Validation of Contamination Control in Rapid Transfer Port Chambers for Pharmaceutical Manufacturing Processes
Abstract
:1. Introduction
- Personnel contamination
- Non-routine activity
- Aseptic assembly
- Human error
- Mechanical failure
- Airborne contaminants
- Improper sanitization: Surface contaminants
- Material transfers: Failure of HEPA (High Efficiency Particulate Air Filter) (0.2 μm filter)
- Improper sterilization
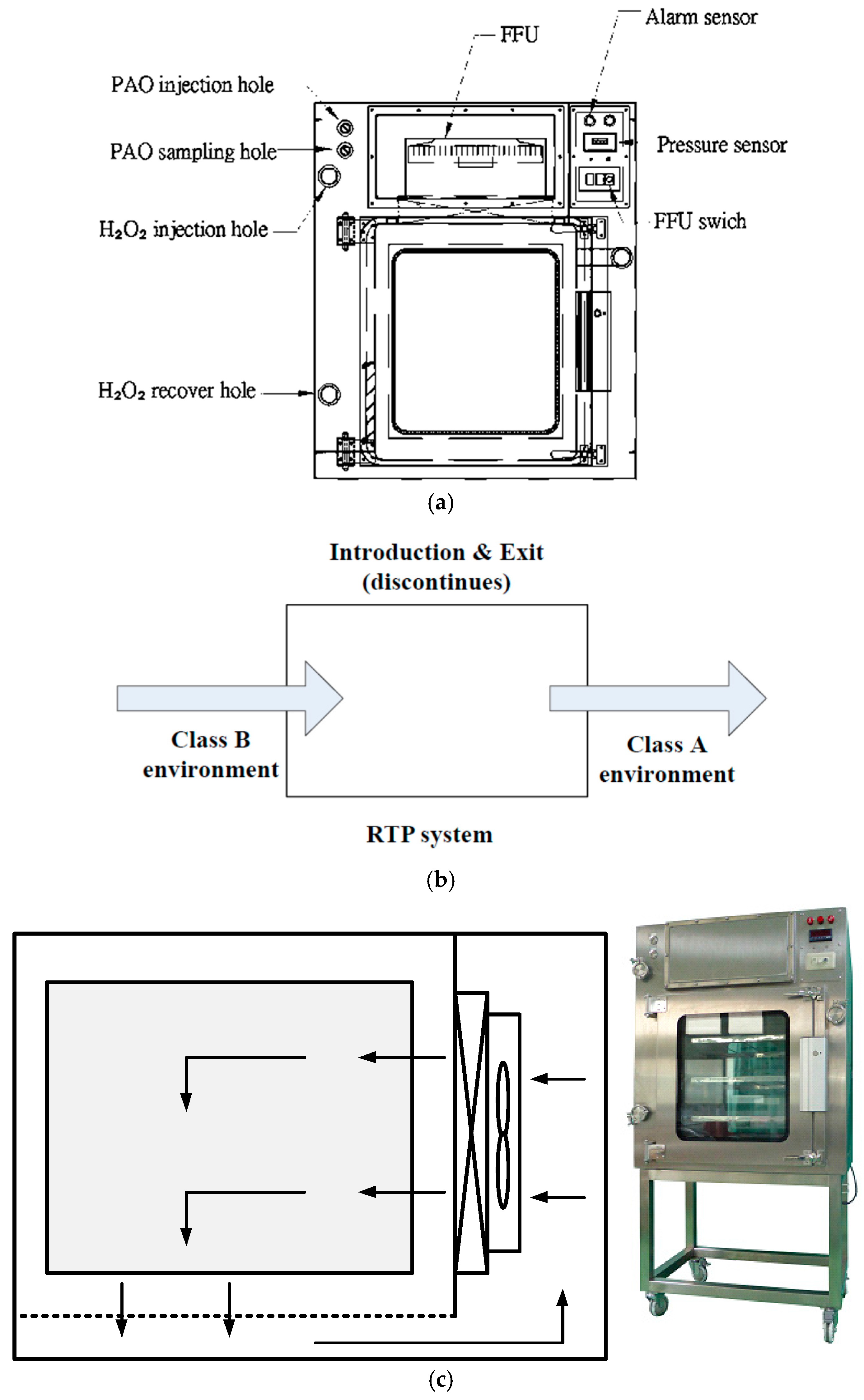
2. Materials and Methods
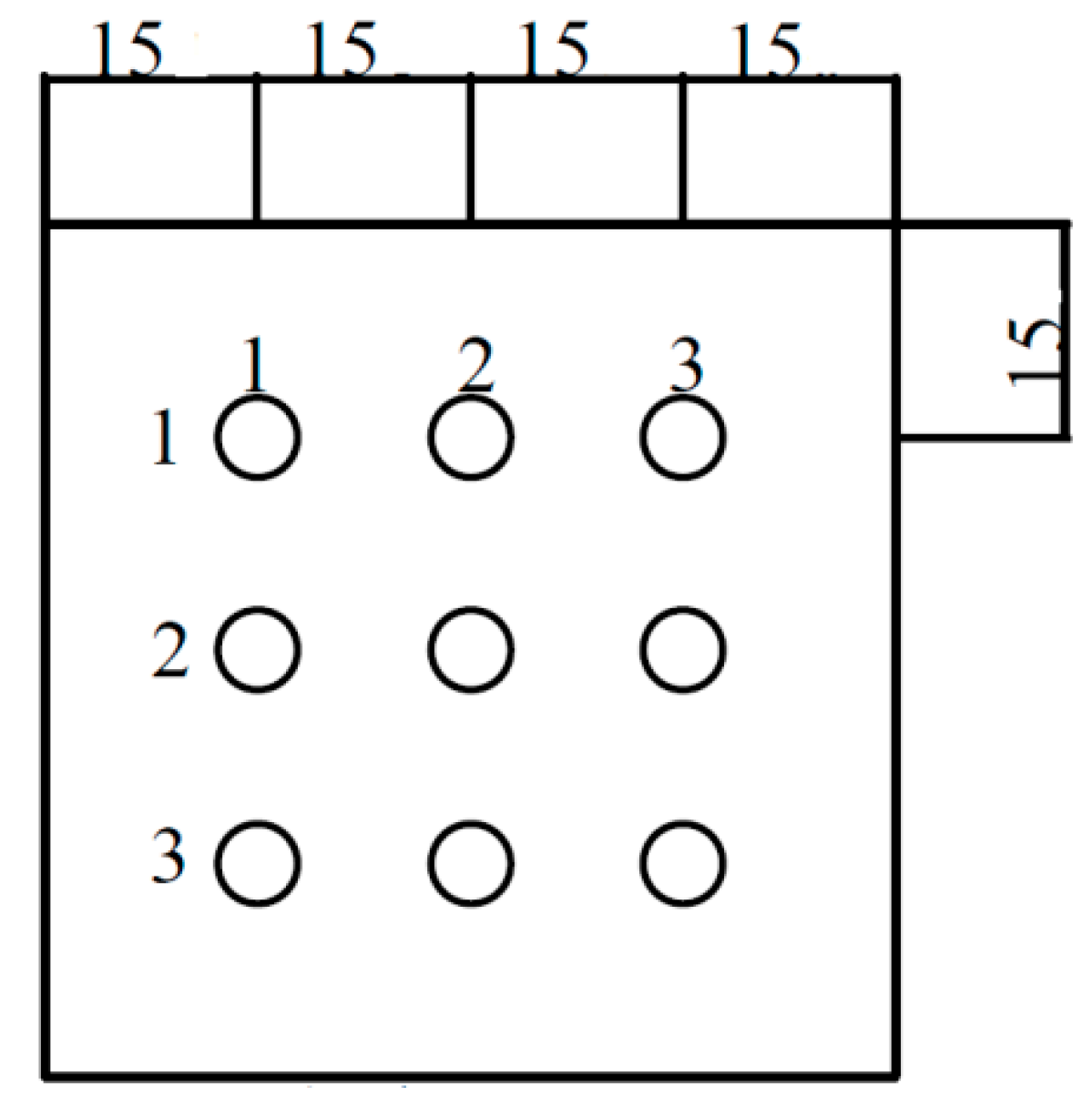
2.1. Airflow Velocity Test
2.2. Particle Count Method
2.3. Pressure Decay Test
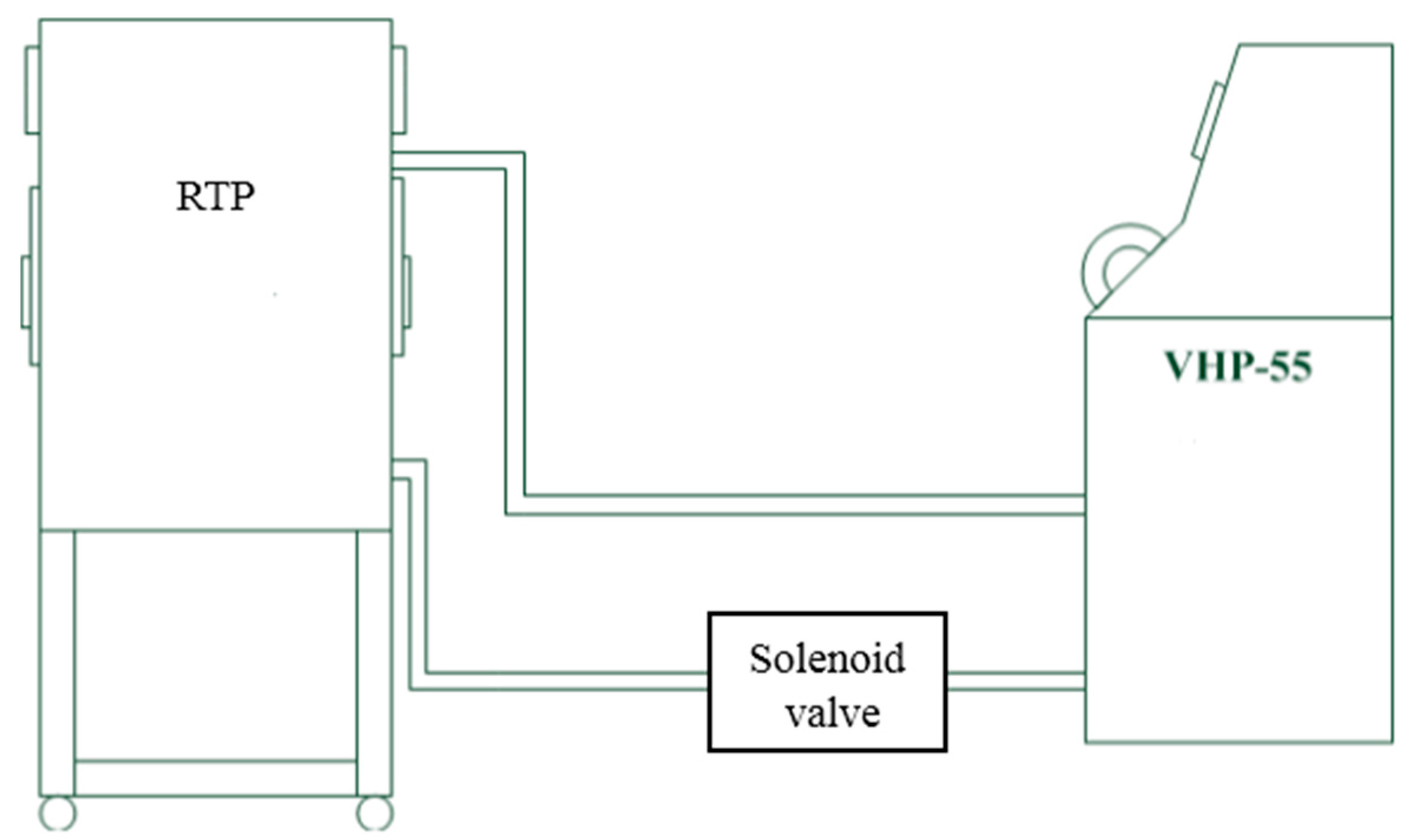
2.4. Vaporized Hydrogen Peroxide Fumigation Tests
3. Results and Discussion
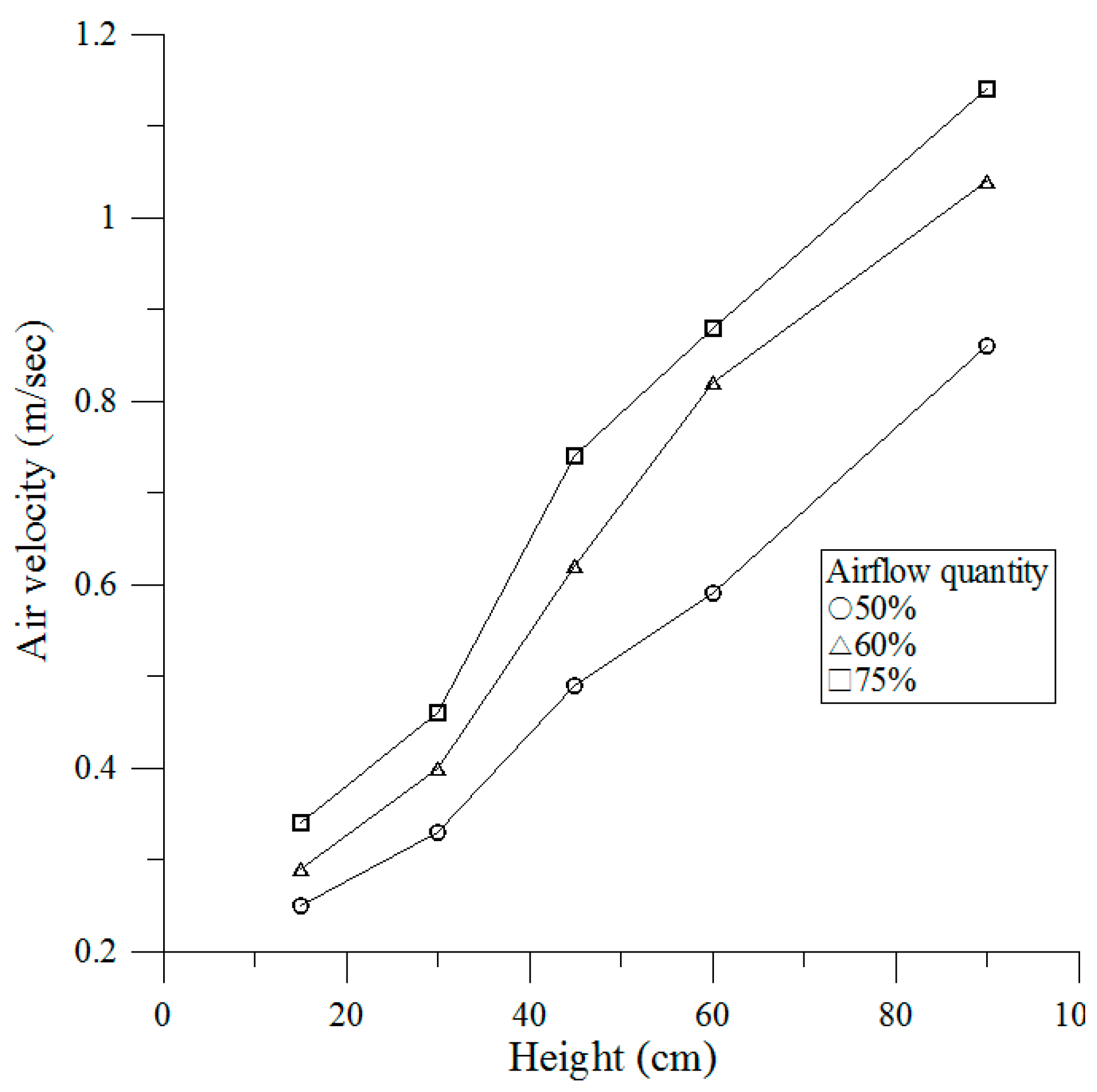
3.1. Airflow Velocity Test
3.2. Particle Count Method
3.3. Pressure Decay Tests
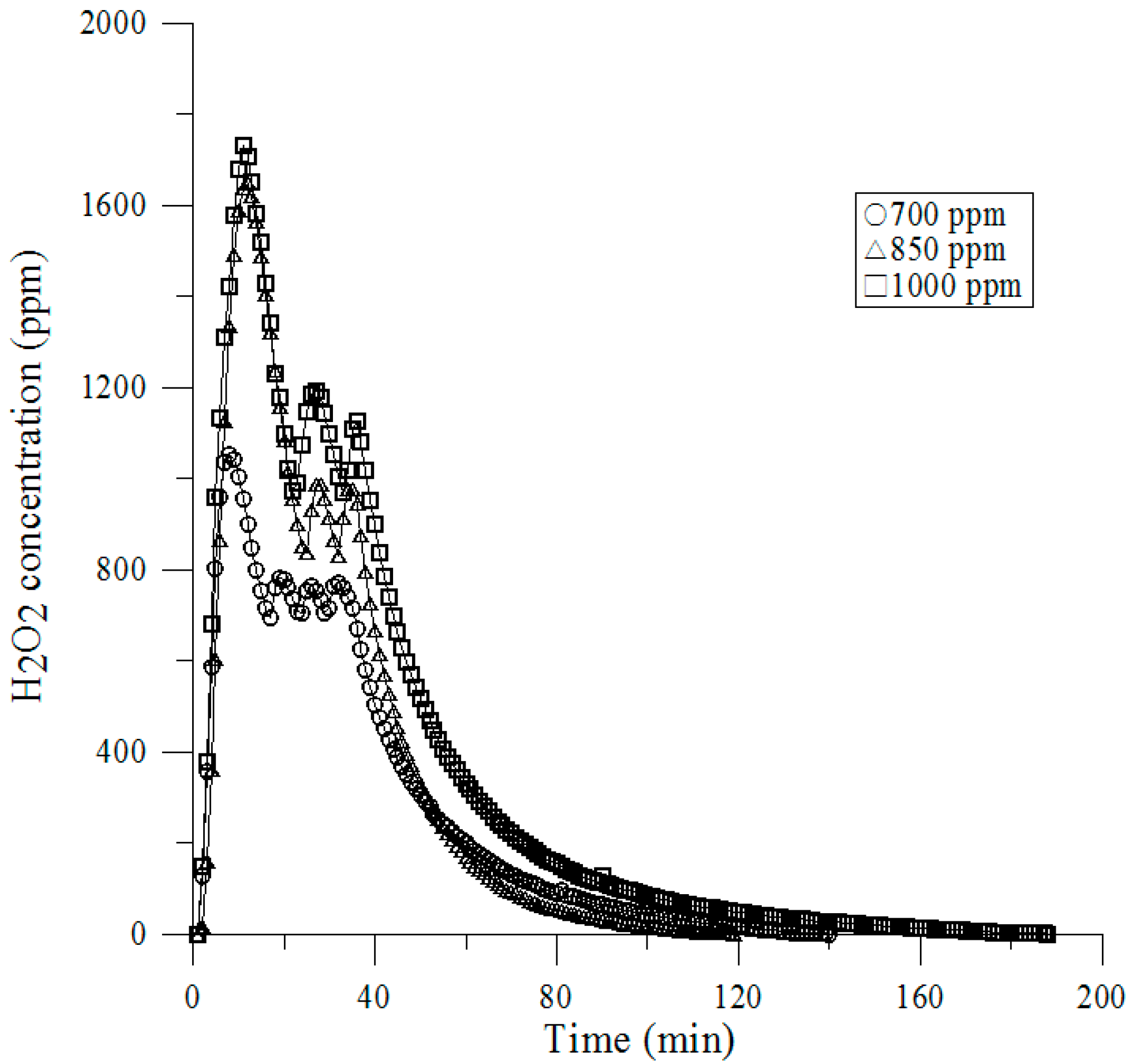
3.4. Vaporized Hydrogen Peroxide Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ISO 14644-1. Cleanrooms and Associated Controlled Environments—Part 1: Classification of Air Cleanliness. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14644:-1:ed-1:v1:en (accessed on 15 August 2016).
- Bioquell Inc. Bio-Decontamination of Material Transfer Systems. Available online: http://www.pharmaceuticalonline.com/doc/bio-decontamination-material-transfer-systems-0001 (accessed on 15 August 2016).
- Jahnke, M. Use of the HACCP concept for the risk analysis of pharmaceutical manufacturing processes. Eur. J. Parenter. Pharm. Sci. 1997, 2, 113–117. [Google Scholar]
- Sandle, T. The use of a risk assessment in the pharmaceutical industry—The application of FMEA to a sterility testing isolator: A case study. Eur. J. Parenter. Pharm. Sci. 2003, 8, 43–49. [Google Scholar]
- Tingley, S.; Baloda, S.; Belongia, B.; Liepold, G. The validation of a rapid sterile transfer port (RsTP) system used in barrier filling lines—An Improved Strategy for Materials Handling in the Pharmaceutical Industry. Pharm. Technol. 2003, 27, 32–40. [Google Scholar]
- Agalloco, J.; Akers, J.; Madsen, R. Current Practices in the Validation of Aseptic Processing 2001. Technical Report No. 36, PDA. J. Pharm. Sci. Technol. 2002, 56, 1–34. [Google Scholar]
- Michael, A.; Szatmary, F.T.; Worth, T.X. Rapid Transfer Port. U.S. Patent 6779567 B1, 2004. [Google Scholar]
- Tsai, C.; Caillet, C.; Hu, H.; Zhou, F.; Ding, H.; Zhang, G.; Zhou, B.; Wang, S.; Lu, S.; Buchy, P.; et al. Measurement of neutralizing antibody responses against H5N1 clades in immunized mice and ferrets using pseudotypes expressing influenza hemagglutinin and neuraminidase. Vaccine 2009, 27, 6777–9670. [Google Scholar] [CrossRef] [PubMed]
- Hillman, P.; Gebremedhin, K.; Warner, R. Ventilation system to minimize airborne bacteria, dust, humidity, and ammonia in calf nurseries. J. Dairy Sci. 1992, 75, 1305–1312. [Google Scholar] [CrossRef]
- Allen, J.R.; Burgess, M.; Aiken, S.C. Container Lid Gasket Protective Strip for Double Door Transfer System. U.S. Patent 0212054 A1, 2009. [Google Scholar]
- Kruse, R.H.; Puckett, W.H.; Richardson, J.H. Biological safety cabinetry. Clin. Microbiol. Rev. 1991, 4, 207–241. [Google Scholar] [CrossRef] [PubMed]
- Wathes, C.M.; Johnson, H.E. Physical protection against airborne pathogens and pollutants by a novel animal isolator in a level 3 containment laboratory. Epidemiol. Infect. 1991, 107, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Huang, R. Isolator of Using Negative Pressure for Big Animals. CN Patent Publication 1625942A, 2005. [Google Scholar]
- Tattershall, S.F. Enclosure for Handling Hazardous Material. U.S. Patent 7077486 B2, 2006. [Google Scholar]
- Advisory Committee on Dangerous Pathogens. Categorisation of Pathogens According to Hazard and Categories of Containment; HMSO: London, UK, 1983.
- Runkle, R.S.; Allendale, N.J.; Marsh, R.C.; Albuquerque, N.M. Unit for Providing Environmental Control of Animals. U.S. Patent 3630174, 1969. [Google Scholar]
- ISO 14644-7. Cleanrooms and Associated Controlled Environments—Part 7: Separative Devices (Clean Air Hoods, Gloveboxes, Isolators and Mini Environments) 2004. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14644:-7:ed-1:v1:en (accessed on 15 August 2016).
- ISO 10648-2. Containment Enclosures—Part 2: Classification According to Leak Tightness and Associated Checking Methods. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10648:-2:ed-1:v1:en (accessed on 15 August 2016).
- ASTM E2930. Standard Practice for Pressure Decay Leak Test Method 2013. Available online: https://www.astm.org/Standards/E2930.htm (accessed on 15 August 2016).
- ISO 14644-3. Cleanrooms and Associated Controlled Environments—Part 3 Test Methods, B.4 Airflow Test 2005. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14644:-3:ed-1:v1:en (accessed on 17 August 2016).
- ISO 14644-3. Cleanrooms and Associated Controlled Environments—Part 3 Test Methods, B.13 Containment Leak Test 2005. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14644:-3:ed-1:v1:en (accessed on 20 August 2016).
- ISO 14644-3. Cleanrooms and Associated Controlled Environments—Part 3 Test Methods, B.5 Air Pressure Difference Test 2005. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14644:-3:ed-1:v1:en (accessed on 17 August 2016).
- Doyle, D.F. Gas System Leakage Pressure Decay. J. Pressure Vessel Technol. 2013. [Google Scholar] [CrossRef]
- Baumeister, T.; Avallone, E.A.; Baumeister, T. Marks Mechanical Engineering Handbook, 8th ed.; McGraw Hill Co.: New York, NY, USA, 1978. [Google Scholar]
- ISO 14644-3. Cleanrooms and Associated Controlled Environments Part 3 Test Methods, C.4 Airflow Test 2005. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14644:-3:ed-1:v1:en (accessed on 15 August 2016).
- USP 797: Pharmaceutical Labs & Sterile Compounding. Available online: http://www.portafab.com/usp-797 (accessed on 15 August 2016).
- Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice. Available online: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070342.pdf (accessed on 15 August 2016).
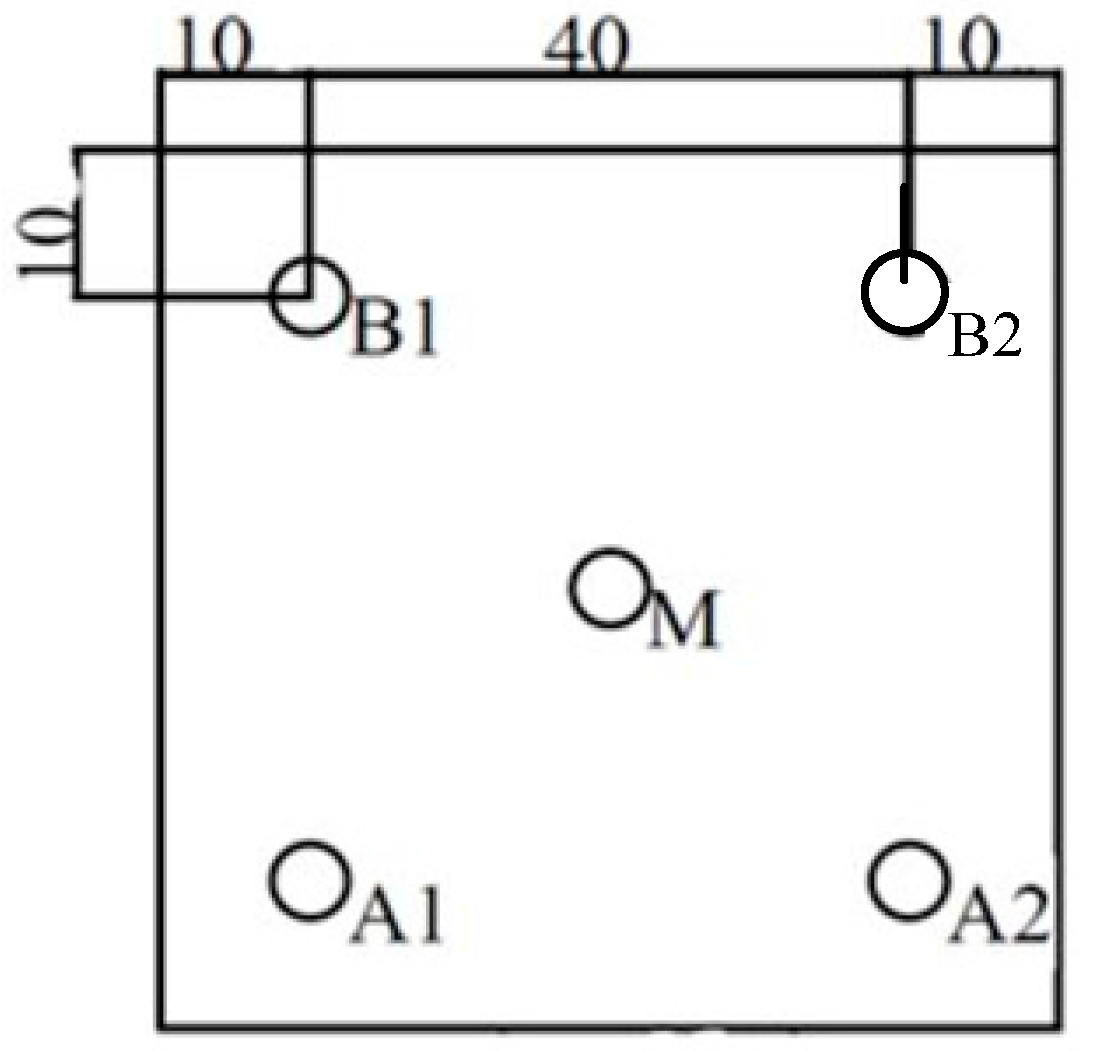

| 20 cm over Platform, 75% Airflow Quantity | |||||
| Measuring points | A1 | B1 | A2 | B2 | M |
| 0.3 µm | 456 | 6634 | 1427 | 301 | 956 |
| 0.5 µm | 33 | 475 | 96 | 26 | 76 |
| 1 µm | 9 | 158 | 38 | 4 | 14 |
| 3 µm | 0 | 12 | 0 | 0 | 1 |
| 5 µm | 1 | 7 | 2 | 0 | 1 |
| 10 µm | 0 | 0 | 0 | 0 | 0 |
| 20 cm over Platform, 60% Airflow Quantity | |||||
| Measuring points | A1 | B1 | A2 | B2 | M |
| 0.3 µm | 436 | 3035 | 2673 | 533 | 393 |
| 0.5 µm | 29 | 197 | 178 | 37 | 30 |
| 1 µm | 8 | 76 | 66 | 10 | 6 |
| 3 µm | 1 | 9 | 3 | 2 | 2 |
| 5 µm | 0 | 2 | 1 | 0 | 1 |
| 10 µm | 0 | 0 | 0 | 0 | 1 |
| 20 cm over Platform, 50% Airflow Quantity | |||||
| Measuring points | A1 | B1 | A2 | B2 | M |
| 0.3 µm | 1165 | 11,781 | 937 | 802 | 520 |
| 0.5 µm | 76 | 854 | 59 | 54 | 34 |
| 1 µm | 19 | 294 | 20 | 18 | 8 |
| 3 µm | 0 | 23 | 0 | 1 | 0 |
| 5 µm | 0 | 9 | 0 | 0 | 0 |
| 10 µm | 0 | 0 | 0 | 0 | 0 |
| 40 cm over Platform, 75% Airflow Quantity | |||||
| Measuring points | A1 | B1 | A2 | B2 | M |
| 0.3 µm | 88 | 30,766 | 27,221 | 2488 | 0 |
| 0.5 µm | 5 | 2387 | 2103 | 205 | 0 |
| 1 µm | 3 | 764 | 705 | 46 | 0 |
| 3 µm | 0 | 42 | 50 | 0 | 0 |
| 5 µm | 0 | 20 | 24 | 2 | 0 |
| 10 µm | 0 | 1 | 0 | 0 | 0 |
| 40 cm over Platform, 60% Airflow Quantity | |||||
| Measuring points | A1 | B1 | A2 | B2 | M |
| 0.3 µm | 1772 | 38,530 | 78,428 | 1712 | 0 |
| 0.5 µm | 130 | 2941 | 5473 | 115 | 0 |
| 1 µm | 38 | 1050 | 1665 | 30 | 0 |
| 3 µm | 6 | 100 | 145 | 3 | 0 |
| 5 µm | 2 | 50 | 75 | 2 | 0 |
| 10 µm | 0 | 2 | 3 | 1 | 0 |
| 40 cm over Platform, 50% Airflow Quantity | |||||
| Measuring points | A1 | B1 | A2 | B2 | M |
| 0.3 µm | 7642 | 7937 | 124,631 | 665 | 84 |
| 0.5 µm | 530 | 575 | 8763 | 50 | 10 |
| 1 µm | 148 | 172 | 2566 | 16 | 2 |
| 3 µm | 11 | 7 | 195 | 1 | 0 |
| 5 µm | 2 | 12 | 74 | 0 | 0 |
| 10 µm | 0 | 0 | 5 | 0 | 0 |
| 60 cm over Platform, 75% Airflow Quantity | |||||
| Measuring points | A1 | B1 | A2 | B2 | M |
| 0.3 µm | 45,210 | 3492 | 4218 | 5322 | 1 |
| 0.5 µm | 3432 | 265 | 295 | 414 | 0 |
| 1 µm | 1092 | 84 | 88 | 96 | 0 |
| 3 µm | 74 | 5 | 7 | 9 | 0 |
| 5 µm | 42 | 3 | 2 | 3 | 1 |
| 10 µm | 3 | 1 | 0 | 1 | 0 |
| 60 cm over Platform, 60% Airflow Quantity | |||||
| Measuring points | A1 | B1 | A2 | B2 | M |
| 0.3 µm | 1971 | 33,288 | 18,861 | 43,865 | 1 |
| 0.5 µm | 159 | 2513 | 1376 | 3361 | 0 |
| 1 µm | 42 | 851 | 370 | 1076 | 0 |
| 3 µm | 4 | 57 | 26 | 76 | 0 |
| 5 µm | 1 | 32 | 13 | 36 | 0 |
| 10 µm | 0 | 1 | 1 | 2 | 0 |
| 60 cm over Platform, 50% Airflow Quantity | |||||
| Measuring points | A1 | B1 | A2 | B2 | M |
| 0.3 µm | 2034 | 89,337 | 15,599 | 4054 | 1 |
| 0.5 µm | 120 | 6895 | 1123 | 262 | 0 |
| 1 µm | 40 | 2174 | 341 | 79 | 0 |
| 3 µm | 6 | 172 | 24 | 3 | 0 |
| 5 µm | 2 | 82 | 10 | 2 | 0 |
| 10 µm | 0 | 2 | 2 | 0 | 0 |
| Pi (Pa) | Pressure (P) vs. Time (t) | Pf (Pa) |
|---|---|---|
| 45 | P = −0.0033 ln(t) + 4.0742, R2 = 0.9612 | 7.2 |
| 30 | P = −0.0036 ln(t) + 3.5563, R2 = 0.961 | 5.6 |
| 15 | P = −0.0242 ln(t) + 3.3191, R2 = 0.9775 | 2.2 |
| Total Concentration of Sterilant | 700 ppm | 850 ppm | 1000 ppm |
|---|---|---|---|
| Positive BIs/Total BIs | 0/120 | 120/120 | 0/120 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.-C.; Shiue, A.; Liu, H.-Y.; Chiu, R.-B. Validation of Contamination Control in Rapid Transfer Port Chambers for Pharmaceutical Manufacturing Processes. Int. J. Environ. Res. Public Health 2016, 13, 1129. https://doi.org/10.3390/ijerph13111129
Hu S-C, Shiue A, Liu H-Y, Chiu R-B. Validation of Contamination Control in Rapid Transfer Port Chambers for Pharmaceutical Manufacturing Processes. International Journal of Environmental Research and Public Health. 2016; 13(11):1129. https://doi.org/10.3390/ijerph13111129
Chicago/Turabian StyleHu, Shih-Cheng, Angus Shiue, Han-Yang Liu, and Rong-Ben Chiu. 2016. "Validation of Contamination Control in Rapid Transfer Port Chambers for Pharmaceutical Manufacturing Processes" International Journal of Environmental Research and Public Health 13, no. 11: 1129. https://doi.org/10.3390/ijerph13111129





