Degradation and Mineralization of Benzohydroxamic Acid by Synthesized Mesoporous La/TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization
2.3. Photocatalytic Degradation of Benzohydroxamic Acid
3. Results and Discussion
3.1. Characterization
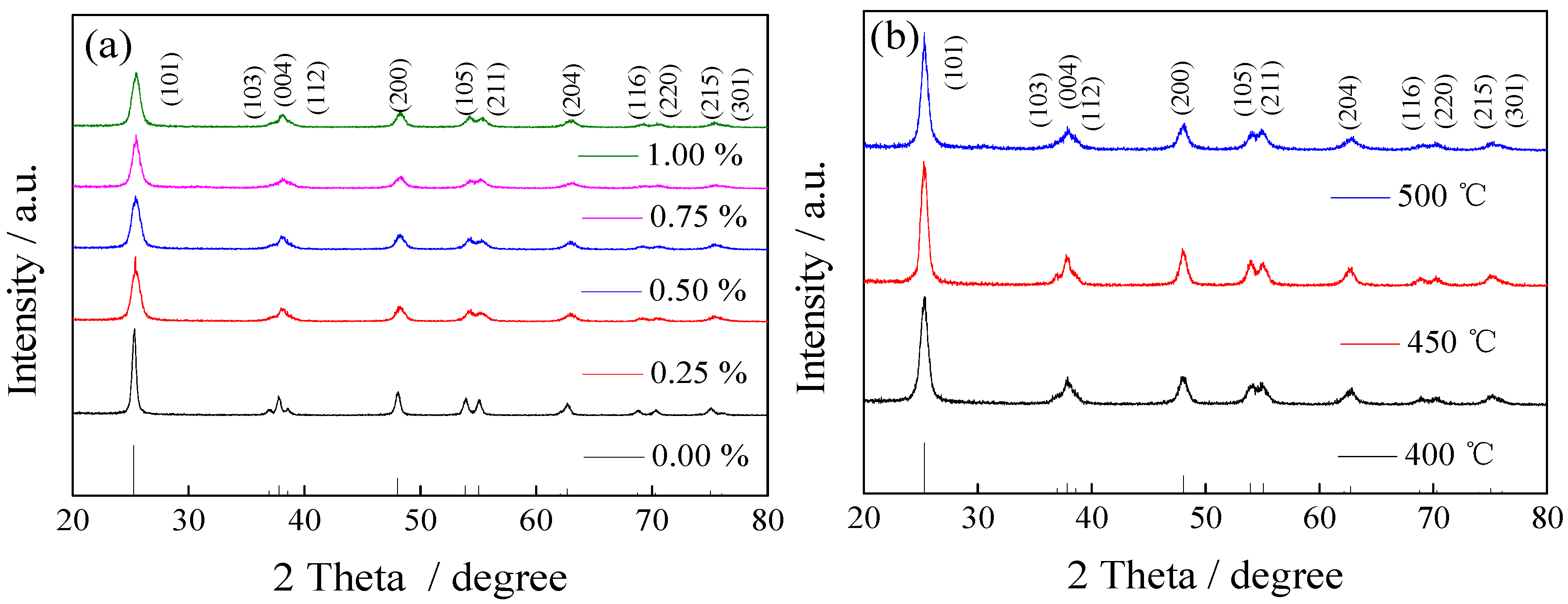
3.1.1. XRD
3.1.2. XPS
3.1.3. UV-vis DRS
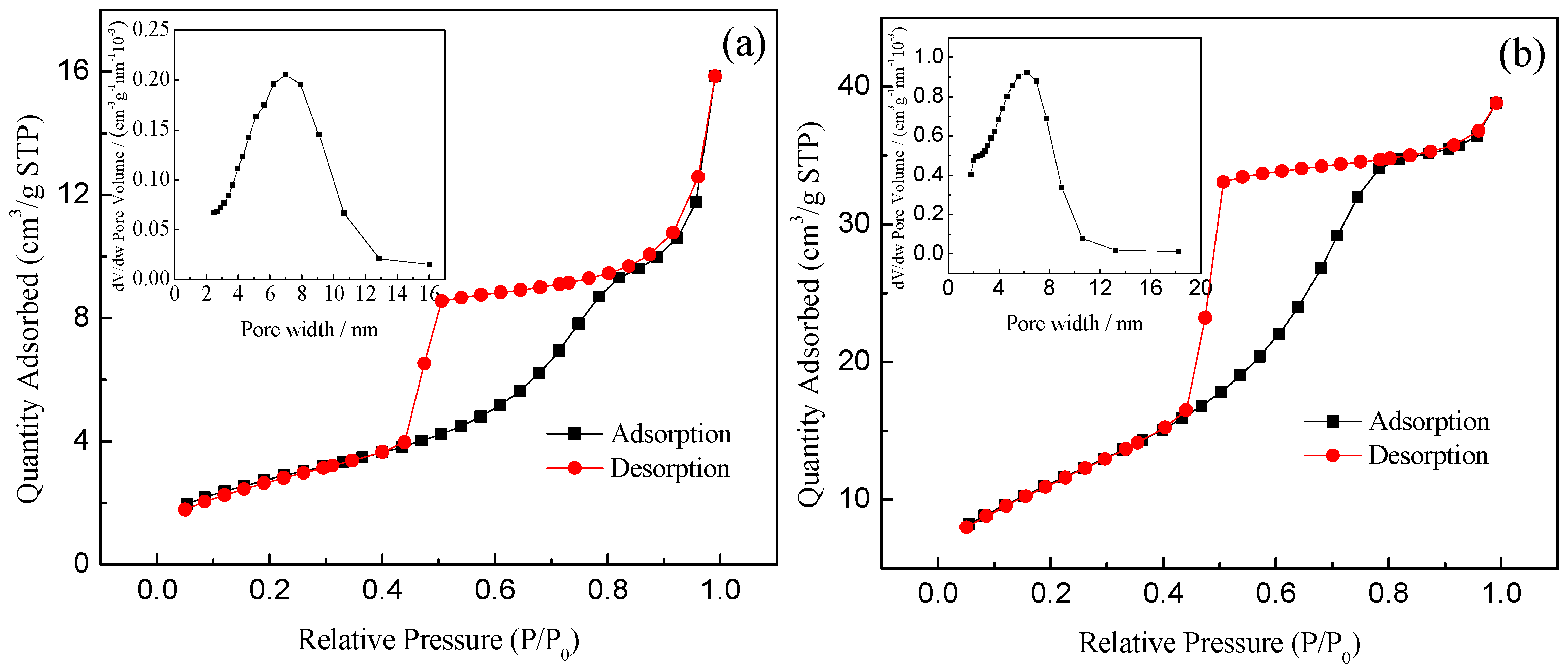
3.1.4. Specific Surface Area and Porosity Analysis
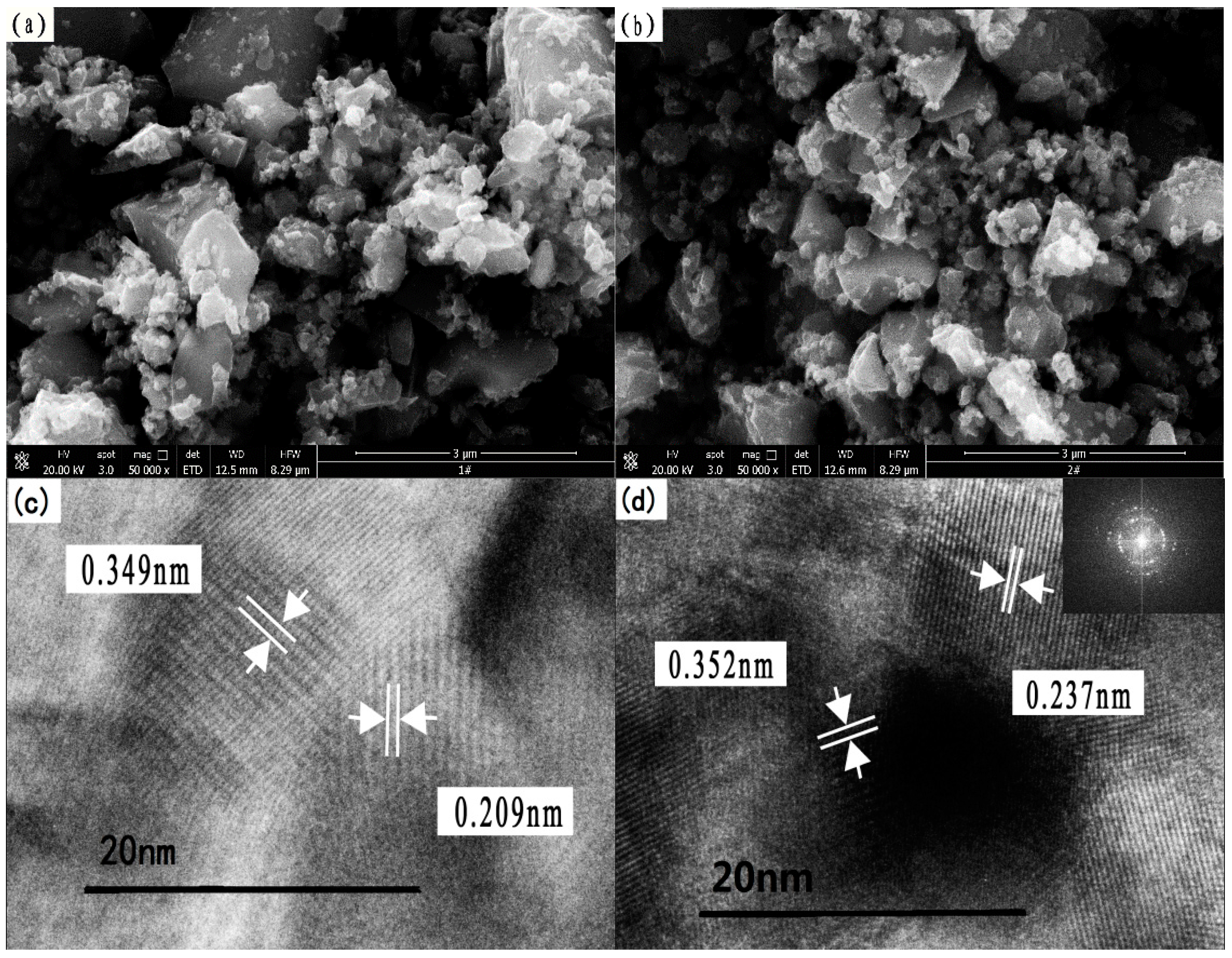
3.1.5. Microstructure Analysis
3.2. Photocatalytic Degradation of Benzohydroxamic Acid
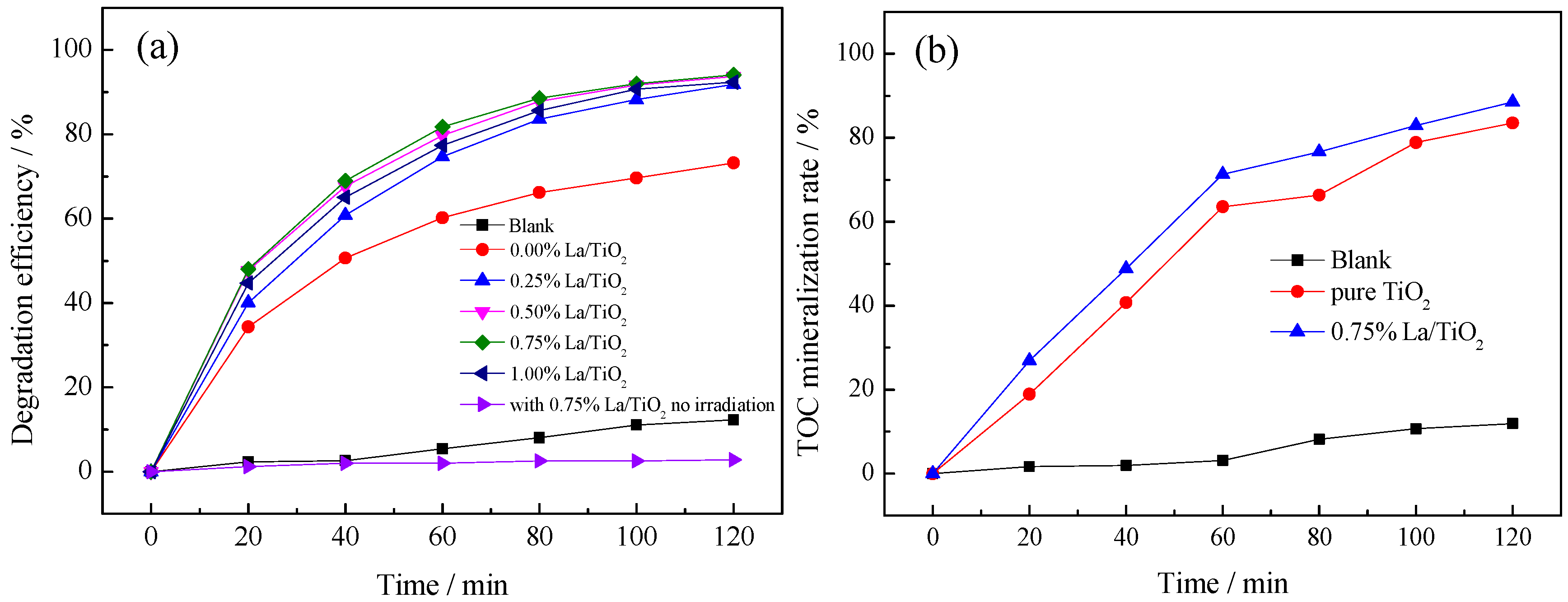
3.2.1. Effect of La3+ Doping Amount
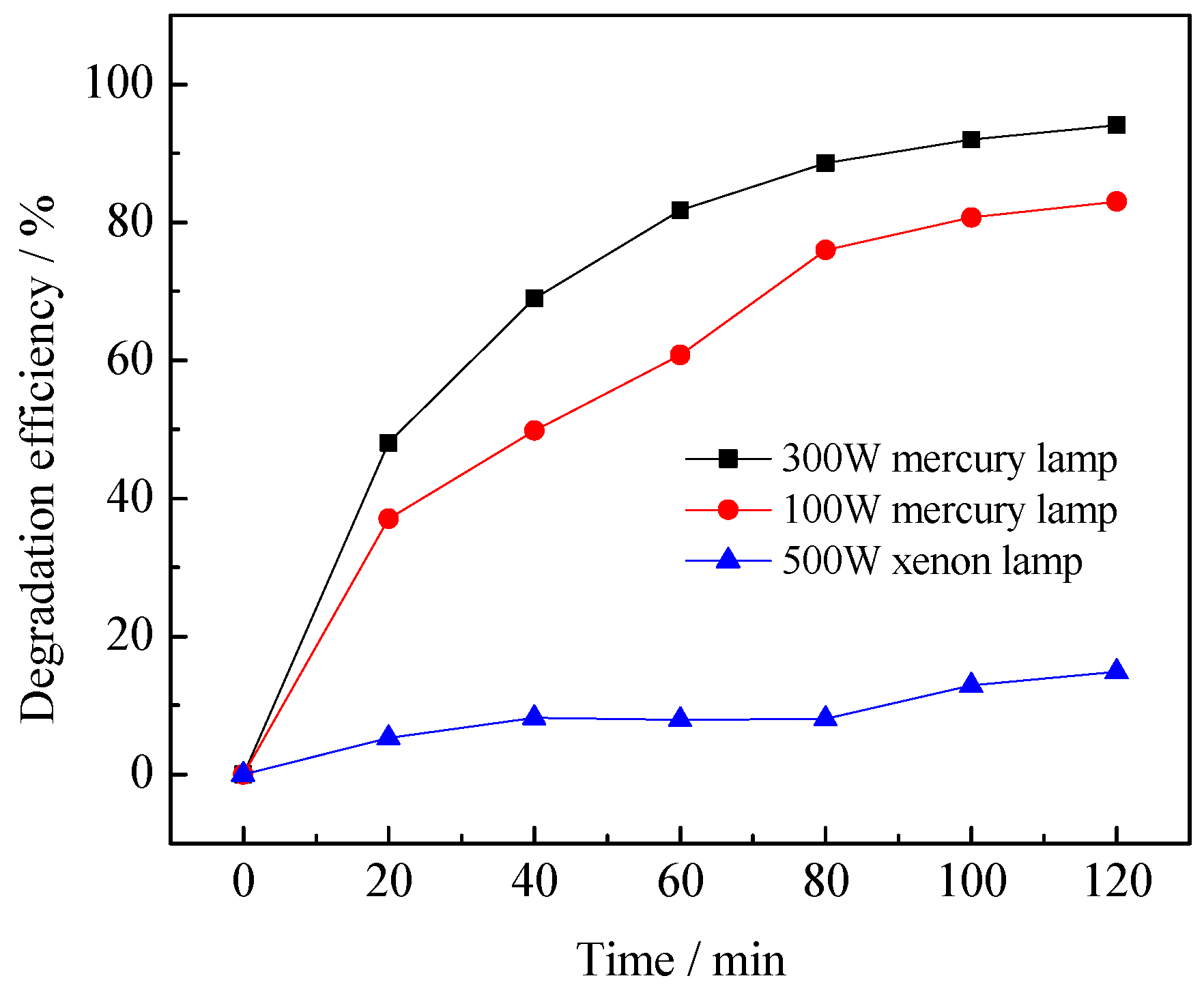
3.2.2. Effect of Light Intensity
3.2.3. Effect of Catalyst Dosage
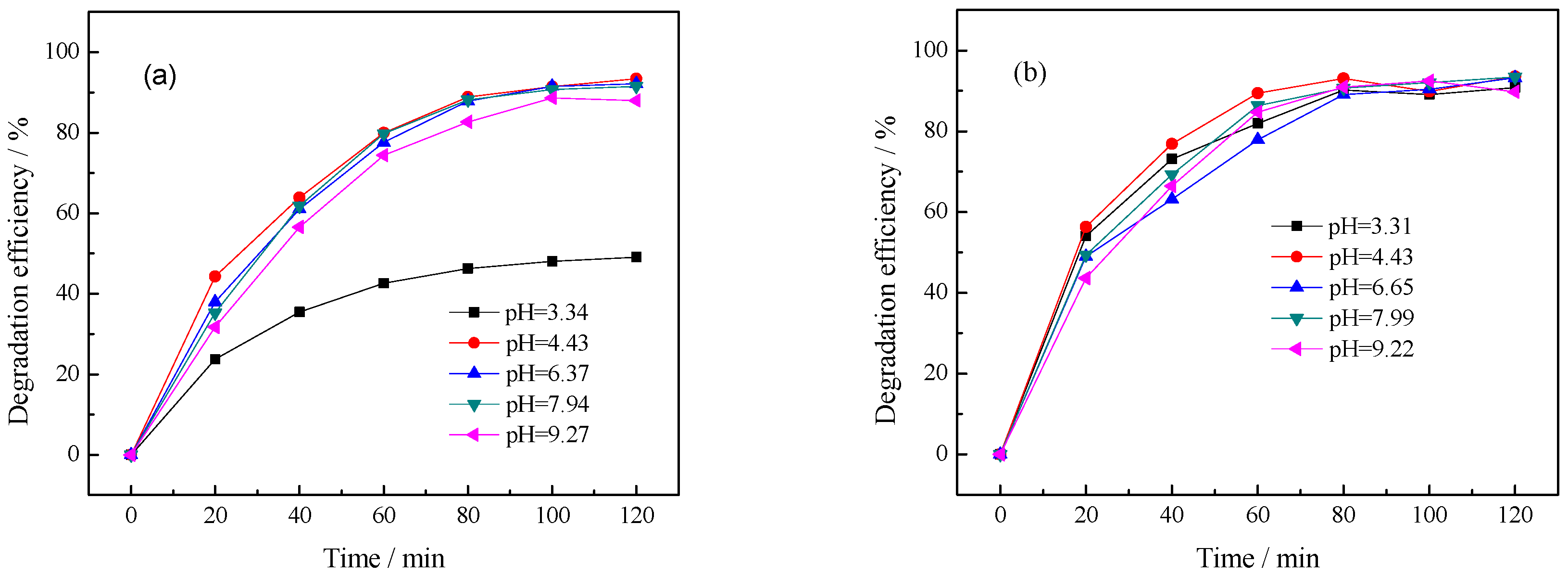
3.2.4. Effect of Initial pH Value of Solution
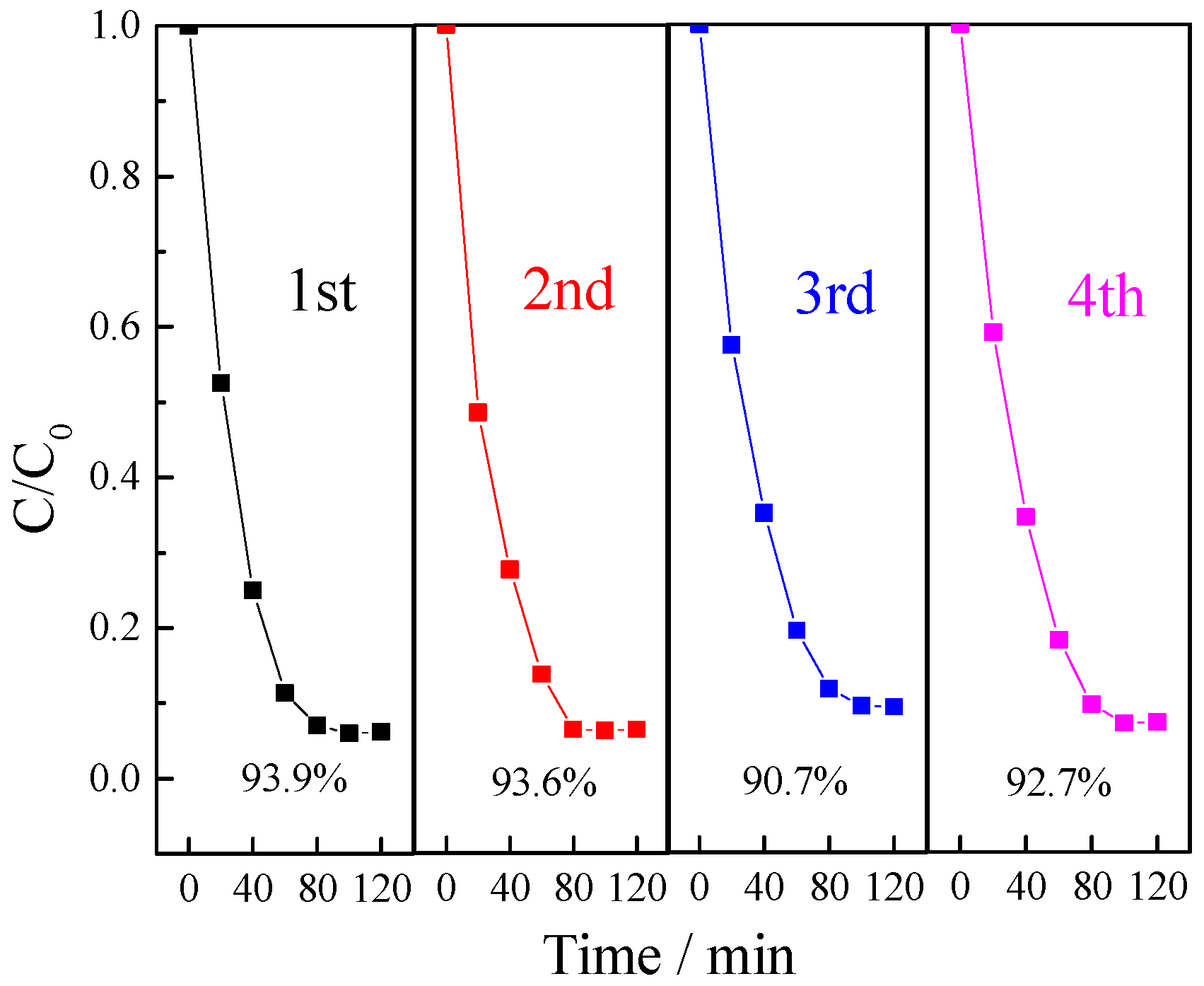
3.2.5. The Reusability of Photocatalyst
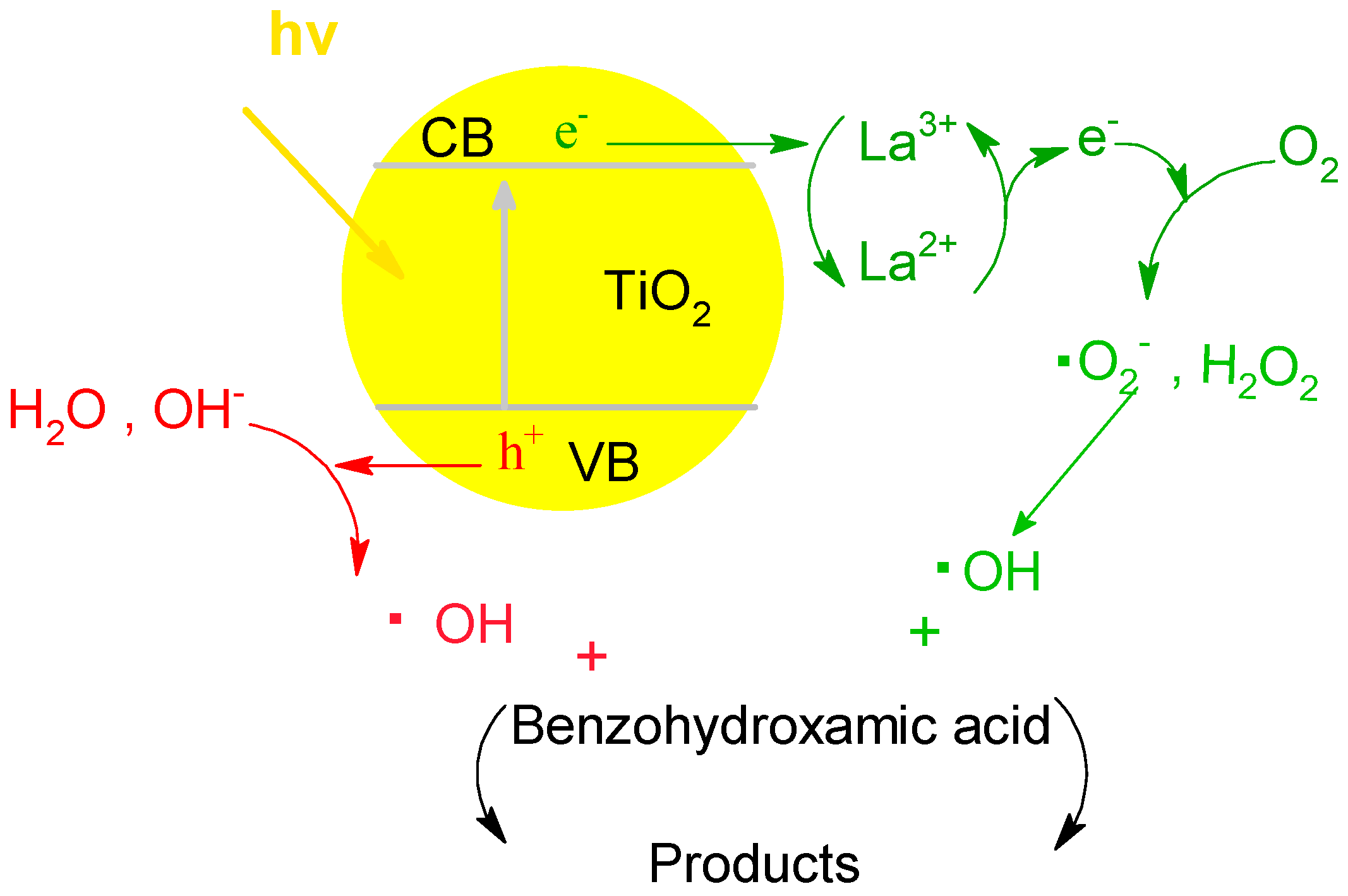
3.2.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis and Interfacial Charge Transfer Processes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Assis, S.M.; Montennegro, L.C.M.; Peres, A.E.C. Utilization of hydroxamates in minerals froth flotation. Miner. Eng. 1996, 9, 103–114. [Google Scholar] [CrossRef]
- Quast, K.B. A review of hematite flotation using 12-carbon chain collectors. Miner. Eng. 2000, 205, 1361–1376. [Google Scholar] [CrossRef]
- Hashimoto, S.; Nakumura, Y. Nuclease activity of a hydroxamic acid derivative in the presence of various metal ions. Chem. Soc. Chem. Commun. 1995, 14, 1413–1414. [Google Scholar] [CrossRef]
- Lee, K.; Archibald, D.; McLean, J. Flotation of mixed copper oxide and sulphidemineral swith xanthate and hydroxamate collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, J.; Zhao, J.; Xi, N.; He, D. Synthesis and crystal structure of N-((3-(2-nitrophenyl) propanoyl)oxy)-N-phenylbenzamide. Chin. J. Struct. Chem. 2015, 34, 688–694. [Google Scholar]
- Sinirtas, E.; Isleyen, M.; Soylu, G.S.P. Photocatalytic degradation of 2,4-dichlorophenol with V2O5-TiO2 catalysts: Effect of catalyst support and surfactant additives. Chin. J. Catal. 2016, 37, 607–615. [Google Scholar] [CrossRef]
- Ako, R.T.; Ekanayake, P.; Tan, A.L. La modified TiO2 photoanode and its effect on DSSC performance: A comparative study of doping and surface treatment on deep and surface charge trapping. Mater. Chem. Phys. 2016, 172, 105–112. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.R.; Pudukudy, M.; Yaakob, Z.; Ismail, N.A. CeO2-TiO2 as a visible light active catalyst for the photoreduction of CO2 to methanol. J. Rare Earths 2015, 33, 1155–1161. [Google Scholar] [CrossRef]
- Salas-Bañales, E.; Quiroz-Segoviano, R.I.Y.; Díaz-Alejo, L.A.; Rojas-González, F.; Estrella-González, A.; Campero, A.; García-Sánchez, M.A. Comparative study of the optical and textural properties of tetrapyrrole macrocycles trapped within ZrO2, TiO2, and SiO2 translucent xerogels. Molecules 2015, 20, 19463–19488. [Google Scholar] [CrossRef] [PubMed]
- Golubovic, A.; Tomic, N.; Fincur, N. Synthesis of pure and La-doped anatase nanopowders by sol-gel and hydrothermal methods and their efficiency in photocatalytic degradation of alprazolam. Ceram. Int. 2014, 40, 13409–13418. [Google Scholar] [CrossRef]
- Huo, Y.; Zhu, J.; Li, J. An active La/TiO2 photocatalyst prepared by ultrasonication-assisted sol-gel method followed by treatment under supercritical conditions. J. Appl. Phys. 2007, 278, 237–243. [Google Scholar] [CrossRef]
- Okuno, T.; Kawamura, G.; Muto, H.; Matsuda, A. Fabrication of shape-controlled Au nanoparticles in a TiO2-containing mesoporous template using UV irradiation and their shape-dependent photocatalysis. J. Mater. Sci. Technol. 2014, 30, 8–12. [Google Scholar] [CrossRef]
- Li, H.; Shi, Z.; Liu, H. Humidity sensing properties of La3+/Ce3+-doped TiO2-20 wt.% SnO2 thin films derived from sol-gel method. J. Rare Earths 2010, 28, 123–127. [Google Scholar] [CrossRef]
- Bingham, S.; Daoud, W.A. Recent advances in making nano-sized TiO2 visible-light active through rare-earth metal doping. J. Mater. Chem. 2011, 21, 2041–2050. [Google Scholar] [CrossRef]
- Stengl, V.; Bakardjieva, S.; Murafa, N. Preparation and photocatalytic activity of rare earth doped TiO2 nanoparticles. Mater. Chem. Phys. 2008, 114, 217–226. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Ismail, A.A.; Mohamed, R.M. Enhancement of titania by doping rare earth for photodegradation of organic dye (Direct Blue). J. Hazard. Mater. 2008, 166, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Bueno-Lopez, A.; Verbaas, M. The effect of surface OH-population on the photocatalytic activity of rare earth-doped P25-TiO2 in methylene blue degradation. J. Catal. 2008, 260, 75–80. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Wang, X.; Yu, T.; Guan, J.; Yu, Z.; Li, Z.; Zou, Z. Increasing the oxygen vacancy density on the TiO2 surface by La-doping for dye-sensitized solar cells. J. Phys. Chem. 2010, 114, 18396–18400. [Google Scholar]
- Grujic-Brojcin, M.; Armakovic, S.; Tomic, N. Surface modification of sol-gel synthesized TiO2 nanoparticles induced by La-doping. Mater. Charact. 2013, 88, 30–41. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Wang, X. Catalytic degradation of 4-chlorophenol with La/TiO2 in a dielectric barrier discharge system. RSC Adv. 2016, 6, 28994–29002. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Revathy, V.; Rosmin, V. Influence of La doping on structural and optical properties of TiO2 nanocrystals. Mater. Charact. 2016, 113, 144–151. [Google Scholar] [CrossRef]
- Leila, E.; Hinda, L.; Ammar, H. Synthesis, characterization and photocatalytic activity of Li−, Cd−, and La-doped TiO2. Mater. Sci. Semicond. Process. 2015, 36, 103–114. [Google Scholar]
- Moradi, S.; Vossoughi, M.; Feilizadeh, M. Photocatalytic degradation of dibenzothiophene using La/PEG-modified TiO2 under visible light irradiation. Res. Chem. Intermed. 2015, 41, 4151–4167. [Google Scholar] [CrossRef]
- Kumaresan, L.; Prabhu, A.; Palanichamy, M.; Arumugam, E. Synthesis and characterization of Zr4+, La3+ and Ce3+ doped mesoporous TiO2: Evaluation of their photocatalytic activity. J. Hazard. Mater. 2010, 186, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.R.; Ahmed, E.; Hong, Z.L.; Ahmad, M. Synthesis and photocatalytic properties of visible light responsive La/TiO2-graphene composites. Appl. Surf. Sci. 2012, 263, 254–259. [Google Scholar] [CrossRef]
- Tanyi, A.R.; Rafieh, A.I.; Ekaneyaka, P. Enhanced efficiency of dye-sensitized solar cells based on Mg and La co-doped TiO2 photoanodes. Electrochim. Acta 2015, 178, 240–248. [Google Scholar] [CrossRef]
- Fan, W.; Bai, H.; Zhang, G.; Yan, Y.; Liu, C.; Shi, W. Titanium dioxide macroporous materials doped with iron: Synthesis and photo-catalytic properties. Cryst. Eng. Comm. 2014, 16, 116–122. [Google Scholar] [CrossRef]
- Sharotri, N.; Sud, D. A greener approach to synthesize visible light responsive nanoporous S-doped TiO2 with enhanced photocatalytic activity. New J. Chem. 2015, 39, 2217–2223. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Selvaganesh, S.V.; Giridhar, V.V.; Bhat, S.D. Iron and nitrogen co-doped titania matrix supported Pt for enhanced oxygen reduction activity in polymer electrolyte fuel cells. RSC Adv. 2016, 6, 39261–39274. [Google Scholar] [CrossRef]
- Komarala, E.V.P.; Doshi, S.; Mohammed, A.; Bahadur, D. Efficient antibacterial activity via protein degradation of a 3D layered double hydroxide-reduced graphene oxide nanohybrid. RSC Adv. 2016, 6, 40389–40398. [Google Scholar] [CrossRef]
- Guerra, V.L.P.; Altamura, D.; Trifiletti, V. Implications of TiO2 surface functionalization on polycrystalline mixed halide perovskite films and photovoltaic devices. J. Mater. Chem. 2015, 3, 20811–20818. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, C.; Chen, J.; Che, H.; Xiao, L.; Gu, W.; Shi, W. Facile synthesis of BiOI/CdWO4 p-n junctions: Enhanced photocatalytic activities and photoelectrochemistry. RSC Adv. 2016, 6, 38290–38299. [Google Scholar] [CrossRef]
- Wen, Q.; Yu, J.; Sun, X. Hydrothermal treatment of a TiO2 film by hydrochloric acid for efficient dye-sensitized solar cells. New J. Chem. 2016, 40, 3233–3237. [Google Scholar] [CrossRef]
- Spadavecchia, F.; Cappelletti, C.; Ardizzone, S. Solar photoactivity of nano-N-TiO2 from tertiary amine: Role of defects and paramagnetic species. Appl. Catal. B 2010, 96, 314–322. [Google Scholar] [CrossRef]
- Gomez, V.; Clemente, A.; Irusta, S.; Balasab, F.; Santamaria, J. Identification of TiO2 nanoparticles using La and Ce as labels: Application to the evaluation of surface contamination during the handling of nanosized matter. Environ. Sci. Nano 2014, 1, 496–503. [Google Scholar]
- Das, S.K.; Bhunia, M.K.; Sinha, A.K.; Bhaumik, A. Characterization, and biofuel application of mesoporous Zirconium oxophosphates. ACS Catal. 2011, 1, 493–501. [Google Scholar] [CrossRef]
- Masika, E.; Mokaya, R. Mesoporous aluminosilicates from a zeolite BEA recipe. Chem. Mater. 2011, 23, 2491–2498. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Chen, X.; Niu, H.; Zhang, T.; Liu, J.; Qu, F. Fabrication of TiO2/porous carbon nanofibers with superior visible photocatalytic activity. New J. Chem. 2015, 39, 7863–7872. [Google Scholar] [CrossRef]
- Bai, S.; Hu, X.; Sun, J.; Ren, B. Preparation and characterization of Ti supported bimodal mesoporous catalysts using a self-assembly route combined with a ship-in-a-bottle method. New J. Chem. 2014, 38, 2128–2134. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, G.; Guo, L. Hierarchically structured TiO2 for Ba-filled skutterudite with enhanced thermoelectric performance. J. Mater. Chem. A 2014, 2, 20629–20635. [Google Scholar] [CrossRef]
- Hasan, M.R.; Hamid, S.B.A.; Basirun, W.J. Ga doped RGO-TiO2 composite on an ITO surface electrode for investigation of photoelectrocatalytic activity under visible light irradiation. New J. Chem. 2015, 39, 369–376. [Google Scholar] [CrossRef]
- Li, N.; Jayaraman, S.; Tee, S.Y. Effect of La-doping on optical bandgap and photoelectrochemical performance of hematite nanostructures. J. Mater. Chem. A 2014, 2, 19290–19297. [Google Scholar] [CrossRef]
- Boulon, G.; Alombert-Goget, G.; Guyot, Y.; Guzik, M.; Epicier, T.; Blanchard, N.P.; Chen, L.; Hud, V.; Chen, W. Conjugation of TEM-EDX and optical spectroscopy tools for the localization of Yb3+, Er3+ and Co2+ dopants in laser glass ceramics composed of MgAl2O4 spinel nano-crystals embedded in SiO2 glass. J. Mater. Chem. C 2014, 2, 9385–9397. [Google Scholar] [CrossRef]
- Yoon, S.; Manthiram, A. Hollow core-shell mesoporous TiO2 spheres for lithium ion storage. J. Phys. Chem. C 2011, 115, 9410–9416. [Google Scholar] [CrossRef]
- Zhou, W. Study on the Biodegradability of Hydroxamic Acid Collectors. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2012. [Google Scholar]
- Hu, C.; Wang, C.; Gong, W.; Mei, G. Study on the biodegradation of three hydroxamic acid collectors. Hubei Agric. Sci. 2013, 52, 2505–2507. [Google Scholar]
- Li, G.; Li, L.; Boerio-Goates, J.; Woodfield, B. High purity anatase TiO2 nanocrystals: Near room-temperature synthesis, grain growth kinetics, and surface hydration chemistry. J. Am. Chem. Soc. 2005, 127, 8659–8666. [Google Scholar] [CrossRef] [PubMed]
- Beaussart, A.; Petrone, L.; Mierczynska-Vasilev, A.; McQuillan, A.; David, A. In situ ATR FTIR study of dextrin adsorption on anatase TiO2. Langmuir 2012, 28, 4233–4240. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Tang, J.; Teng, C.; Cai, T.; Deng, Q. La-modified mesoporous TiO2 nanoparticles with enhanced photocatalytic activity for elimination of VOCs. J. Porous Mater. 2015, 22, 361–367. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wang, J.; Wang, C.; Zhu, S.; Li, Z.; Tang, X.; Wu, M. Degradation and Mineralization of Benzohydroxamic Acid by Synthesized Mesoporous La/TiO2. Int. J. Environ. Res. Public Health 2016, 13, 997. https://doi.org/10.3390/ijerph13100997
Luo X, Wang J, Wang C, Zhu S, Li Z, Tang X, Wu M. Degradation and Mineralization of Benzohydroxamic Acid by Synthesized Mesoporous La/TiO2. International Journal of Environmental Research and Public Health. 2016; 13(10):997. https://doi.org/10.3390/ijerph13100997
Chicago/Turabian StyleLuo, Xianping, Junyu Wang, Chunying Wang, Sipin Zhu, Zhihui Li, Xuekun Tang, and Min Wu. 2016. "Degradation and Mineralization of Benzohydroxamic Acid by Synthesized Mesoporous La/TiO2" International Journal of Environmental Research and Public Health 13, no. 10: 997. https://doi.org/10.3390/ijerph13100997
APA StyleLuo, X., Wang, J., Wang, C., Zhu, S., Li, Z., Tang, X., & Wu, M. (2016). Degradation and Mineralization of Benzohydroxamic Acid by Synthesized Mesoporous La/TiO2. International Journal of Environmental Research and Public Health, 13(10), 997. https://doi.org/10.3390/ijerph13100997





