An Application of the Multivariate Linear Mixed Model to the Analysis of Shoulder Complexity in Breast Cancer Patients
Abstract
:1. Introduction
- (1)
- to compare shoulder muscle activity in the affected and unaffected shoulder during elevation of the arm;
- (2)
- to explore the relationship between any observed differences in muscle activity and patients report of pain and dysfunction;
- (3)
- to explore the relationship between any observed differences in muscle activity and key clinical variables.
2. Data Set
3. Statistical Hypotheses Testing and Statistical Modelling
3.1. Statistical Test of Clinical Hypotheses
- H01: The altered muscle activity is not associated with the patient’s report of pain and dysfunction;
- H02: Clinical risk factors have no effect on the activities of the muscles;
- H03: The effect of clinical risk factors is the same on the affected arm as with that on the unaffected arm.
- H04: The activity of the four key muscles acting at the scapula on the affected arm is the same as from those acting on the unaffected arm.
3.2. The Multivariate Linear Mixed Model
4. Empirical Results
4.1. Model Comparison
4.2. Statistical Inference of Clinical Hypotheses
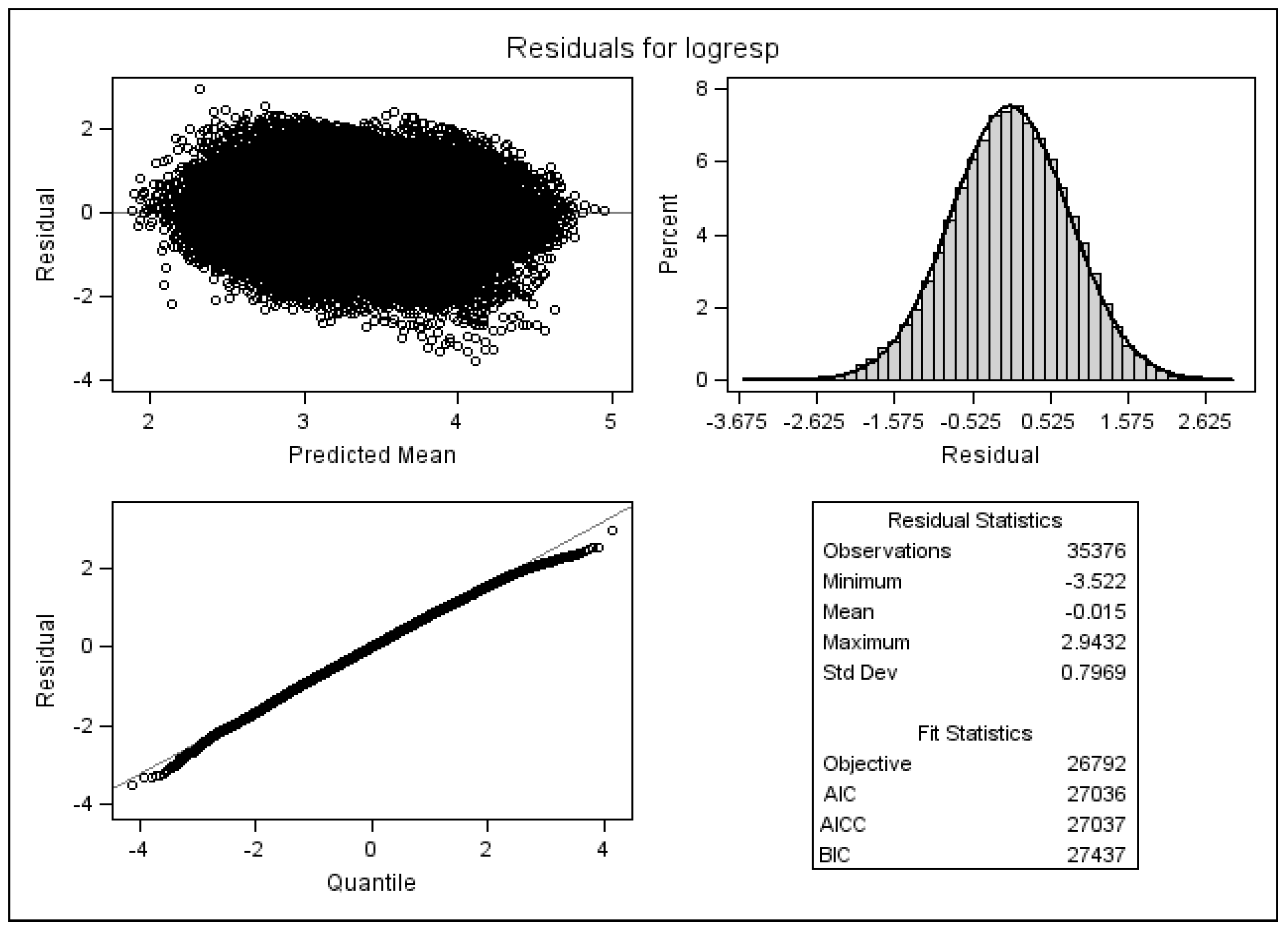
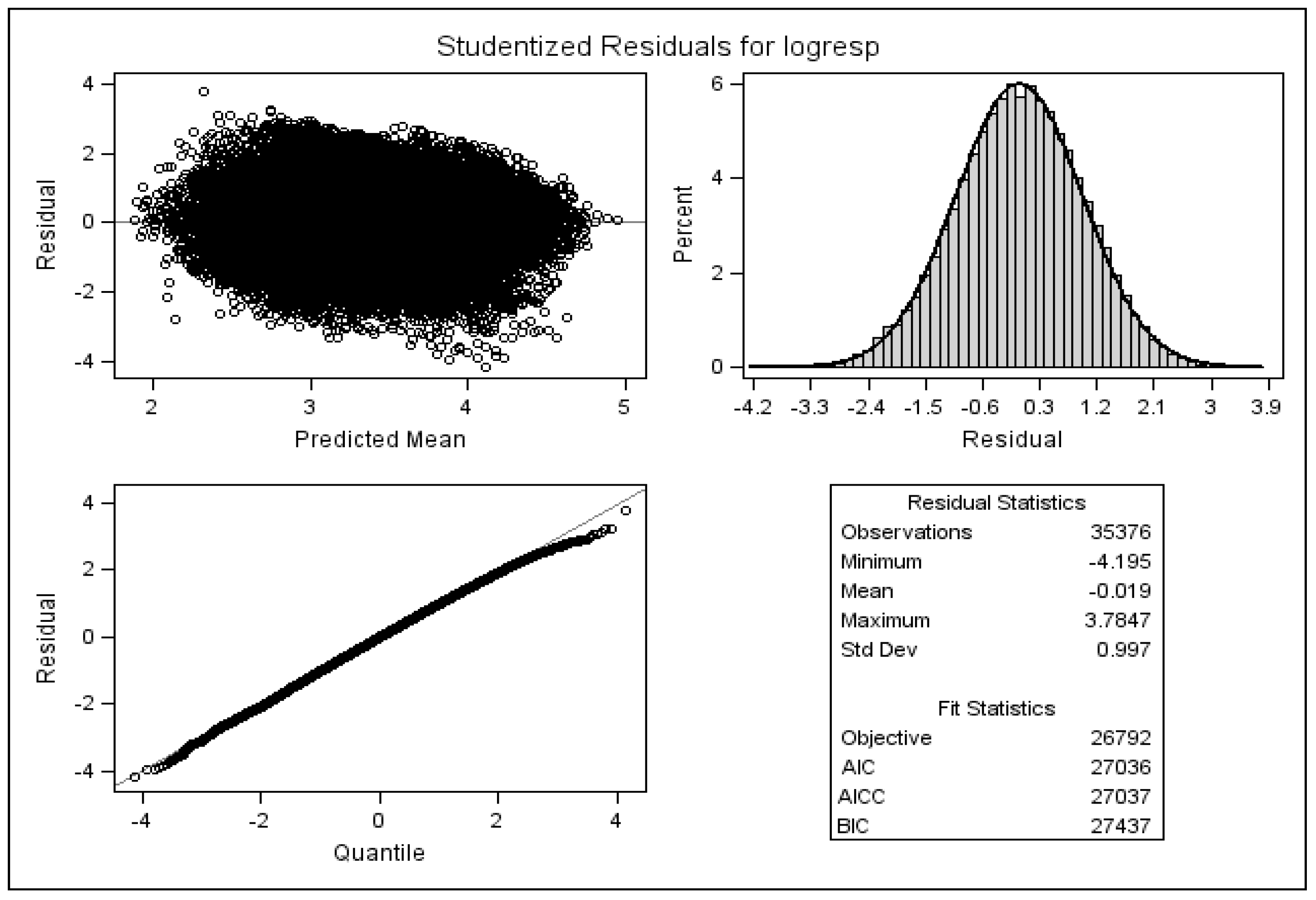
4.3. Checking Model Assumptions
5. Discussions
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Box, R.C.; Reul-Hirche, H.M.; Bullock-Saxton, J.E.; Furnival, C.M. Shoulder movement after breast cancer surgery: Results of a randomised controlled study of postoperative physiotherapy. Breast Cancer Res. Treat. 2002, 75, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Rietman, J.S.; Dijkstra, P.U.; Hoekstra, H.J.; Eisma, W.H.; Szabo, B.G.; Groothoff, J.W.; Geertzen, J.H.B. Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: A systematic review. Eur. J. Surg. Oncol. 2003, 29, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.; Rouffaer, L.; Vanhelden, P.; Piot, W.; Troosters, T.; Christiaens, M.-R. Recovery of upper limb function after axillary dissection. J. Surg. Oncol. 2003, 83, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M.; Hopewell, J.W.; Robbins, M.E. Initiation of non-neoplastic late effects: The role of endothelium and connective tissue. Stem. Cells Dayt. Ohio 1995, 13 (Suppl. 1), 248–256. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, K.R.; Benson, E.A. Non-tumour morbidity and mortality after modified radical mastectomy. Ann. R. Coll. Surg. Engl. 1992, 74, 314–317. [Google Scholar] [PubMed]
- Soulen, R.L.; Romero, J.A.; Chuba, P.J.; Evelhoch, J.L.; Simpson, R.E.; Forman, J.D. Musculoskeletal complications of neutron therapy for prostate cancer. Radiat. Oncol. Investig. 1997, 5, 81–91. [Google Scholar] [CrossRef]
- Ryttov, N.; Blichert-Toft, M.; Madsen, E.L.; Weber, J. Influence of adjuvant irradiation on shoulder joint function after mastectomy for breast carcinoma. Acta Radiol. Oncol. 1983, 22, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M.; Overgaard, M.; Thames, H.D. Fractionation sensitivity of a functional endpoint: Impaired shoulder movement after post-mastectomy radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 531–537. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gaze, M.N.; Rodger, A.; Chetty, U.; Forrest, A.P. Arm morbidity within a trial of mastectomy and either nodal sample with selective radiotherapy or axillary clearance. Br. J. Surg. 1989, 76, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Shamley, D.R.; Srinanaganathan, R.; Weatherall, R.; Oskrochi, R.; Watson, M.; Ostlere, S.; Sugden, E. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res. Treat. 2007, 106, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Roach, K.E.; Budiman-Mak, E.; Songsiridej, N.; Lertratanakul, Y. Development of a shoulder pain and disability index. Arthritis Care Res. Off. J. Arthritis Health Prof. Assoc. 1991, 4, 143–149. [Google Scholar] [CrossRef]
- Coull, B.A.; Agresti, A. Random effects modeling of multiple binomial responses using the multivariate binomial logit-normal distribution. Biometrics 2000, 56, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Fieuws, S.; Verbeke, G.; Molenberghs, G. Random-effects models for multivariate repeated measures. Stat. Methods Med. Res. 2007, 16, 387–397. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS; SAS Inst.: Cary, NC, USA, 2011. [Google Scholar]
- Graven-Nielsen, T.; Svensson, P.; Arendt-Nielsen, L. Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function. Electroencephalogr. Clin. Neurophysiol. Mot. Control. 1997, 105, 156–164. [Google Scholar] [CrossRef]
- Cahalan, T.D.; Johnson, M.E.; Chao, E.Y. Shoulder strength analysis using the Cybex II isokinetic dynamometer. Clin. Orthop. 1991, 271, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.E.; Johnson, M.E.; O’Driscoll, S.W.; An, K.N. Age-related changes in normal isometric shoulder strength. Am. J. Sports Med. 1999, 27, 651–657. [Google Scholar] [PubMed]
- Murray, M.P.; Gore, D.R.; Gardner, G.M.; Mollinger, L.A. Shoulder motion and muscle strength of normal men and women in two age groups. Clin. Orthop. 1985, 192, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Otis, J.C.; Warren, R.F.; Backus, S.I.; Santner, T.J.; Mabrey, J.D. Torque production in the shoulder of the normal young adult male. The interaction of function, dominance, joint angle, and angular velocity. Am. J. Sports Med. 1990, 18, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Shamley, D.; Lascurain-Aguirrebeña, I.; Oskrochi, R.; Srinaganathan, R. Shoulder morbidity after treatment for breast cancer is bilateral and greater after mastectomy. Acta Oncol. 2012, 51, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.C. Perry’s The Chemotherapy Source Book, 5th Revised ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2012; p. 848. [Google Scholar]
- Binda, D.; Vanhoutte, E.K.; Cavaletti, G.; Cornblath, D.R.; Postma, T.J.; Frigeni, B.; Alberti, P.; Bruna, J.; Velasco, R.; Argyriou, A.A.; et al. Rasch-built Overall Disability Scale for patients with chemotherapy-induced peripheral neuropathy (CIPN-R-ODS). Eur. J. Cancer 2013, 49, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Lin, Y.-L.; Kuo, Y.F.; Hortobagyi, G.N.; Goodwin, J.S. Decline in the use of anthracyclines for breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Kyritsis, A.P.; Makatsoris, T.; Kalofonos, H.P. Chemotherapy-induced peripheral neuropathy in adults: A comprehensive update of the literature. Cancer Manag. Res. 2014, 6, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Polychronopoulos, P.; Koutras, A.; Iconomou, G.; Iconomou, A.; Kalofonos, H.P.; Chroni, E. Peripheral neuropathy induced by administration of cisplatin- and paclitaxel-based chemotherapy. Could it be predicted? Support. Care Cancer 2005, 13, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Gomide, L.B.; Matheus, J.P.C.; Candido dos Reis, F.J. Morbidity after breast cancer treatment and physiotherapeutic performance. Int. J. Clin. Pract. 2007, 61, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Beurskens, C.H.; Uden, C.J.; van Strobbe, L.J.; Oostendorp, R.A.; Wobbes, T. The efficacy of physiotherapy upon shoulder function following axillary dissection in breast cancer, a randomized controlled study. BMC Cancer 2007, 7, 166. [Google Scholar] [CrossRef] [PubMed]
| Clinical Measures | Parameter Estimates | p-Value | |||
|---|---|---|---|---|---|
| Ln(PM) | Ln(UT) | Ln(SA) | Ln(RH) | Overall Effect | |
| Intercept | 2.9812 | 3.07 | 2.7905 | 2.1944 | <0.0001 |
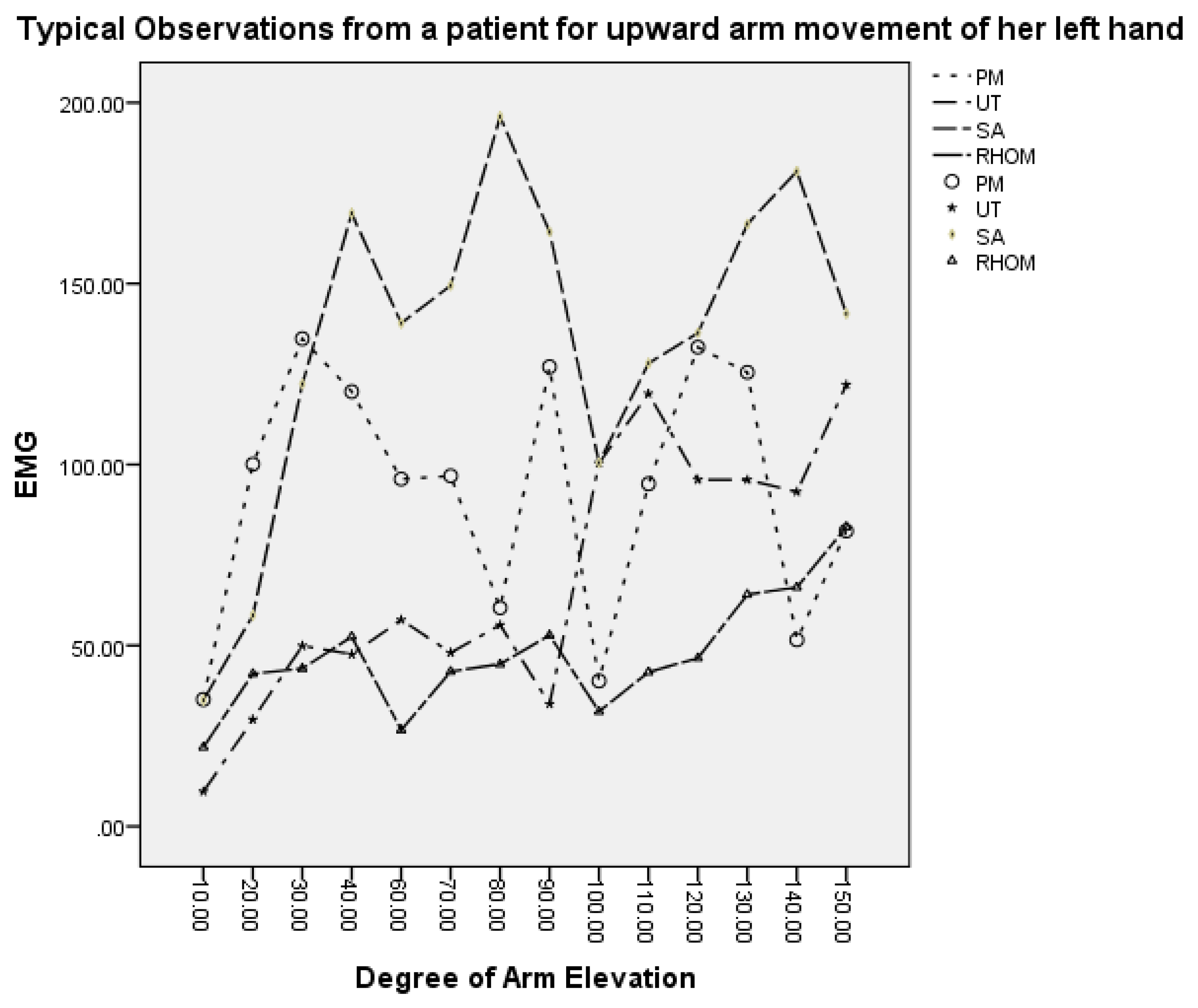
| Humeral elevation × 100 | 0.5053 | 0.5868 | 0.8403 | 0.7097 | <0.0001 |
| Move up | 0.1175 | 0.287 | 0.1286 | 0.1746 | <.0001 |
| Affected | −0.1897 | 0.02896 | 0.4124 | −0.0995 | 0.1855 |
| Handleft | 0.2908 | 0.1677 | 0.1959 | 0.1686 | <0.0001 |
| Dominant | 0.07338 | 0.1233 | 0.07865 | 0.07744 | 0.4888 |
| Agex100 | −0.00037 | 0.4736 | −0.503 | 0.4888 | 0.0386 |
| Duration × 100 | −0.01 | 0.0024 | 0.0119 | 0.0125 | 0.0286 |
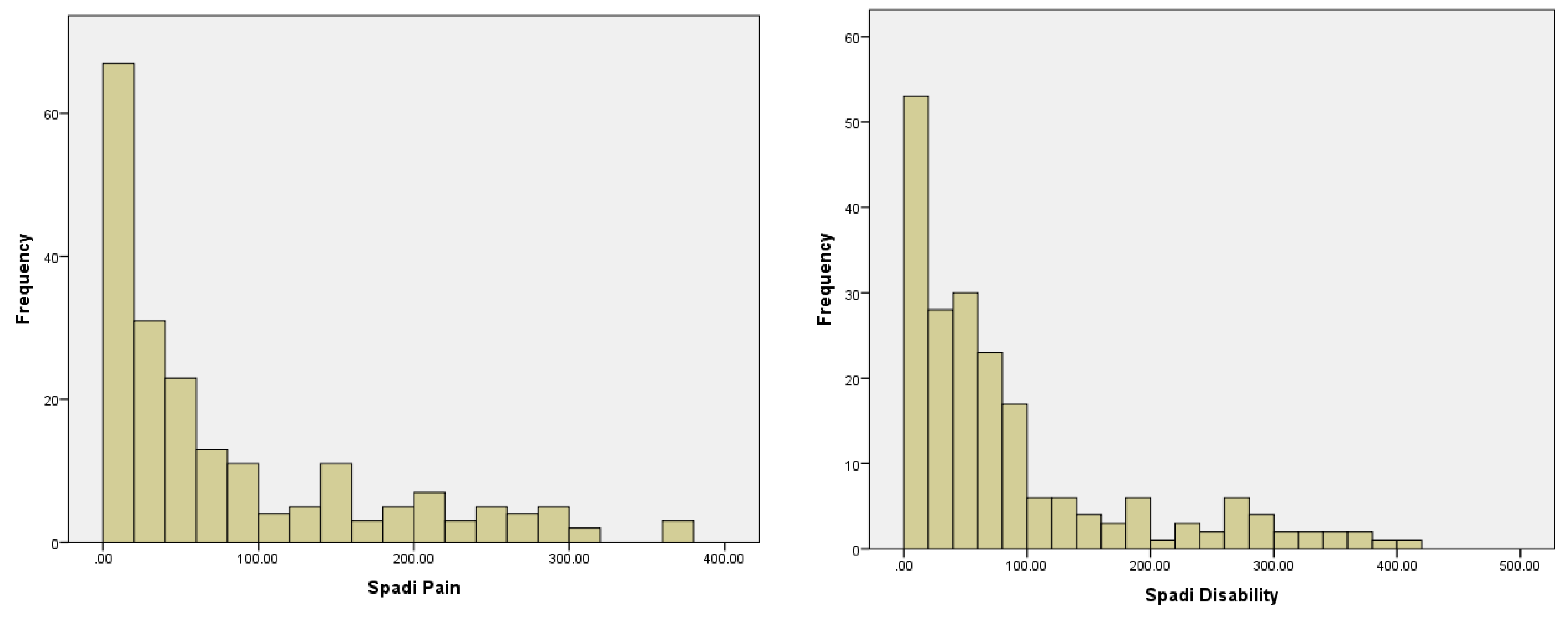
| Spadipai × 100 | −0.146 | −0.156 | −0.061 | −0.095 | 0.0005 |
| Spadidis × 100 | 0.0591 | 0.0726 | 0.0707 | −0.042 | 0.1284 |
| Wle | −0.2556 | −0.1913 | −0.2544 | −0.2267 | <0.0001 |
| Chemocat | −0.1107 | −0.0803 | −0.4658 | −0.1233 | <0.0001 |
| Inter_humeral elevation * × 100 | 0.0161 | 0.0837 | 0.019 | 0.1194 | 0.2037 |
| Inter_dominant * | −0.2211 | −0.1212 | −0.3452 | −0.2614 | <0.0001 |
| Inter_duration * × 100 | 0.0037 | −0.004 | −0.021 | −0.007 | 0.1410 |
| Inter_spadipai * × 100 | 0.1083 | 0.1046 | −0.051 | 0.0948 | 0.0239 |
| Exercise 6 m ** | −0.00577 | −0.0644 | −0.05941 | 0.01678 | 0.0349 |
| Exercise now ** | 0.06808 | 0.05504 | 0.08655 | 0.01458 | <0.0001 |
| Physio now ** | 0.307 | 0.5523 | 0.1583 | 0.3082 | 0.1207 |
| Physio ever ** | −0.01245 | −0.0663 | 0.1235 | −0.1439 | 0.4765 |
| ρ | 0.89 | 0.92 | 0.90 | 0.91 | N/A |
| σ2 | 0.62 | 0.73 | 0.61 | 0.63 | N/A |
| –2 log likelihood of the model with interaction terms –2 log likelihood of the model without interaction | 29,181.2 29,898.4 | ||||
| Clinical Measures | Parameter Estimates | p-Value | |||
|---|---|---|---|---|---|
| Ln(PM) | Ln(UT) | Ln(SA) | Ln(RH) | Overall Effect | |
| Intercept | 3.0631 | 3.1468 | 2.8339 | 2.2456 | <0.0001 |
| Humeral elevation × 100 | 0.4999 | 0.5733 | 0.8288 | 0.6937 | <0.0001 |
| Move up | 0.1357 | 0.2985 | 0.136 | 0.2008 | <0.0001 |
| Affected | –0.06145 | 0.07579 | 0.5562 | 0.1457 | 0.0113 |
| Handleft | 0.2825 | 0.154 | 0.199 | 0.1627 | <0.0001 |
| Dominant | 0.07379 | 0.1044 | 0.07393 | 0.07766 | 0.6258 |
| Agex100 | –0.088 | 0.4317 | −0.542 | 0.4361 | 0.5757 |
| Duration × 100 | –0.011 | 0.0022 | 0.0113 | 0.0119 | 0.2323 |
| Spadipai × 100 | –0.147 | –0.155 | –0.063 | –0.104 | 0.1053 |
| Spadidis × 100 | 0.0532 | 0.0676 | 0.0589 | –0.035 | 0.8000 |
| Wle | –0.2831 | −0.199 | −0.2626 | –0.2262 | <0.0001 |
| Chemocat | –0.1611 | –0.1034 | –0.4759 | −0.1365 | 0.0231 |
| Inter_humeral elevation * × 100 | 0.0029 | 0.0774 | 0.0047 | 0.1091 | 0.1146 |
| Inter_dominant * | –0.2326 | –0.1192 | –0.3466 | –0.2746 | 0.0654 |
| Inter_duration * × 100 | 0.0022 | –0.007 | –0.024 | −0.011 | <0.0001 |
| Inter_spadipai * × 100 | 0.1042 | 0.1195 | −0.023 | 0.1221 | <0.0001 |
| Exercise 6 m ** | –0.02449 | –0.04658 | –0.05968 | 0.003809 | 0.0142 |
| Exercise now ** | 0.06914 | 0.01997 | 0.05287 | –0.03337 | <0.0001 |
| Physio now ** | 0.01201 | 0.4788 | 0.1107 | 0.5951 | 0.0060 |
| Physio ever ** | 0.002231 | –0.1651 | 0.01827 | –0.2481 | 0.0163 |
| ρ | 0.79 | 0.85 | 0.82 | 0.80 | N/A |
| σ2 | 0.31 | 0.37 | 0.33 | 0.29 | N/A |
| Random effect variance | 0.33 | 0.38 | 0.28 | 0.35 | N/A |
| –2 log likelihood of the model with interaction terms –2 log likelihood of the model without interaction | 27,645.1 27,862.8 | ||||
| Clinical Measures | Parameter Estimates | p-Value | |||
|---|---|---|---|---|---|
| Ln(PM) | Ln(UT) | Ln(SA) | Ln(RH) | Overall Effect | |
| Intercept | 3.0662 | 3.1566 | 2.8413 | 2.2559 | <0.0001 |
| Humeral elevation × 100 | 0.5012 | 0.5761 | 0.8314 | 0.694 | <0.0001 |
| Move_up | 0.1357 | 0.299 | 0.1362 | 0.201 | <0.0001 |
| Affected | –0.03653 | 0.1271 | 0.5837 | 0.1368 | 0.0054 |
| Handleft | 0.2837 | 0.1537 | 0.1983 | 0.1608 | <0.0001 |
| Dominant | 0.07391 | 0.1057 | 0.07586 | 0.07687 | 0.8796 |
| Age × 100 | –0.093 | 0.4211 | –0.549 | 0.4292 | 0.1775 |
| Duration × 100 | –0.011 | 0.0021 | 0.0112 | 0.0119 | 0.0227 |
| Spadipai × 100 | –0.148 | –0.155 | –0.064 | –0.103 | 0.4220 |
| Spadidis × 100 | 0.0533 | 0.0679 | 0.0604 | –0.037 | 0.5270 |
| Wle | –0.283 | –0.2028 | –0.2668 | –0.2306 | 0.0272 |
| Chemocat | –0.1598 | –0.1078 | –0.4812 | –0.1446 | 0.0205 |
| Inter_humeral elevation * × 100 | 5.07E-04 | 0.0737 | 0.0036 | 0.1096 | 0.1219 |
| Inter_dominant * | –0.2347 | –0.1247 | –0.3505 | –0.2764 | 0.2958 |
| Inter_duration * × 100 | 0.0015 | −0.008 | –0.024 | –0.011 | <0.0001 |
| Inter_spadipai * × 100 | 0.1115 | 0.1211 | –0.023 | 0.1212 | <0.0001 |
| Exercise 6 m ** | –0.02175 | –0.05333 | –0.06286 | 0.006382 | 0.0049 |
| Exercise now ** | 0.05879 | 0.01727 | 0.05089 | –0.03237 | <0.0001 |
| Physio now ** | 0.08553 | 0.4907 | 0.1158 | 0.5001 | 0.0177 |
| Physio ever ** | –0.0337 | –0.1669 | 0.02314 | –0.2484 | 0.0121 |
| ρ | 0.79 | 0.85 | 0.82 | 0.80 | N/A |
| σ2 | 0.31 | 0.37 | 0.33 | 0.29 | N/A |
| Random effect variance | 0.33 | 0.38 | 0.29 | 0.35 | N/A |
| –2 log likelihood of the model with interaction terms –2 log likelihood of the model without interaction | 26,833.2 27,522.8 | ||||
| Variable | EMG Effect (Significant) | |||
|---|---|---|---|---|
| Pectoralis Major | Upper Trapezius | Serratus Anterior | Rhomboid | |
| Humeral Elevation | ↑ * | ↑ * | ↑ * | ↑ * |
| Left vs. Right Shoulder | ↑ * | ↑ * | ↑ * | ↑ * |
| Time Since Surgery (DURATION) | ↓ | ↑ | ↑ | ↑ |
| WLE (vs. Other Treatment Modalities) | ↓ * | ↓ * | ↓ * | ↓ * |
| Chemotherapy | ↓ | ↓ | ↓ * | ↓ |
| Variable | EMG Effect (Significant) | |||
|---|---|---|---|---|
| Pectoralis Major | Upper Trapezius | Serratus Anterior | Rhomboid | |
| Increasing Pain Score | ↑ * | ↑ * | ↓ | ↑ * |
| Exercise Last 6 Months | ↓ | ↓ * | ↓ * | ↑ |
| Current Exercise | ↑ * | ↑ | ↑ * | ↓ * |
| Current Physiotherapy | ↑ | ↑ * | ↑ | ↑ * |
| Time Since Surgery | ↑ | ↓ | ↓ * | ↓ * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oskrochi, G.; Lesaffre, E.; Oskrochi, Y.; Shamley, D. An Application of the Multivariate Linear Mixed Model to the Analysis of Shoulder Complexity in Breast Cancer Patients. Int. J. Environ. Res. Public Health 2016, 13, 274. https://doi.org/10.3390/ijerph13030274
Oskrochi G, Lesaffre E, Oskrochi Y, Shamley D. An Application of the Multivariate Linear Mixed Model to the Analysis of Shoulder Complexity in Breast Cancer Patients. International Journal of Environmental Research and Public Health. 2016; 13(3):274. https://doi.org/10.3390/ijerph13030274
Chicago/Turabian StyleOskrochi, Gholamreza, Emmanuel Lesaffre, Youssof Oskrochi, and Delva Shamley. 2016. "An Application of the Multivariate Linear Mixed Model to the Analysis of Shoulder Complexity in Breast Cancer Patients" International Journal of Environmental Research and Public Health 13, no. 3: 274. https://doi.org/10.3390/ijerph13030274





