21.7) | 250 (97.3) | 7 (2.7) | 0.240 (0.110, 0.525) | <0.001 |
| Body mass index (kg/m2) | |||||
| <25 | 451 (38.0) | 403 (89.4) | 48 (10.6) | Reference | |
| 25–29 | 571 (48.1) | 527 (92.3) | 44 (7.7) | 0.701 (0.456, 1.077) | 0.214 |
| >30 | 165 (13.9) | 153 (92.7) | 12 (7.3) | 0.658 (0.341, 1.273) | 0.854 |
| Number of additional illnesses | |||||
| ≤1 | 883 (77.6) | 837 (94.8) | 46 (5.2) | Reference | |
| ≥2 | 255 (22.4) | 210 (82.4) | 45 (17.6) | 3.899 (2.516, 6.041) | <0.001 |
| Variables | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Gender | |||
| Male | 1 (reference) | ||
| Female | 1.984 | 1.077, 3.656 | 0.028 |
| Annual income (CNY/year) | |||
| ≤5000 | 1 (reference) | ||
| 5000–20,000 | 0.483 | 0.276, 0.847 | 0.011 |
| >20,000 | 0.356 | 0.181, 0.700 | 0.003 |
| Sleep duration (h/d) | |||
| ≤7 | 1 (reference) | ||
| 7–8 | 0.453 | 0.233, 0.881 | 0.020 |
| 8–9 | 0.883 | 0.451, 1.727 | 0.715 |
| >9 | 1.315 | 0.592, 2.921 | 0.502 |
| Number of additional illness | |||
| ≤1 | 1 (reference) | ||
| ≥2 | 3.080 | 1.886, 5.028 | <0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Yang, H.; Guo, X.; Zheng, L.; Sun, Y. Prevalence of Depression among Rural Residents with Diabetes Mellitus: A Cross-Sectional Study from Northeast China. Int. J. Environ. Res. Public Health 2016, 13, 542. https://doi.org/10.3390/ijerph13060542
Yu S, Yang H, Guo X, Zheng L, Sun Y. Prevalence of Depression among Rural Residents with Diabetes Mellitus: A Cross-Sectional Study from Northeast China. International Journal of Environmental Research and Public Health. 2016; 13(6):542. https://doi.org/10.3390/ijerph13060542
Chicago/Turabian StyleYu, Shasha, Hongmei Yang, Xiaofan Guo, Liqiang Zheng, and Yingxian Sun. 2016. "Prevalence of Depression among Rural Residents with Diabetes Mellitus: A Cross-Sectional Study from Northeast China" International Journal of Environmental Research and Public Health 13, no. 6: 542. https://doi.org/10.3390/ijerph13060542





