Co-Formulants in Glyphosate-Based Herbicides Disrupt Aromatase Activity in Human Cells below Toxic Levels
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Cell Lines and Treatments
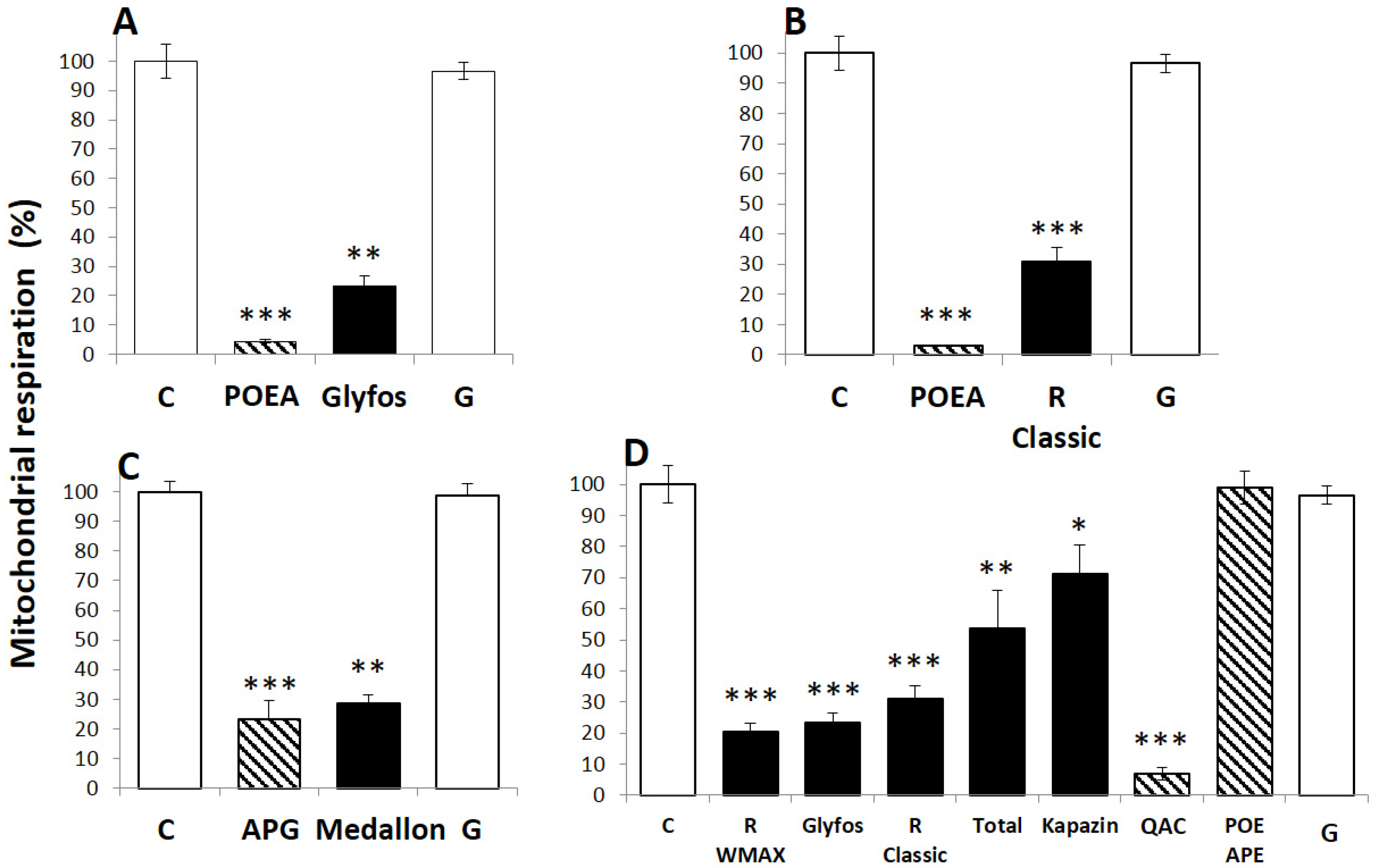
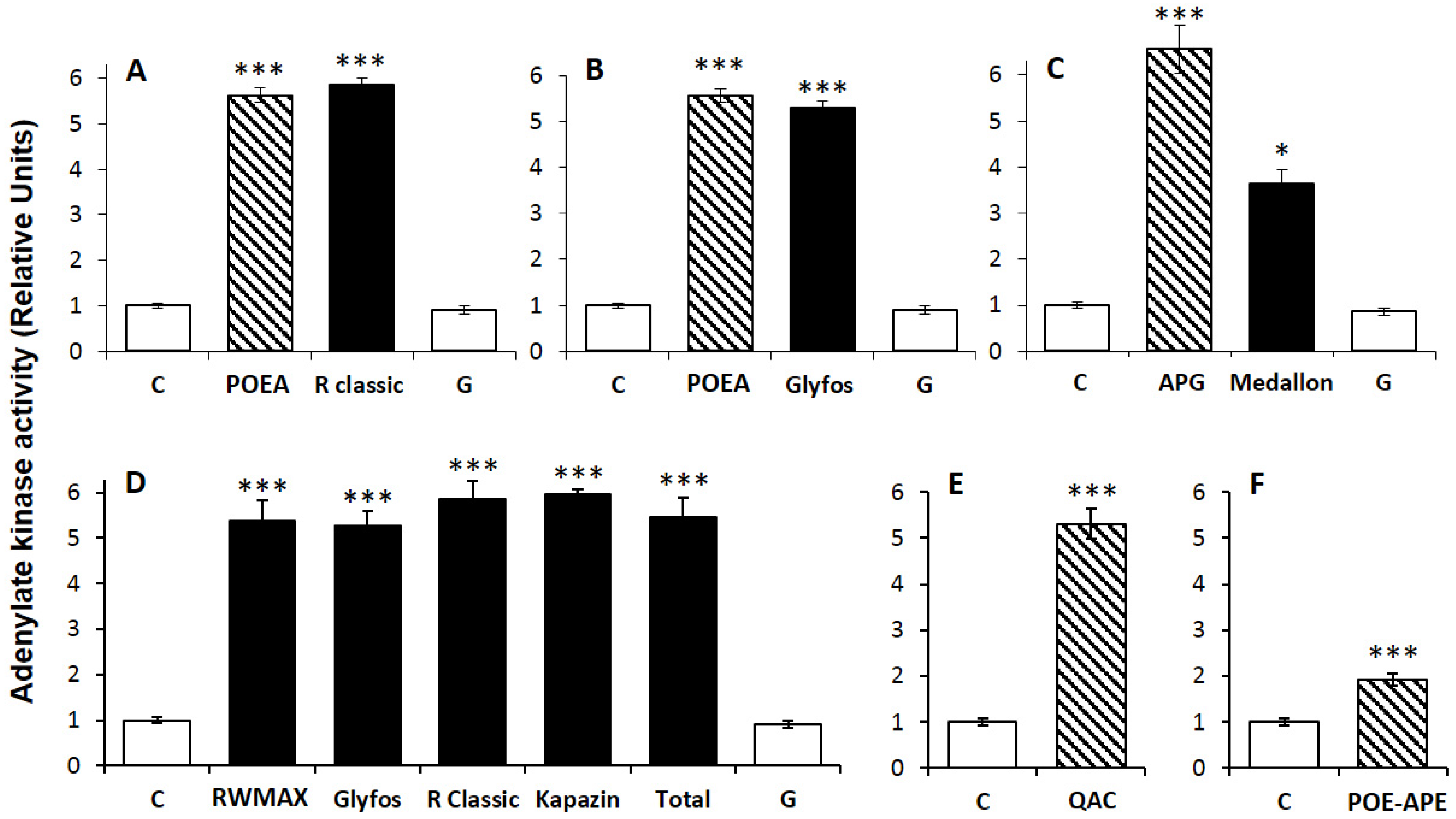
2.3. Cell Treatments and Cytotoxicity Biomarkers
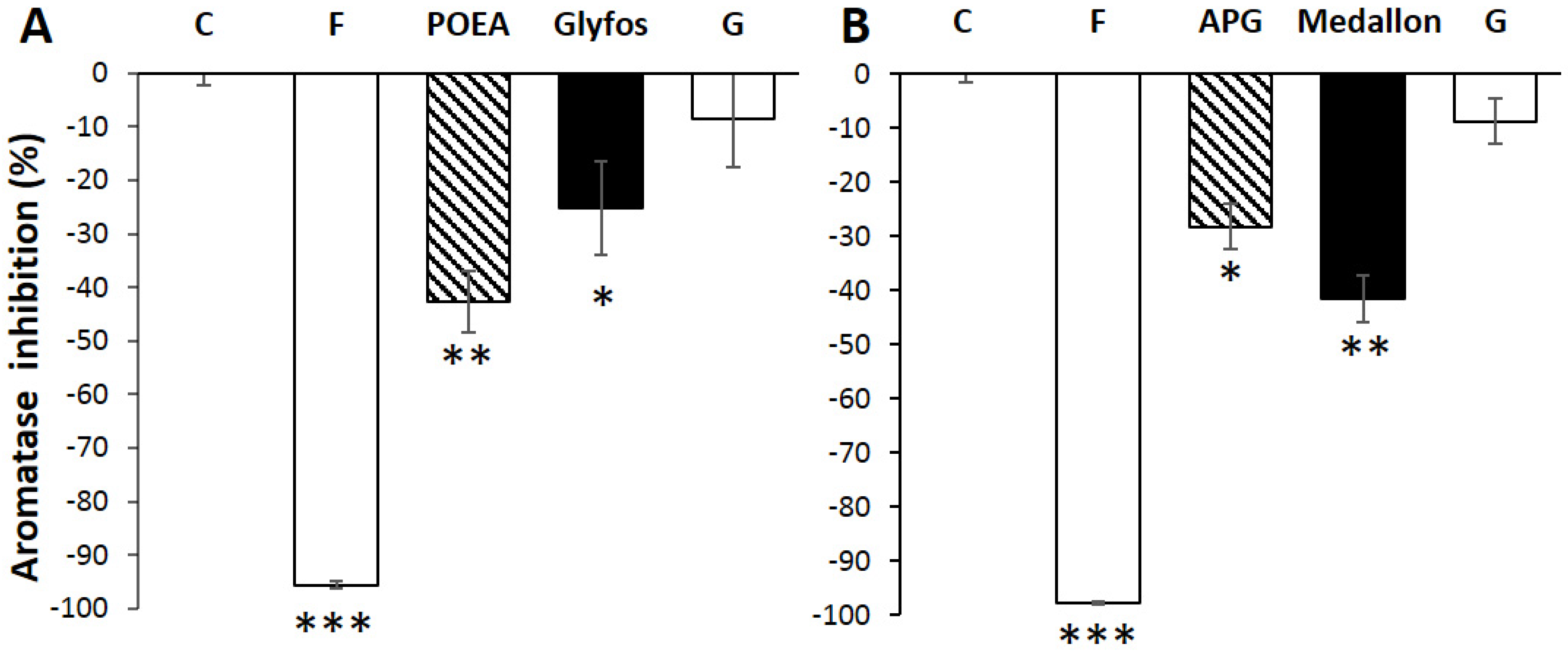
2.4. Aromatase Activity Measurement
2.5. Statistical Analysis
3. Results
3.1. Toxicity Thresholds
3.2. Comparative Toxicities within and between Formulations
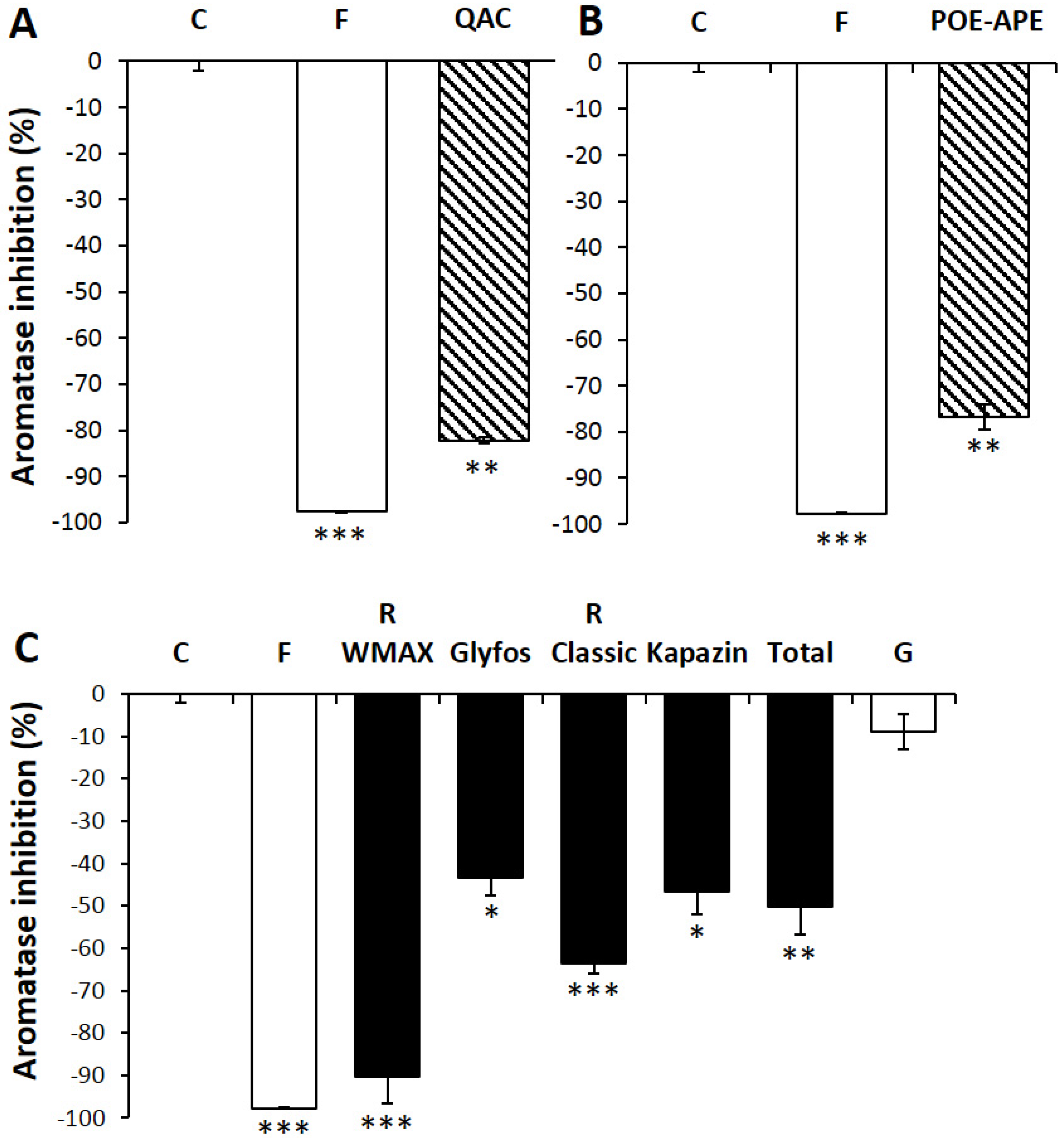
3.3. Aromatase Inhibition by Co-Formulants within and between Formulations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Main, K.M.; Skakkebaek, N.E.; Virtanen, H.E.; Toppari, J. Genital anomalies in boys and the environment. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Toppari, J.; Larsen, J.C.; Christiansen, P.; Giwercman, A.; Grandjean, P.; Guillette, L.J., Jr.; Jegou, B.; Jensen, T.K.; Keiding, N.; Leffers, H.; et al. Male reproductive health and environmental xenoestrogens. Environ. Health Perspect. 1996, 104, 741–803. [Google Scholar] [CrossRef]
- Toppari, J.; Juul, A. Trends in puberty timing in humans and environmental modifiers. Mol. Cell Endocrinol. 2010, 324, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Relyea, R.A. The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol. Appl. 2005, 15, 618–627. [Google Scholar] [CrossRef]
- Haefs, R.; Schmitz-Eiberger, M.; Mainx, H.G.; Mittelstaedt, W.; Noga, G. Studies on a new group of biodegradable surfactants for glyphosate. Pest Manag. Sci. 2002, 58, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Nobels, I.; Spanoghe, P.; Haesaert, G.; Robbens, J.; Blust, R. Toxicity ranking and toxic mode of action evaluation of commonly used agricultural adjuvants on the basis of bacterial gene expression profiles. PLoS ONE 2011, 6, e24139. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Defarge, N.; Spiroux, V.J.; Seralini, G.E. Major pesticides are more toxic to human cells than their declared active principles. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Bernay, B.; Seralini, G.E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef] [PubMed]
- ANSES. Evaluation of the Risks of Pesticide Residues in Water Distribution. Available online: http://www.observatoire-pesticides.fr/upload/bibliotheque/230206191572191620118401138686/ORP-Ra-PesticidesEau.pdf (accessed on 17 February 2016).
- Székács, A.; Darvas, B. Forty years with glyphosate. In Herbicides—Properties, Synthesis and Control of Weeds; Hasaneen, M.N., Ed.; Elsevier: Burlington, MA, USA, 2012. [Google Scholar]
- James, C. Global Status of Commercialized Biotech/GM Crops: 2011; ISAAA Brief: Ithaca, NY, USA, 2011. [Google Scholar]
- Bøhn, T.; Cuhra, M.; Traavik, T.; Sanden, M.; Fagan, J.; Primicerio, R. Compositional differences in soybeans on the market: Glyphosate accumulates in Roundup Ready GM soybeans. Food Chem. 2014, 153, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zolla, L.; Rinalducci, S.; Antonioli, P.; Righetti, P. Proteomics as a complementary tool for identifying unintended side effects occurring in transgenic maize seeds as a result of genetic modifications. J. Proteome Res. 2008, 7, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- FAO. Maximum Residues Limits in Food/Feed for Glyphosate. Available online: http://www.codexalimentarius.net/pestres/data/pesticides/details.html?id=158 (accessed on 1 November 2015).
- Benachour, N.; Sipahutar, H.; Moslemi, S.; Gasnier, C.; Travert, C.; Seralini, G.E. Time- and dose-dependent effects of roundup on human embryonic and placental cells. Arch. Environ. Contam. Toxicol. 2007, 53, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Benachour, N.; Seralini, G.E. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 2009, 22. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, C.; Benachour, N.; Clair, E.; Travert, C.; Langlois, F.; Laurant, C.; Decroix-Laporte, C.; Seralini, G.E. Dig1 protects against cell death provoked by glyphosate-based herbicides in human liver cell lines. J. Occup. Med. Toxicol. 2010, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, C.; Laurant, C.; Decroix-Laporte, C.; Mesnage, R.; Clair, E.; Travert, C.; Seralini, G.E. Defined plant extracts can protect human cells against combined xenobiotic effects. J. Occup. Med Toxicol. 2011, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Clair, E.; Mesnage, R.; Travert, C.; Seralini, G.E. A glyphosate-based herbicide induces necrosis and apoptosis in mature rat testicular cells in vitro, and testosterone decrease at lower levels. Toxicol. Vitro 2012, 26, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Folmar, L.C.; Sanders, H.O.; Julin, A.M. Toxicity of the herbicide glyphosphate and several of its formulations to fish and aquatic invertebrates. Arch. Environ. Contam. Toxicol. 1979, 8, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Bidwell, J.R. The toxicity of glyphosate and several glyphosate formulations to four species of southwestern Australian frogs. Arch. Environ. Contam. Toxicol. 1999, 36, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.T.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Marc, J.; Le Breton, M.; Cormier, P.; Morales, J.; Belle, R.; Mulner-Lorillon, O. A glyphosate-based pesticide impinges on transcription. Toxicol. Appl. Pharmacol. 2005, 203, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, S.M.; Proudfoot, A.T.; Vale, J.A. Glyphosate poisoning. Toxicol. Rev. 2004, 23, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.; Marzuki, A.; Abdul, R.H.; Abdul, A.M. The oral and intratracheal toxicities of ROUNDUP and its components to rats. Vet. Human Toxicol. 1997, 39, 147–151. [Google Scholar]
- Romano, R.M.; Romano, M.A.; Bernardi, M.M.; Furtado, P.V.; Oliveira, C.A. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch. Toxicol. 2010, 84, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.A.; Romano, R.M.; Santos, L.D.; Wisniewski, P.; Campos, D.A.; De Souza, P.B.; Viau, P.; Bernardi, M.M.; Nunes, M.T.; De Oliveira, C.A. Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch. Toxicol. 2012, 86, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Watson, R.E.; DeSesso, J.M. Developmental and reproductive outcomes in humans and animals after glyphosate exposure: A critical analysis. J Toxicol. Environ. Health B Crit. Rev. 2012, 15, 39–96. [Google Scholar] [CrossRef] [PubMed]
- Cassault-Meyer, E.; Gress, S.; Seralini, G.E.; Galeraud-Denis, I. An acute exposure to glyphosate-based herbicide alters aromatase levels in testis and sperm nuclear quality. Environ. Toxicol. Pharmacol. 2014, 38. [Google Scholar] [CrossRef] [PubMed]
- Gehin, A.; Guillaume, Y.C.; Millet, J.; Guyon, C.; Nicod, L. Vitamins C and E reverse effect of herbicide-induced toxicity on human epidermal cells HaCaT: A biochemometric approach. Int. J. Pharm. 2005, 288, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, K.; Yapar, K.; Oruc, E.; Yalcin, E. Protective effect of Ginkgo biloba L. leaf extract against glyphosate toxicity in Swiss albino mice. J. Med. Food. 2011, 14, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Prasad, S.; Mahmood, Z.; Shukla, Y. Studies on glyphosate-induced carcinogenicity in mouse skin: A proteomic approach. J. Proteomics 2010, 73, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.P.E. Occupational risk for male infertility. Occup. Environ. Med. 2014, 71, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Contardo-Jara, V.; Klingelmann, E.; Wiegand, C. Bioaccumulation of glyphosate and its formulation Roundup Ultra in Lumbriculus variegatus and its effects on biotransformation and antioxidant enzymes. Environ. Pollut. 2009, 157, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-Lorence, S.; Amarnrh, B.; Fisher, C.R.; Michael, M.D.; Mendelson, C.R.; et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994, 15, 342–355. [Google Scholar] [PubMed]
- OECD. Draft Guidance Document on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption. Available online: http://www.oecd.org/chemicalsafety/testing/50459967.pdf (accessed on 17 February 2016).
- Richard, S.; Moslemi, S.; Sipahutar, H.; Benachour, N.; Seralini, G.E. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ. Health Perspect. 2005, 113, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.C.; Seralini, G.E. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Letcher, R.J.; Van Holsteijn, I.; Drenth, H.J.; Norstrom, R.J.; Bergman, A.; Safe, S.; Pieters, R.; Van den Berg, M. Cytotoxicity and aromatase (CYP19) activity modulation by organochlorines in human placental JEG-3 and JAR choriocarcinoma cells. Toxicol. Appl. Pharmacol. 1999, 160, 10–20. [Google Scholar] [CrossRef] [PubMed]
- L’Azou, B.; Fernandez, P.; Bareille, R.; Beneteau, M.; Bourget, C.; Cambar, J.; Bordenave, L. In vitro endothelial cell susceptibility to xenobiotics: Comparison of three cell types. Cell Biol. Toxicol. 2005, 21, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- FDA. GRAS EXEMPTION CLAIM Alkyl Polyglycoside Surfactants; EAS: Alexandria, VA, USA, 2007.
- Crouch, S.P.; Kozlowski, R.; Slater, K.J.; Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Meth. 1993, 160, 81–88. [Google Scholar] [CrossRef]
- Thompson, E.A., Jr.; Siiteri, P.K. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J. Biol. Chem. 1974, 249, 5364–5372. [Google Scholar] [PubMed]
- Dintinger, T.; Gaillard, J.L.; Moslemi, S.; Zwain, I.; Silberzahn, P. Androgen and 19-norandrogen aromatization by equine and human placental microsomes. J. Steroid Biochem. 1989, 33, 949–954. [Google Scholar] [CrossRef]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 2014, 9, e77547. [Google Scholar] [CrossRef] [PubMed]
- Székács, I.; Fejes, Á.; Klátyik, S.; Takács, E.; Patkó, D.; Pomóthy, J.; Mörtl, M.; Horváth, R.; Madarász, E.; Darvas, B.; et al. Environmental and toxicological impacts of glyphosate with its formulating adjuvant. Int. J. Biol. Food Vet. Agri. Eng. 2014, 8, 210–216. [Google Scholar]
- Cox, C. Herbicide factsheet—Glyphosate. J. Pestic. Reform. 2004, 24, 10–15. [Google Scholar]
- Defarge, N.; Mesnage, R.; Gress, S.; Seralini, G.E. Letter to the editor: Developmental and reproductive outcomes of roundup and glyphosate in humans and animals. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Belle, R.; Marc, J.; Morales, J.; Cormier, P.; Mulner-Lorillon, O. Letter to the editor: Toxicity of roundup and glyphosate. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. Toxicology for the twenty-first century. Nature 2009, 460, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Dallegrave, E.; Mantese, F.D.; Oliveira, R.T.; Andrade, A.J.; Dalsenter, P.R.; Langeloh, A. Pre- and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch. Toxicol. 2007, 81, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Salem, M.H.; Ibrahim, H.Z.; Helmi, S.; Seehy, M.A.; Bertheussen, K. Toxic effects of carbofuran and glyphosate on semen characteristics in rabbits. J. Environ. Sci. Health B 1995, 30, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Seralini, G.-E.; Clair, E.; Mesnage, R.; Gress, S.; Defarge, N.; Malatesta, M.; Hennequin, D.; De Vendomois, J.S. Republished study: Long-term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environ. Sci. Europ. 2014, 26, 14. [Google Scholar] [CrossRef]
- Bonefeld-Jorgensen, E.C.; Long, M.; Hofmeister, M.V.; Vinggaard, A.M. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: New data and a brief review. Environ. Health Perspect. 2007, 115 (Suppl. 1), 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Defarge, N.; Spiroux, V.J.; Seralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.L.; Han, Z.; Liu, J.; Farmer, D.R.; Papadopoulos, V. Disrupting mitochondrial function with surfactants inhibits MA-10 Leydig cell steroidogenesis. Cell Biol. Toxicol. 2007, 23, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Cserhati, T. Alkyl ethoxylated and alkylphenol ethoxylated nonionic surfactants: Interaction with bioactive compounds and biological effects. Environ. Health Perspect. 1995, 103, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wu, J.; Huang, Y.; Shen, S.; Han, X. Nonylphenol induces apoptosis in rat testicular Sertoli cells via endoplasmic reticulum stress. Toxicol. Lett. 2009, 186, 84–95. [Google Scholar] [CrossRef] [PubMed]
- De Liz Oliveira Cavalli, V.L.; Cattani, D.; Rieg, H.C.E.; Pierozan, P.; Zanatta, L.; Parisotto, E.B.; Filho, D.W.; Silva, F.R.M.B.; Pessoa-Pureur, R.; Zamoner, A. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radic. Biol. Med. 2013, 65, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Garry, V.F. In vitro studies of cellular and molecular developmental toxicity of adjuvants, herbicides, and fungicides commonly used in Red River Valley, Minnesota. J. Toxicol. Environ. Health A 2000, 60, 423–439. [Google Scholar] [PubMed]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Brausch, J.M.; Smith, P.N. Toxicity of three polyethoxylated tallowamine surfactant formulations to laboratory and field collected fairy shrimp, Thamnocephalus platyurus. Arch. Environ. Contam. Toxicol. 2007, 52, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.A.; Halling-Sorensen, B.; Mogensen, B.B.; Vejrup, K.V. Environmental properties and effects of nonionic surfactant adjuvants in pesticides: A review. Chemosphere 2003, 50, 871–901. [Google Scholar] [CrossRef]
- Eddleston, M.; Street, J.M.; Self, I.; Thompson, A.; King, T.; Williams, N.; Naredo, G.; Dissanayake, K.; Yu, L.M.; Worek, F.; et al. A role for solvents in the toxicity of agricultural organophosphorus pesticides. Toxicology 2012, 294, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.A.; Vejrup, K.V.; Mogensen, B.B.; Halling-Sørensen, B. Liquid chromatography-mass spectrometry method to determine alcohol ethoxylates and alkylamine ethoxylates in soil interstitial water, ground water and surface water samples. J. Chromatogr. 2002, 957, 45–57. [Google Scholar] [CrossRef]
- Vincent, M.D.; Sneddon, J. Nonylphenol: An overview and its determination in oysters and wastewaters and preliminary degradation results from laboratory experiments. Microchem. J. 2009, 92, 112–118. [Google Scholar] [CrossRef]
- Bergé, A.; Cladière, M.; Gasperi, J.; Coursimault, A.; Tassin, B.; Moilleron, R. Meta-analysis of environmental contamination by alkylphenols. Environ. Sci. Pollut. Res. 2012, 19, 3798–3819. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Han, H.; Li, D.; Ma, Y.; Tu, X.; Wu, Y. Analysis of alkylphenol and bisphenol A in meat by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem. 2007, 105, 1236–1241. [Google Scholar] [CrossRef]
- Ferrer, E.; Santoni, E.; Vittori, S.; Font, G.; Mañes, J.; Sagratini, G. Simultaneous determination of bisphenol A, octylphenol, and nonylphenol by pressurised liquid extraction and liquid chromatography—Tandem mass spectrometry in powdered milk and infant formulas. Food Chem. 2011, 126, 360–367. [Google Scholar] [CrossRef]
- She, Y.; Wang, J.; Zheng, Y.; Cao, W.; Wang, R.; Dong, F.; Liu, X.; Qian, M.; Zhang, H.; Wu, L. Determination of nonylphenol ethoxylate metabolites in vegetables and crops by high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2012, 132, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mullin, C.A. Determination of nonylphenol ethoxylate and octylphenol ethoxylate surfactants in beehive samples by high performance liquid chromatography coupled to mass spectrometry. Food Chem. 2014, 158, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Burn, R.W.; Thorpe, K.; Williams, R.; Tyler, C. Statistical modeling suggests that antiandrogens in effluents from wastewater treatment works contribute to widespread sexual disruption in fish living in English rivers. Environ. Health Perspect. 2009, 117, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Twaddle, N.C.; Churchwell, M.I.; Chang, H.C.; Newbold, R.R.; Delclos, K.B. Mass spectrometric determination of p-nonylphenol metabolism and disposition following oral administration to Sprague-Dawley rats. Reprod Toxicol. 2002, 16, 45–56. [Google Scholar] [CrossRef]
- Mehling, A.; Kleber, M.; Hensen, H. Comparative studies on the ocular and dermal irritation potential of surfactants. Food Chem. Toxicol. 2007, 45, 747–758. [Google Scholar] [CrossRef] [PubMed]
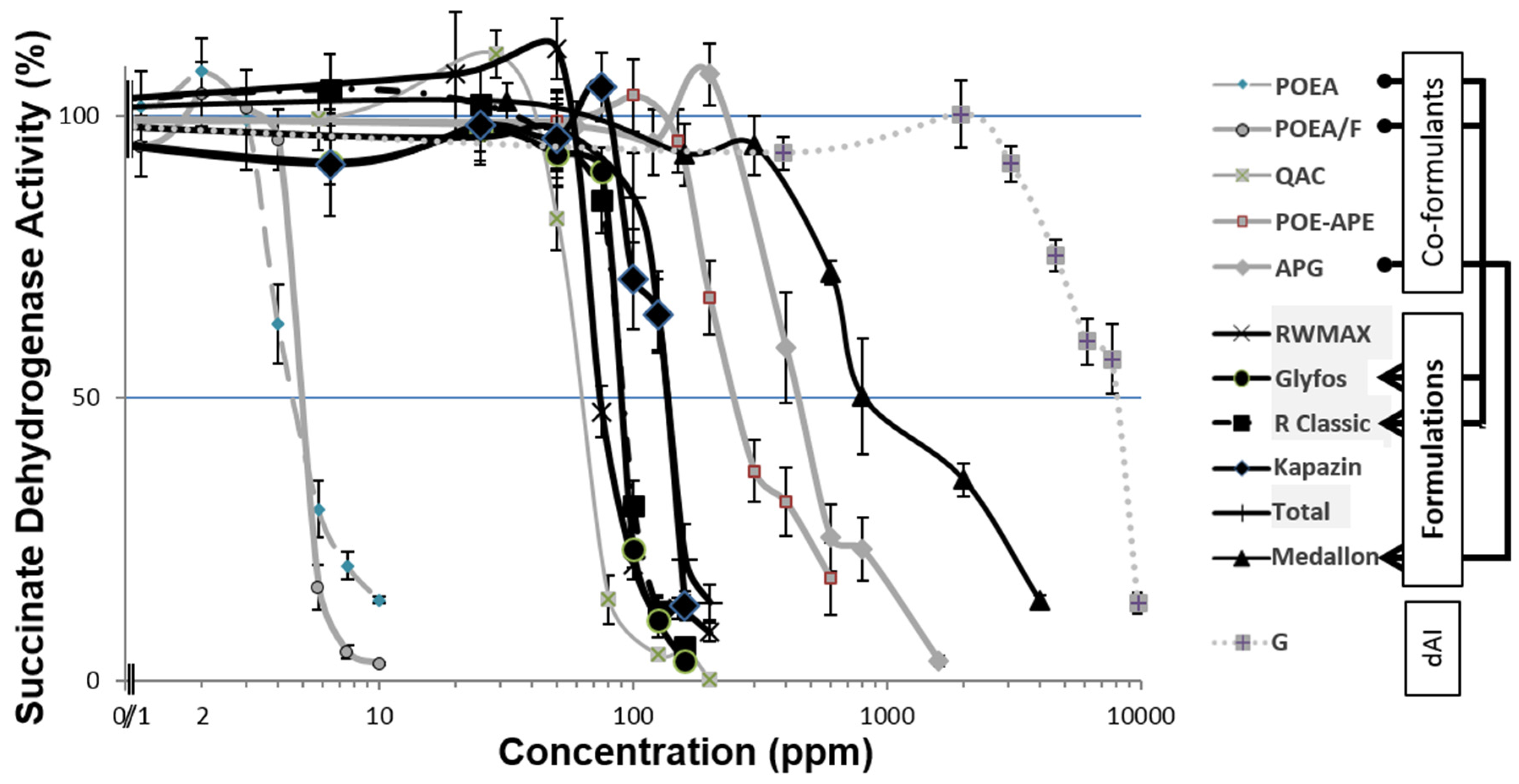
| Products | Trade Name (Manufacturer, Country) | Declared Active Ingredient (dAI) | dAI (%) | Present in | (ppm) | NOEC | LOEC | LC50 | |
|---|---|---|---|---|---|---|---|---|---|
| Co-formulants | POEA | Emulson AG GPE 3SS (Lamberti, Ita) | Polyethoxylated tallow amine | 100 | Roundup Classic, Glyfos | 3.0 | 3.5 | 3.9 | |
| POEA/F | Emulson AG GPE 3/SSM (Lamberti, Ita) | Polyethoxylated tallow amine | 70 | Roundup Classic, Glyfos | 4.0 | 4.5 | 4.7 | ||
| QAC | Emulson AG CB 30 (Lamberti, Ita) | Quaternary ammonium compound | 30 | other herbicides | 35 | 50 | 58 | ||
| POE-APE | Rolfen Bio (Lamberti, Ita) | POE alkyl phosphate ether | 70 | other herbicides | 150 | 200 | 222 | ||
| APG | Plantapon LGC (The Soap kitchen, UK) | Alkyl polyglucoside | 28.5–34.0 | Medallon Premium | 200 | 400 | 421 | ||
| G salt of (g/L) | G (g/L) | Co-formulants (%) | |||||||
| Formulations | RWMAX | Roundup WeatherMAX (Monsanto, Can) | Potassium (660) | 540 | Petroleum distillate /Transorb2 | 60 | 70 | 71 | |
| Glyfos | Glyfos (Cheminova, Hun) | IPA (486) | 360 | 9% POEA | 75 | 85 | 86 | ||
| R Classic | Roundup Classic (Monsanto, Hun) | IPA (486) | 360 | 15,5% POEA | 75 | 80 | 89 | ||
| Kapazin | Kapazin (Arysta, Hun) | IPA (486) | 360 | C8-10 ethoxylated alcohol (<2 g/L), Triethylene glycol monobutyl ether (<2 g/L) | 75 | 85 | 128 | ||
| Total | Total (Sinon Corporation, Hun) | IPA (486) | 360 | 58.5% unknown surfactant | 100 | 125 | 130 | ||
| Medallon | Medallon Premium (Syngenta, Hun) | diammonium (433) | 360 | 10%–20% APG (150 g/L) | 500 | 600 | 1268 | ||
| dAI | G | Glyphosate isopropyl ammonium (Hun) | IPA (486) | 360 | 3100 | 4600 | 7878 | ||
| Chemical Structure | CAS RN * | Chemical Class of Substance Group/Substance Name |
|---|---|---|
| Co-Formulants | ||
 | 61791-26-2 | polyethoxylated tallowamine (POEA) (R = C14–C18) (n + m= 2–28) |
 | 383178-66-3 + 110615-47-9 | alkyl polyglucosides (APG) (n < 3, m = 3–6) |
 | 68130-47-2 + 50769-39-6 | polyoxyethylene alkyl ether phosphates (POE-APE) (n = 6–10, m = 2–3) |
 | 66455-29-6 | quaternary ammonium compound (QAC) |
| Active Ingredient | ||
 | 386411-94-0 | isopropylamine salt of glyphosate |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Defarge, N.; Takács, E.; Lozano, V.L.; Mesnage, R.; Spiroux de Vendômois, J.; Séralini, G.-E.; Székács, A. Co-Formulants in Glyphosate-Based Herbicides Disrupt Aromatase Activity in Human Cells below Toxic Levels. Int. J. Environ. Res. Public Health 2016, 13, 264. https://doi.org/10.3390/ijerph13030264
Defarge N, Takács E, Lozano VL, Mesnage R, Spiroux de Vendômois J, Séralini G-E, Székács A. Co-Formulants in Glyphosate-Based Herbicides Disrupt Aromatase Activity in Human Cells below Toxic Levels. International Journal of Environmental Research and Public Health. 2016; 13(3):264. https://doi.org/10.3390/ijerph13030264
Chicago/Turabian StyleDefarge, Nicolas, Eszter Takács, Verónica Laura Lozano, Robin Mesnage, Joël Spiroux de Vendômois, Gilles-Eric Séralini, and András Székács. 2016. "Co-Formulants in Glyphosate-Based Herbicides Disrupt Aromatase Activity in Human Cells below Toxic Levels" International Journal of Environmental Research and Public Health 13, no. 3: 264. https://doi.org/10.3390/ijerph13030264
APA StyleDefarge, N., Takács, E., Lozano, V. L., Mesnage, R., Spiroux de Vendômois, J., Séralini, G.-E., & Székács, A. (2016). Co-Formulants in Glyphosate-Based Herbicides Disrupt Aromatase Activity in Human Cells below Toxic Levels. International Journal of Environmental Research and Public Health, 13(3), 264. https://doi.org/10.3390/ijerph13030264








