Ambient Fine Particulate Matter Exposure and Risk of Cardiovascular Mortality: Adjustment of the Meteorological Factors
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Setting
2.2. Data Collection
2.3. Statistical Analysis
2.3.1. Analytic Plan
2.3.2. Associations and Exposure-Response Relationships between PM2.5 and Mortality
2.3.3. Impacts of Extensive Adjustment of Temperature and Relative Humidity
2.4. Sensitivity Analysis
3. Results
3.1. Descriptive Results
3.2. Associations and Exposure-Response Relationships between PM2.5 and Mortality in Whole Population and Subgroup Population from Base Models
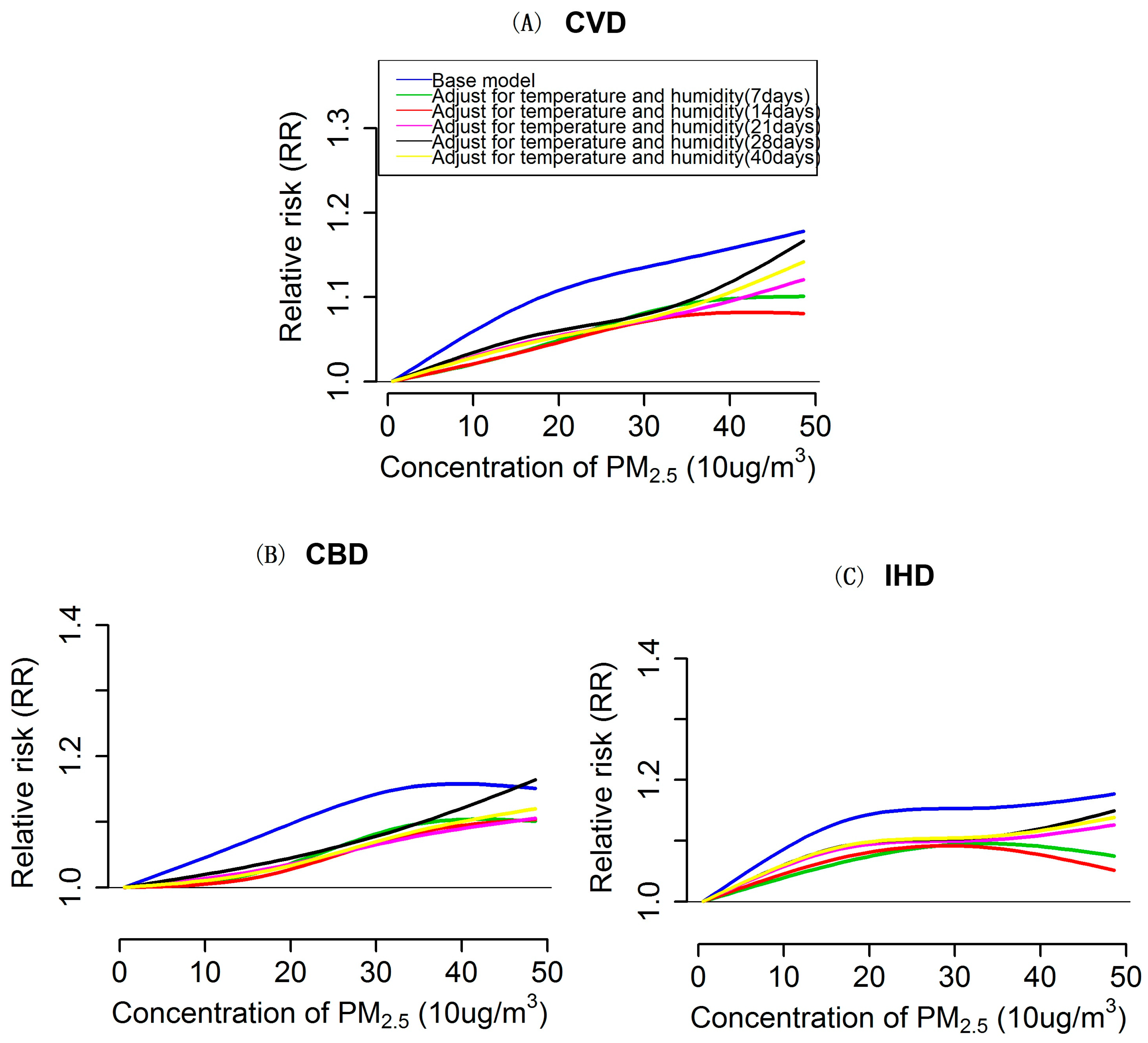
3.3. Impacts of Extensively Adjustment of Temperature and Humidity on PM2.5 Effects Estimation and Exposure-Response Relationships
3.4. Sensitivity Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pope, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Hu, J.L.; Goldberg, D.; Reynolds, P.; Hertz, A.; Bernstein, L.; Kleeman, M.J. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: Results from the California teachers study cohort. Environ. Health Perspect. 2015, 123, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Samoli, E.; Alessandrini, E.; Cadum, E.; Ostro, B.; Berti, G.; Faustini, A.; Jacquemin, B.; Linares, C.; Pascal, M.; et al. Short-term associations between fine and coarse particulate matter and hospitalizations in southern europe: Results from the med-particles project. Environ. Health Perspect. 2013, 121, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J. The effect of fine and coarse particulate air pollution on mortality: A national analysis. Environ. Health Perspect. 2009, 117, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Koutrakis, P.; Coull, B.; Kloog, I.; Schwartz, J. Acute effect of fine particulate matter on mortality in three southeastern states from 2007–2011. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Honda, Y.; Hashizume, M.; Guo, Y.L.; Wu, C.F.; Kan, H.; Jung, K.; Lim, Y.H.; Yi, S.; Kim, H. Short-term exposure to fine and coarse particles and mortality: A multicity time-series study in East Asia. Environ. Pollut. 2015, 207, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.D.; London, S.J.; Chen, G.H.; Zhang, Y.H.; Song, G.X.; Zhao, N.Q.; Jiang, L.L.; Chen, B.H. Differentiating the effects of fine and coarse particles on daily mortality in Shanghai, China. Environ. Int. 2007, 33, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xin, J.Y.; Wang, Y.S.; Wang, S.G.; Li, G.X.; Pan, X.C.; Liu, Z.R.; Wang, L.L. The acute effects of fine particles on respiratory mortality and morbidity in Beijing, 2004–2009. Environ. Sci. Pollut. Res. 2013, 20, 6433–6444. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Chen, R.J.; Pan, G.W.; Xu, X.H.; Song, W.M.; Chen, B.H.; Kan, H.D. Fine particulate air pollution and daily mortality in Shenyang, China. Sci. Total Environ. 2011, 409, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Samet, J.M.; Richardson, D.B. Commentary: Does air pollution confound studies of temperature? Epidemiology 2014, 25, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Guo, Y.M.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.L.; Rocklov, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Kan, H.D.; Chen, R.J.; Tong, S.L. Ambient air pollution, climate change, and population health in China. Environ. Int. 2012, 42, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Welty, L.J.; Zeger, S.L. Are the acute effects of particulate matter on mortality in the national morbidity, mortality, and air pollution study the result of inadequate control for weather and season? A sensitivity analysis using flexible distributed lag models. Am. J. Epidemiol. 2005, 162, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.X.; Peng, X.W.; Huang, W.; Chen, R.J.; Xu, Z.C.; Chen, B.H.; Kan, H.D. A time-stratified case-crossover study of fine particulate matter air pollution and mortality in Guangzhou, China. Int. Arch. Occup. Environ. Health 2012, 85, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Analitis, A.; Touloumi, G.; Schwartz, J.; Anderson, H.R.; Sunyer, J.; Bisanti, L.; Zmirou, D.; Vonk, J.M.; Pekkanen, J.; et al. Estimating the exposure-response relationships between particulate matter and mortality within the aphea multicity project. Environ. Health Perspect. 2005, 113, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Annual Statistical Reports of Beijing. Available online: http://www.bjstats.gov.cn/ (accessed on 12 May 2016).
- Xie, W.X.; Li, G.; Zhao, D.; Xie, X.Q.; Wei, Z.H.; Wang, W.; Wang, M.; Li, G.X.; Liu, W.R.; Sun, J.Y.; et al. Relationship between fine particulate air pollution and ischaemic heart disease morbidity and mortality. Heart 2015, 101, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zou, T.; Guo, B.; Li, S.; Zhang, H.Z.; Zhang, S.Y.; Huang, H.; Chen, S.X. Assessing Beijing’s PM2.5 pollution: Severity, weather impact, APEC and winter heating. Proc. R. Soc. A Math. Phys. Eng. 2015. [Google Scholar] [CrossRef]
- Junger, W.L.; de Leon, A.P. Imputation of missing data in time series for air pollutants. Atmos. Environ. 2015, 102, 96–104. [Google Scholar] [CrossRef]
- Janes, H.; Sheppard, L.; Lumley, T. Case-crossover analyses of air pollution exposure data: Referent selection strategies and their implications for bias. Epidemiology 2005, 16, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zeger, S.L. On the equivalence of case-crossover and time series methods in environmental epidemiology. Biostatistics 2007, 8, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Symons, J.M.; Geyh, A.S.; Zeger, S.L. An approach to checking case-crossover analyses based on equivalence with time-series methods. Epidemiology 2008, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.G.; Gasparrini, A.; Tobias, A. Conditional poisson models: A flexible alternative to conditional logistic case cross-over analysis. BMC Med. Res. Methodol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Distributed lag non-linear models. Stat. Med. 2010, 29, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Barnett, A.G.; Pan, X.C.; Yu, W.W.; Tong, S.L. The impact of temperature on mortality in Tianjin, China: A case-crossover design with a distributed lag nonlinear model. Environ. Health Perspect. 2011, 119, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Stafoggia, M.; Rodopoulou, S.; Ostro, B.; Declercq, C.; Alessandrini, E.; Diaz, J.; Karanasiou, A.; Kelessis, A.G.; Le Tertre, A.; et al. Associations between fine and coarse particles and mortality in mediterranean cities: Results from the med-particles project. Environ. Health Perspect. 2013, 121, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Forastiere, F.; Faustini, A.; Biggeri, A.; Bisanti, L.; Cadum, E.; Cernigliaro, A.; Mallone, S.; Pandolfi, P.; Serinelli, M.; et al. Susceptibility factors to ozone-related mortality a population-based case-crossover analysis. Am. J. Respir. Crit. Care Med. 2010, 182, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. Assessing confounding, effect modification, and thresholds in the association between ambient particles and daily deaths. Environ. Health Perspect. 2000, 108, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. The distributed lag between air pollution and daily deaths. Epidemiology 2000, 11, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J.; Samoli, E.; Gryparis, A.; Touloumi, G.; Atkinson, R.; Le Tertre, A.; Bobros, J.; Celko, M.; Goren, A.; et al. The temporal pattern of mortality responses to air pollution: A multicity assessment of mortality displacement. Epidemiology 2002, 13, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J.; Samoli, E.; Gryparis, A.; Touloumi, G.; Peacock, J.; Anderson, R.H.; Le Tertre, A.; Bobros, J.; Celko, M.; et al. The temporal pattern of respiratory and heart disease mortality in response to air pollution. Environ. Health Perspect. 2003, 111, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Samet, J.M.; Dominici, F. Time-series studies of particulate matter. Annu. Rev. Public Health 2004, 25, 247–280. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Gasparrini, A.; Armstrong, B.; Li, S.S.; Tawatsupa, B.; Tobias, A.; Lavigne, E.; Coelho, M.D.Z.S.; Leone, M.; Pan, X.C.; et al. Global variation in the effects of ambient temperature on mortality a systematic evaluation. Epidemiology 2014, 25, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; Tong, S.; Clements, A.C.A. What measure of temperature is the best predictor of mortality? Environ. Res. 2010, 110, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A. Distributed lag linear and non-linear models in R: The package dlnm. J. Stat. Softw. 2011, 43, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wood, S. Mgcv: GAMs and generalized ridge regression for R. R News 2001, 1, 20–25. [Google Scholar]
- Peng, R.D.; Dominici, F.; Louis, T.A. Model choice in time series studies of air pollution and mortality. J. R. Stat. Soc. Ser. A Stat. 2006, 169, 179–198. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Gasparrini, A.; Hajat, S.; Smeeth, L.; Armstrong, B. Time series regression studies in environmental epidemiology. Int. J. Epidemiol. 2013, 42, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.; Zeger, S.; Kelsall, J.; Xu, J.; Kalkstein, L. Does weather confound or modify the association of particulate air pollution with mortality? An analysis of the philadelphia data, 1973–1980. Environ. Res. 1998, 77, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Li, S.S.; Tian, Z.X.; Pan, X.C.; Zhang, J.L.; Williams, G. The burden of air pollution on years of life lost in Beijing, China, 2004–2008: Retrospective regression analysis of daily deaths. Br. Med. J. 2013. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yin, P.; Zhou, M.; Ou, C.Q.; Li, M.; Li, J.; Liu, X.; Gao, J.; Liu, Y.; Qin, R.; et al. The burden of stroke mortality attributable to cold and hot ambient temperatures: Epidemiological evidence from China. Environ. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Turner, M.C.; Cohen, A.; Krewski, D.; Jerrett, M.; Gapstur, S.M.; Thun, M.J. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: Shape of the exposure-response relationships. Environ. Health Perspect. 2011, 119, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Krewski, D.; Jerrett, M.; Shi, Y.L.; Calle, E.E.; Thun, M.J. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke shape of the exposure-response relationship. Circulation 2009, 120, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.A.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.F.; Zanobetti, A.; Schwartz, J. The time course of weather-related deaths. Epidemiology 2001, 12, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, G.X.; Zhao, L.; Jiang, G.H.; Pan, X.C. Characteristics of the exposure-response relationship of particulate matter and mortality a time series analysis of 7 cities in China. J. Occup. Environ. Med. 2015, 57, e93–e100. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.W.; Hu, X.F.; Sayer, A.M.; Levy, R.; Zhang, Q.; Xue, Y.G.; Tong, S.L.; Bi, J.; Huang, L.; Liu, Y. Satellite-based spatiotemporal trends in PM2.5 concentrations: China, 2004–2013. Environ. Health Perspect. 2016, 124, 184–192. [Google Scholar] [CrossRef] [PubMed]


| Factor | Mean ± SD | Percentiles | ||||
|---|---|---|---|---|---|---|
| Min | P25 | P50 | P75 | Max | ||
| Outcomes | ||||||
| CVD | 99.57 ± 20.36 | 54 | 85 | 97 | 113 | 173 |
| CBD | 46.25 ± 10.32 | 22 | 39 | 45 | 53 | 82 |
| IHD | 44.87 ± 11.18 | 20 | 37 | 44 | 52 | 92 |
| Environmental Data | ||||||
| PM2.5 (μg/m3) | 95.68 ± 70.83 | 5.83 | 41.79 | 80.09 | 127.92 | 492.75 |
| Temperature (°C) | 13.21 ± 11.34 | −12.5 | 2.2 | 14.9 | 24.0 | 34.5 |
| Barometric Pressure (kPa) | 101.24 ± 1.03 | 98.97 | 100.41 | 101.18 | 102.05 | 103.93 |
| Relative Humidity (%) | 50.86 ± 19.97 | 9 | 34 | 52 | 67 | 95 |
| Wind (m/s) | 2.23 ± 0.93 | 0.5 | 1.6 | 2.1 | 2.7 | 6.4 |
| Mortality | Lag Days | Whole Population | Gender | Age Group | ||
|---|---|---|---|---|---|---|
| Male | Female | ≥65 | <65 | |||
| CVD | ||||||
| Lag 0–1 | 0.42 (0.28, 0.56) | 0.47 (0.29, 0.64) | 0.36 (0.16, 0.56) | 0.46 (0.31, 0.62) | 0.23 (−0.06, 0.52) | |
| Lag 2–5 | −0.15 (−0.29, −0.02) | −0.10 (−0.27, 0.07) | −0.22 (−0.41, −0.03) | −0.14 (−0.29, 0.01) | −0.20 (−0.47, 0.08) | |
| Lag 0–5 | 0.24 (0.05, 0.43) | 0.34 (0.10, 0.58) | 0.12 (−0.15, 0.39) | 0.30 (0.08, 0.51) | −0.01 (−0.41, 0.39) | |
| CBD | ||||||
| Lag 0–1 | 0.42 (0.23, 0.62) | 0.46 (0.21, 0.70) | 0.38 (0.10, 0.66) | 0.45 (0.24, 0.66) | 0.31 (−0.12, 0.75) | |
| Lag 2–5 | −0.16 (−0.34, 0.02) | −0.03 (−0.26, 0.20) | −0.33 (−0.60, −0.06) | −0.14 (−0.35, 0.06) | −0.23 (−0.64, 0.18) | |
| Lag 0–5 | 0.23 (−0.03, 0.50) | 0.39 (0.05, 0.72) | 0.03 (−0.36, 0.42) | 0.29 (0.00, 0.58) | −0.02 (−0.62, 0.58) | |
| IHD | ||||||
| Lag 0–1 | 0.47 (0.26, 0.67) | 0.58 (0.31, 0.84) | 0.34 (0.05, 0.62) | 0.52 (0.29, 0.74) | 0.23 (−0.21, 0.66) | |
| Lag 2–5 | −0.23 (−0.43, −0.04) | −0.26 (−0.51, −0.01) | −0.20 (−0.47, 0.07) | −0.21 (−0.42, 0.01) | −0.35 (−0.77, 0.06) | |
| Lag 0–5 | 0.22 (−0.06, 0.50) | 0.28 (−0.08, 0.64) | 0.16 (−0.24, 0.55) | 0.28 (−0.02, 0.59) | −0.07 (−0.67, 0.53) | |
| Outcome | Population | Base Model a | Extensive Adjusted Model b | ||||
|---|---|---|---|---|---|---|---|
| 7 Days | 14 Days | 21 Days | 28 Days | 40 Days | |||
| CVD | Whole population | 0.42 (0.28, 0.56) | 0.25 (0.11, 0.40) | 0.22 (0.07, 0.37) | 0.24 (0.09, 0.38) | 0.27 (0.13, 0.42) | 0.25 (0.11, 0.39) |
| Male | 0.47 (0.29, 0.64) | 0.26 (0.07, 0.44) | 0.21 (0.02, 0.40) | 0.25 (0.06, 0.44) | 0.28 (0.10, 0.47) | 0.26 (0.08, 0.43) | |
| Female | 0.36 (0.16, 0.56) | 0.25 (0.04, 0.46) | 0.22 (0.01, 0.44) | 0.21 (0.00, 0.43) | 0.26 (0.05, 0.46) | 0.24 (0.04, 0.44) | |
| ≥65 | 0.46 (0.31, 0.62) | 0.28 (0.11, 0.44) | 0.25 (0.08, 0.41) | 0.25 (0.08, 0.41) | 0.29 (0.13, 0.45) | 0.28 (0.12, 0.43) | |
| <65 | 0.14 (−0.16, 0.43) | 0.15 (−0.17, 0.47) | 0.10 (−0.22, 0.42) | 0.18 (−0.14, 0.50) | 0.19 (−0.13, 0.50) | 0.13 (−0.18, 0.43) | |
| CBD | |||||||
| Whole population | 0.42 (0.23, 0.62) | 0.26 (0.05, 0.47) | 0.22 (0.01, 0.43) | 0.21 (0.00, 0.42) | 0.27 (0.06, 0.47) | 0.23 (0.03, 0.42) | |
| Male | 0.46 (0.21, 0.70) | 0.22 (−0.04, 0.49) | 0.15 (−0.11, 0.42) | 0.16 (−0.11, 0.43) | 0.19 (−0.07, 0.45) | 0.17 (−0.08, 0.42) | |
| Female | 0.38 (0.10, 0.66) | 0.31 (0.00, 0.61) | 0.31 (0.00, 0.62) | 0.28 (−0.03, 0.60) | 0.35 (0.05, 0.67) | 0.30 (0.01, 0.60) | |
| ≥65 | 0.45 (0.24, 0.66) | 0.28 (0.05, 0.51) | 0.23 (0.01, 0.46) | 0.21 (−0.02, 0.43) | 0.26 (0.04, 0.48) | 0.22 (0.01, 0.43) | |
| <65 | 0.31 (−0.12, 0.75) | 0.17 (−0.30, 0.65) | 0.18 (−0.30, 0.67) | 0.25 (−0.23, 0.74) | 0.31 (−0.16, 0.78) | 0.26 (−0.19, 0.72) | |
| IHD | |||||||
| Whole population | 0.47 (0.26, 0.67) | 0.27 (0.05, 0.49) | 0.25 (0.03, 0.47) | 0.30 (0.08, 0.53) | 0.33 (0.11, 0.54) | 0.33 (0.12, 0.54) | |
| Male | 0.58 (0.31, 0.84) | 0.36 (0.05, 0.62) | 0.35 (0.06, 0.64) | 0.43 (0.14, 0.72) | 0.47 (0.18, 0.75) | 0.46 (0.19, 0.73) | |
| Female | 0.34 (0.05, 0.62) | 0.16 (−0.14, 0.47) | 0.14 (−0.18, 0.45) | 0.15 (−0.16, 0.47) | 0.16 (−0.14, 0.47) | 0.18 (−0.12, 0.47) | |
| ≥65 | 0.52 (0.29, 0.74) | 0.28 (0.04, 0.52) | 0.28 (0.03, 0.52) | 0.31 (0.07, 0.56) | 0.35 (0.12, 0.59) | 0.37 (0.14, 0.60) | |
| <65 | 0.23 (−0.21, 0.66) | 0.23 (−0.25, 0.71) | 0.14 (−0.35, 0.62) | 0.25 (−0.24, 0.74) | 0.21 (−0.27, 0.69) | 0.15 (−0.31, 0.61) | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, K.; Li, W.; Zhang, R.; Li, R.; Xu, Q.; Cao, Y. Ambient Fine Particulate Matter Exposure and Risk of Cardiovascular Mortality: Adjustment of the Meteorological Factors. Int. J. Environ. Res. Public Health 2016, 13, 1082. https://doi.org/10.3390/ijerph13111082
Luo K, Li W, Zhang R, Li R, Xu Q, Cao Y. Ambient Fine Particulate Matter Exposure and Risk of Cardiovascular Mortality: Adjustment of the Meteorological Factors. International Journal of Environmental Research and Public Health. 2016; 13(11):1082. https://doi.org/10.3390/ijerph13111082
Chicago/Turabian StyleLuo, Kai, Wenjing Li, Ruiming Zhang, Runkui Li, Qun Xu, and Yang Cao. 2016. "Ambient Fine Particulate Matter Exposure and Risk of Cardiovascular Mortality: Adjustment of the Meteorological Factors" International Journal of Environmental Research and Public Health 13, no. 11: 1082. https://doi.org/10.3390/ijerph13111082
APA StyleLuo, K., Li, W., Zhang, R., Li, R., Xu, Q., & Cao, Y. (2016). Ambient Fine Particulate Matter Exposure and Risk of Cardiovascular Mortality: Adjustment of the Meteorological Factors. International Journal of Environmental Research and Public Health, 13(11), 1082. https://doi.org/10.3390/ijerph13111082








